Introduction
Among the most poorly known high-Andean cricetids is Neomicroxus, a genus recently erected to encompass small-bodied akodont-like sigmodontines previously placed in Akodon and Microxus. Neomicroxus was based on Microxus latebricola, originally described from a single specimen collected in Ambato, on the eastern Cordillera in Ecuador (Anthony 1924). Another species, Acodon bogotensis, is also included in the genus, being up to date exclusive from Colombia and Venezuela (Alvarado-Serrano and D’Elía 2013, 2015).
Paradoxically, both species of Neomicroxus remained taxonomically unexplored, although they are abundant and easy to catch in high-Andean environments (e. g., Corporación Suna Hisca 2003; Vianchá et al. 2012; Brito 2013; Curay 2019; Ojala-Barbour et al. 2019). The deconstruction of the genus Microxus, after the peak of its complexity during the ’30 (i. e., involving affinis, bogotensis, iheringi, lanosus, latebricola, mimus, and torques; Gyldenstolpe 1932), was a slow and hesitant process. As late as the beginnings of the present century, the taxonomic situation of bogotensis and latebricola was summarized by Voss (2003:21) as follows “This species latebricola closely resembles Akodon sic bogotensis Thomas (1895), another eastern-Andean species that was formerly referred to the genus Microxus. Among other shared similarities, both species differ from typical Akodon by their … Although phylogenetic analyses of mitochondrial DNA sequences do not support the separate generic status of Microxus (as represented by the type species mimus Thomas; seeSmith and Patton 1993and references cited therein), sequence data from latebricola and bogotensis have not been analyzed. Despite their current generic classification, these two northern-Andean endemics clearly form a distinct clade that merits nomenclatural recognition.” The advent of the first molecular data for latebricola was the keystone to crystallize what the acute morphological eye of Voss (2003) envisioned; Neomicroxus was erected with latebricola as type species (Alvarado-Serrano and D’Elía 2013). However, molecular findings retrieved an additional and previously unsuspected result; N. latebricola was neither an Akodon nor an Akodontini (Alvarado-Serrano and D’Elía 2013).
Almost nothing is known about the potential variability within Neomicroxus along the > 10º degrees of latitude which encompasses its range. Both species are found in the northern Andes at elevations above 2,400 masl and reaching as high as 3,900 masl (Alvarado-Serrano and D’Elía 2015). N. bogotensis is endemic to the Cordillera Oriental of Colombia and the Cordillera de Mérida and Páramo de Tamá in Venezuela, while N. latebricola occurs to high elevations of the eastern Andes in Ecuador, from Tungurahua to Carchi provinces (Alvarado-Serrano and D’Elía 2013, 2015). Recently, Curay (2019) revealed morphological variation within the Ecuadorian populations that supports the recognition of geographic structure in what is currently understood as N. latebricola. It is in agreement with the finding of populations of the species, traditionally known and restricted only to the eastern Andes, in western locations from the Cordillera Occidental such as the Páramo de Frailejones (Brito 2013). In this contribution, we undertook a reappraisal of the systematics of Neomicroxus, including for the first time sequences of N. bogotensis. We analyzed two DNA markers and morphometric variables as a first attempt to explore of the alpha-taxonomy of the genus.
Materials and methods
Sequence acquisition. We obtained DNA sequences from specimens of Neomicroxus from Colombia (n = 3, Cundinamarca and Santander departments) and Ecuador (n = 2, Carchi province; Appendix 1, Appendix 2). The new molecular data consisted of five nucleotide sequences of the first portion of the mitochondrial cytochrome b gene (cytb, 801bp) and four of the first exon of the interphotoreceptor retinoid binding protein (IRBP, 1514bp). Here, we included for the first time in any phylogenetic study sequences of three specimens of Neomicroxus bogotensis. The monophyly of the genus, the identity of the sister group, and its phylogenetic position into the Sigmodontinae have not been fully corroborated (Alvarado-Serrano and D’Elía 2013). Therefore, we included sequences for representatives of the several tribes of Sigmodontinae and some outgroup taxa (other Cricetidae, Nesomyidae and Spalacidae) retrieved from GenBank. For those terminals that miss information, we completed the matrix with missing data or ambiguous state characters (i.e. N). All analyzed taxa as well as the vouchers of their cytb and IRBP sequences are listed in Appendix 2.
DNA of high molecular weight was extracted from the Ecuadorian specimens (N. latebricola) using the protocol of the Wizard Genomic DNA Purification kit, with fresh tissues as starting material. In the case of the Colombian specimens also DNA of high molecular weight was extracted from fresh tissues, as well as degraded DNA from ancient material (small fragments of rehydrated soft tissue adhered to cranial bones of museum specimens); a GeneJet Genomic DNA Purification Kit (Thermo Fisher Scientific) was used indistinctly for both processes. However, the ancient material was previously subjected to a repetitive washing protocol (Giarla et al. 2010) in order to remove foreign DNA and potential PCR inhibitors. Primer pairs used for amplification and sequencing of the mitochondrial fragment from the Ecuadorian specimens were MVZ05 and MVZ16 (Smith and Patton 1993), while for the IRBP locus we used the A1 and F1 (Jansa and Voss 2000). Amplification conditions followed Da Silva and Patton (1993) for cytb and Jansa and Voss (2000) for IRBP. For the Colombian specimens with DNA of high molecular weight (UIS-MZ 1299 and 1596), a fragment of + 800 bp of the cytb was amplified with the flanking primer L14724 (Irwin et al. 1991), and the internal primer O700 H (Handson and Bradley 2008), while for the specimen with degraded DNA (IAvH 5777), only + 400 base pairs of the gene were amplified with the flanking primer MVZ05, and the internal primer MVZ04 (Smith and Patton 1993). For these cytb sequences we modified the amplification conditions of Hanson and Bradley (2008). The IRBP locus only was amplified from the Colombian specimens with DNA of high molecular weight using the primers IRBP217 and IRBP1351 (Stanhope et al. 1992) and we followed the amplification conditions mentioned there. All reactions included negative controls. Amplicons from Ecuadorian specimens were purified and sequenced at the external service of Macrogen, Inc. (Seoul, Korea), whereas those from Colombian specimens at the Servicio de Secuenciación y Análisis Molecular SSiGMol, Universidad Nacional de Colombia (Bogotá, Colombia).
Descriptive and phylogenetic analysis. Before conducting phylogenetic analyses, we checked the quality of each DNA sequence in the edition process with CodonCode Aligner (Codon-Code 2014). Subsequently, sequences were aligned using default options in ClustalX 2.0 (Larkin et al. 2007). New DNA sequences were deposited in GenBank (accession numbers cytb: MT240520-MT240524, IRBP: MT249797-MT249800). Observed values of cytb sequence divergence (p distance) were calculated with MEGA7 (Kumar et al. 2016) ignoring those sites with missing data (Appendix 3). Phylogenetic analyses were conducted using the concatenated matrix and subjected to Maximum Parsimony (MP; Farris 1982), Maximum Likelihood (ML; Felsenstein 1981) and Bayesian Inference (BI; Huelsenbeck et al. 2001) approaches. MP analysis was carried out in PAUP* version 4.0 (Swofford 2000) with characters treated as unordered and equally weighted, 200 replicates of heuristic searches with random addition of sequences and tree bisection-reconnection (TBR) branch swapping. Nodal support was estimated by 1,000 bootstrap replicates with five replicates of sequence addition each (BT1). For the ML analysis, we used IQ-TREE version 1.6.0 software (Nguyen et al. 2015) implemented in the IQ-TREE webserver (Trifinopoulos et al. 2016) using LG+I+G4 substitution as the best-fit model. Statistical support for each individual node of the ML phylogenetic tree was estimated using 1,000 iterations of the ultrafast bootstrap value (BT2). Bayesian analyses were conducted in MrBayes 3.2 (Ronquist et al. 2012). We performed two independent runs, each with three heated and one cold Markov chains, were allowed to proceed for 107 iterations and were sampled every 1,000 generations. We used the GTR+G+I substitution model previously determined by Akaike information criterion (AIC) in jModeltest (Posada 2008). Log-likelihood values against generation time for each run were plotted in Tracer v1.7.1 (Rambaut et al. 2018). The first 25 % of the trees obtained were discarded as burn-in, and the remaining trees were used to construct a 50 % majority rule consensus tree and obtain the support for each clade as posterior probability (PP) values. Outgroups used in the phylogenetic analyses include taxa of Sigmodontinae and representatives of another rodents families (i. e., Cricetidae, Nesomyidae and Spalacidae).
Studied specimens. We examined the external and craniodental morphology of 55 specimens of Neomicroxus, including skulls, skins, and fluid-preserved animals (see Appendix 1). Studied specimens are deposited in the following institutional collections: Argentina: Colección de Mamíferos del Centro Nacional Patagónico (CNP; Puerto Madryn, Chubut). Colombia: Colección de Mamíferos del Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH; Villa de Leyva, Boyacá). Colección de Mamíferos Alberto Cadena García” del Instituto de Ciencias Naturales de la Universidad Nacional de Colombia (ICN: Bogotá). Colección de Mamíferos del Museo de Historia Natural de la Universidad Industrial de Santander (UIS-MZ; Bucaramanga, Santander). Ecuador: Museo de Zoología de la Pontificia Universidad Católica del Ecuador (QCAZ; Quito). Instituto Nacional de Biodiversidad (INABIO-MECN; Quito). Instituto de Ciencias Biológicas de la Escuela Politécnica Nacional (MEPN; Quito). United States: National Museum of Natural History of Smithsonian Institute (USNM; Washington).
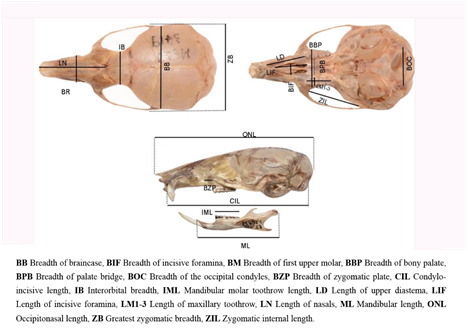
Morphometrics. Taking into account the important degree of hypsodonty showed by Neomicroxus, we established an ad-hoc classification composed by six tooth-wear stages (TWC, Figure 1), which based on dental wear on the cusps and the differentiation of the main structures. In this context, we considered as adults those specimens belonging to the TWC 4 to 6. These animals were employed in morphological qualitative assessment and ulterior statistical analyses based on 18 craniodental measurements (Appendix 4), taken with a digital caliper and expressed in millimeters. For descriptive purposes, univariate statistics for each measurement were calculated. To perform a multivariate exploration, we used a sample composed by 12 specimens of N. bogotensis and 21 of N. latebricola (sexes pooled) as input data for a principal component analysis (PCA; Johnson and Wichern 1999; Carleton and Musser 1989). Raw data were standardized by transformation to their natural logarithms and the first three principal components were calculated on the resultant covariance matrix. To test the potential variation of N. bogotensis through its range, we added to the analysis several Venezuelan specimens (Mérida and Táchira states) with incomplete measurements because they were assessed with a different goal by the senior author. For this reason and to avoid calculations with missing data, we reduced the number of variables considered to eight (ONL, BZP, LD, LIF, LM1-3, BB, CIL, IML), and worked on a matrix composed by 12 individuals. For N. latebricola, the PCA was executed with 21 individuals and 18 variables (Appendix 3). Additionally, to assess the differentiation between the molecular recovered groups, we perform a Discriminant Analysis (DA) employing the same log-transformed data removing missing values (25 individuals, 16 variables). Group assignments were validated by a jackknife resampling. For all morphometrical analyses, we used the free software Past version 4.0 (Hammer et al. 2001).
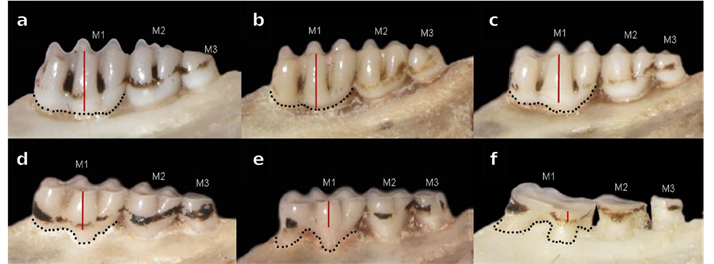
Figure 1 Neomicroxus age classification estimated by the dental wear on the cusps and the differentiation of the main structures. a) TWC1: M1-M2 cusps pronounced with flexus and fossettes visible. Features associated with the procingulum are notorious. M3 erupted, no wear, b) TWC2: M1-M2 similar to TWC1, but M3 shows slight wear worn although still complex in morphology, c) TWC3: M1 has a vestige of posteroloph, roots still are not very visible. M2 retaining some structures, but possesses some fossetes and vestige of the anteroloph. M3 structures are less distinguishable, d) TWC4: Smaller flexus and posteroloph in M1, with noticeable roots. M3 is almost flat with anteroloph and posteroloph barely visible or not distinguishable, e) TWC5: Molar surfaces with scarce occlusal structures, M1 anteromedian flexus not differentiated, M2 flexus slightly evident, and M3 fully flattened, and f) TWC6: Molar surfaces without occlusal structures, roots very visible. M3 is totally worn, clearly exposing the dentin.
Results
Phylogenetic relationships and genetic divergence. Phylogenetic analyses recovered well-resolved topologies within Oryzomyalia (sensuSteppan et al. 2004), with tribal relationships mostly concordant with previous studies (e. g.,Alvarado-Serrano and D’Elía 2013; Salazar-Bravo et al. 2016; Gonçalves et al. 2018). The genus Neomicroxus was found to be monophyletic with high support values (Figure 2a; BT1 - BT2 - PP = 99 - 99 - 1.0), but without affiliation to any recognized tribe in the MP and BI topologies, and sister to Abrotrichini + Wiedomyini in the ML analysis but weakly supported. In all approaches (MP, ML, and BI), two major clades strongly supported were retrieved within the genus. One clade is formed by individuals from Colombia (100 - 96 - 0.9) and can be referred to what is currently understood as N. bogotensis; another clade is represented by sequences from Ecuadorian Cordillera Oriental and Occidental (100 - 74 - 0.9), and can be confidently associated to N. latebricola. The overall mean divergence at the cytb gene for Neomicroxus reaches 6.3 %, meanwhile, the genetic distance between the two main clades is 11 % (see Appendix 3). In the bogotensis clade, the phyletic relationships show a profound divergence between individuals from Santander and Cundinamarca departments (> 6 %). Our sampling is insufficient to evaluate the demography of the species, however, the analyzed localities are geographically close, so we can affirm that the divergence observed between northern and central (Cundinamarca) Colombia is not due to a phenomenon of isolation by distance. This deep divergence (also reflected in the branch lengths), suggests that the populations of N. bogotensis here analyzed are older, possibly demographically stable, with a strong barrier (geographical or ecological) that interrupts gene flow.
On the other hand, within the latebricola clade, we recovered a shallow genealogy with two minor groups or subclades which diverge by 1.4 %. One subclade is composed of the same haplotype shared by QCAZ4160 and QCAZ4167 individuals, both from the Ecuadorian Napo province. Meanwhile the other subclade groups three different haplotypes, slightly divergent, from Carchi (MECN3727 - MECN3734, QCAZ9801) and Napo (QCAZ4121) provinces (Appendix 3). Conversely to the observed variation in N. bogotensis, the genealogical relationships and the divergence values between and within N. latebricola subclades reveal the existence of current genetic flow between populations, reflected by the lack of reciprocal monophyly between the provinces, which also is a sign of populations in the process of expansion.
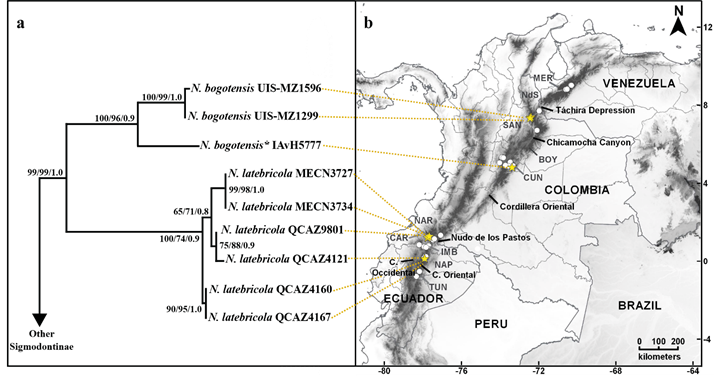
Figure 2. a) Phylogenetic tree of concatenated matrix related to the b) geographical distribution of Neomicroxus specimens from the Andes of Ecuador, Colombia and Venezuela. Support values (MP - ML - BI) are indicate next to each node. Yellow stars indicate specimens used in the phylogenetic analyses.
Morphometric analyses. The univariate morphometric analysis reveals little differences among the samples, being N. bogotensis who possesses lower values to several of the craniodental variables here recorded (Table 1). The PCA for N. bogotensis showed a clear separation between specimens from north of Colombia (Santander and Norte de Santander departments), Cundinamarca, and Venezuela (Figure 3). The 88.6 % of the total variation is summarized in the first two principal components, none of which can be interpreted as a size factor because they include positive and negative coefficients for some variables (Table 2). The largest contribution of the first component is attributed to the following variables: breadth of zygomatic plate, length of incisive foramina, length of upper diastema, occipitonasal length, while for the PC2 are the lengths of incisive foramina and the upper diastema. The N. latebricola PCA retrieves two partially overlapping groups from the Cordillera Oriental (Napo province) and Occidental (Carchi province; Figure 3). These results are congruent with the molecular results (see above); 77.2 % of the variation is explained by the PC1-2. The craniodental variables with the greatest contribution are the breadth of incisive foramina and breadth of the bony palate on the PC1, and breadth of zygomatic plate and length of incisive foramina on the PC2 (Table 2).
Table 1 Univariate statistics for selected craniodental measurements in Neomicroxus. Values provided are mean ± standard deviation and minimum-maximum range. All measurements are expressed in millimeters.
| N. latebricola Occidente | N. latebricola Oriente | N. bogotensis North Colombia | N. bogotensis Cundinamarca | N. bogotensis Venezuela | |
|---|---|---|---|---|---|
| n = 13 | n = 9 | n = 3 | n = 1 | n = 9 | |
| Occipitonasal length - ONL | 25.53 ± 0.4 (24.95 - 26.21) | 25.18 ± 0.22 (24.89 - 25.65) | 23.52 ± 0.71 (22.75 - 24.14) | 24.33 | 23.18 ± 0.39 (22.49 - 23.74) |
| Condylo-incisive length - CIL | 23.18 ± 0.49 (22.49 - 23.91) | 22.62 ± 0.24 (22.15 - 22.93) | 20.57 ± 0.67 (19.9 - 21.24) | 21.45 | 20.43 ± 0.31 (20.03 - 21.14) |
| Greatest zygomatic breadth - ZB | 11.97 ± 0.15 (11.73 - 12.34) | 12.04 ± 0.12 (11.82 -12.21) | --- | 11.67 | 11.25 ± 0.21 (11.01 - 11.56) |
| Interorbital breadth - IB | 4.70 ± 0.1 (4.41 - 4.87) | 4.82 ± 0.12 (4.69 -5.06) | 4.45 ± 0.19 (4.25 - 4.63) | 4.75 | --- |
| Breadth of zygomatic plate - BZP | 1.39 ± 0.06 (1.3 - 1.5) | 1.38 ± 0.1 (1.26 -1.52) | 1.33 ± 0.04 (1.29 - 1.37) | 1.67 | 1.36 ± 0.1 (1.23 -1.54) |
| Length of upper diastema - LD | 6.26 ± 0.19 (6 - 6.68) | 6.38 ± 0.2 (6.09 - 6.74) | 5.75 ± 0.16 (5.58 -5.9) | 6.01 | 5.6 ± 0.13 (5.39 - 5.83) |
| Breadth of bony palate - BBP | 5.39 ± 0.1 (5.24 - 5.6) | 5.36 ± 0.09 (5.26 - 5.51) | 5.24 ± 0.35 (4.99 - 5.64) | 5.59 | --- |
| Length of incisive foramina - LIF | 4.75 ± 0.18 (4.44 - 5) | 4.73 ± 0.25 (4.46 - 5.15) | 4.12 ± 0.71 (3.3 - 4.56) | 4.5 | 3.97 ± 0.14 (3.76 - 4.17) |
| Breadth of incisive foramina - BIF | 1.66 ± 0.1 (1.55 - 1.84) | 2.02 ± 0.14 (1.83 - 2.2) | 1.76 ± 0.13(1.62 - 1.85) | 1.87 | --- |
| Length of maxillary toothrow - LM1-3 | 3.63 ± 0.1 (3.41 - 3.83) | 3.5 ± 0.13 (3.26 - 3.69) | 3.46 ± 0.06 (3.42 - 3.53) | 3.73 | 3.57 ± 0.09 (3.43 - 3.69) |
| Breadth of first upper molar - BM | 1.17 ± 0.04 (1.08 - 1.25) | 1.12 ± 0.04 (1.03 - 1.17) | 1.14 ± 0.09 (1.04 - 1.2) | 1.15 | --- |
| Breadth of palatal bridge - BPB | 2.65 ± 0.16 (2.46 - 3.1) | 2.99 ± 0.17 (2.75 - 3.31) | 2.68 ± 0.49 (2.29 -3.23) | 3.13 | --- |
| Length of nasals - LN | 10.11 ± 0.16 (9.66 - 10.27) | 10.1 ± 0.12 (9.87 - 10.24) | 9.24 ± 0.37 (8.84 - 9.58) | 9.52 | --- |
| Breadth of braincase - BB | 11.65 ± 0.20 (11.21 - 11.96) | 11.65 ± 0.15 (11.28 - 11.79) | 10.93 ± 0.37 (10.53 -11.25) | 11.26 | 11.11 ± 0.21 (10.72 - 11.38) |
| Breadth of the occipital condyles - BOC | 6.14 ± 0.12 (5.92 - 6.39) | 6.14 ± 0.09 (6.04 - 6.3) | 5.82 ± 0.24 (5.55 - 5.98) | 5.77 | --- |
| Zygomatic internal length - ZIL | 7.03 ± 0.17 (6.75 - 7.36) | 7.08 ± 0.1 (6.85 - 7.18) | --- | 6.61 | --- |
| Mandibular length - ML | 12.76 ± 0.33 (11.98 - 13.25) | 13.04 ± 0.37 (12.6 - 13.59) | 11.41 ± 0.29 (11.19 - 11.74) | 12.15 | --- |
| Mandibular molar toothrow length - IML | 3.8 ± 0.08 (3.63 - 3.94) | 3.65 ± 0.13 (3.43 - 3.78) | 3.77 ± 0.03 (3.74 - 3.79) | 3.87 | 3.77 ± 0.11 (3.63 - 3.9) |
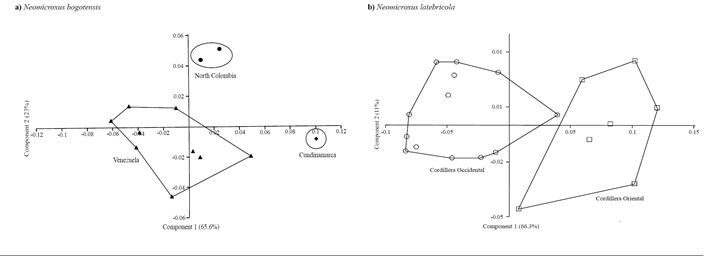
Figure 3. Principal component analysis, components 1 and 2, of the 8 log-transformed craniodental measurements for in N. bogotensis (n=12), and 18 for N. latebricola (n=21). ♦: N. bogotensis Cundinamarca, ●: N. bogotensis North Colombia (Norte de Santander and Santander), ▲: N. bogotensis Venezuela, ○: N. latebricola Ecuadorian Cordillera Occidental, and □: N. latebricola Ecuadorian Cordillera Oriental.
The Discriminant Analysis confirms the separation of N. bogotensis from the north of Colombia (i.e. Norte de Santander and Santander departments) and the specimen of Cundinamarca department. Similarly, the samples of N. latebricola from the Oriental and Occidental cordilleras of Ecuador are clearly differentiated (Figure 4). For both species, the recovered groups are completely concordant with the molecular arrangements. According to jackknife resampling, the predefined groups (i.e. N. bogotensis: North Colombia Norte de Santander and Santander departments, Cundinamarca, and Venezuela Mérida, N. latebricola: Ecuadorian Cordillera Oriental and Occidental ) are correctly classified in a 72 % when Venezuela is excluded, and a 70 % when it is included (Appendix 5). The variables which most contributed to the discrimination among these groups were the interorbital breadth, breadth of bony palate, breadth of incisive foramina, and breadth of first upper molar.
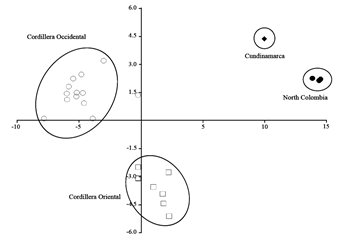
Figure 4. P Plot of canonical discriminant analysis based on 16 craniodental measurements from Colombian and Ecuadorian specimens of Neomicroxus. ♦: N. bogotensis Cundinamarca, ●: N. bogotensis North Colombia (Norte de Santander and Santander), ▲: N. bogotensis Venezuela, ○: N. latebricola Ecuadorian Cordillera Occidental, and □: N. latebricola Ecuadorian Cordillera Oriental.
Discussion
Neomicroxus uniqueness and phylogeny. The distinction of Neomicroxus as a new entity was based on molecular data from a few specimens of N. latebricola, cemented with a shallow morphological review at generic level, mostly pointed to disconnect Neomicroxus from Akodon (Alvarado-Serrano and D’Elía 2013). Since then, only Curay (2019) ventured to evaluate the variability of N. latebricola studying several populationa samples in Ecuador. This approach highlighted the occurrence of N. latebricola in western locations from the cordillera Occidental, a finding previously reported by Brito (2013; overlooked in Alvarado-Serrano and D’Elía 2015) and revealed unsuspected geographical variation.
Despite these findings, the non-inclusion of N. bogotensis in a formal phylogenetic analysis has limited the confirmation of hypothesis advanced by Voss (2003) and Alvarado-Serrano and D’Elía (2013) about the generic status of Neomicroxus. The monophyly of Neomicroxus is not an unsuspected result since both species have been traditionally considered very close due to morphological similarity (Voss 2003). In turn, the novelty molecular data for N. bogotensis, added to those of N. latebricola, strengthens the consideration that the genus does not appear closely related to any other lineage, placing Neomicroxus as a Sigmodontinae incertae sedis (Alvarado-Serrano and D’Elía 2013). This finding invites to the recognition of a new clade on Andean rodents with tribal rank.
Table 2 Results of the principal component analysis based on measurements of Neomicroxus specimens. Scheme and names of taken measurements are illustrated in the Supporting information S3.
| N. bogotensis (n=12) | ||
|---|---|---|
| PC 1 | PC 2 | |
| ONL | 0.18529 | 0.12481 |
| BZP | 0.79866 | -0.51397 |
| LD | 0.23801 | 0.22664 |
| LIF | 0.46159 | 0.76969 |
| LM1-3 | 0.1036 | -0.25169 |
| BB | 0.004621 | 0.023879 |
| CIL | 0.19013 | 0.07015 |
| IML | 0.10575 | -0.087326 |
| Eigenvalue | 0.0019788 | 0.0006965 |
| % variance | 65.571 | 23.081 |
| N. latebricola (n=21) | ||
| PC 1 | PC 2 | |
| ONL | -0.010971 | 0.11015 |
| ZB | -0.006378 | -0.025584 |
| IB | 0.09867 | 0.063224 |
| BZP | 0.041397 | 0.66519 |
| LD | 0.15393 | 0.24007 |
| BBP | 0.0086206 | 0.071494 |
| LIF | 0.067016 | 0.50184 |
| BIF | 0.78061 | -0.11472 |
| LM1-3 | -0.096618 | 0.21955 |
| BM1 | -0.12144 | 0.16342 |
| BPB | 0.5329 | 0.031021 |
| LN | 0.024229 | 0.089939 |
| BB | -0.018767 | -0.077084 |
| BOC | 0.041391 | -0.034099 |
| CIL | -0.026062 | 0.19255 |
| ZIL | 0.074836 | 0.088529 |
| MH | 0.13971 | 0.20511 |
| IML | -0.11945 | 0.18693 |
| Eigenvalue | 0.00417 | 0.00069 |
| % variance | 66.307 | 10.977 |
An additional issue is to explain the differential genealogical structure detected in each species of Neomicroxus, suggesting contrasting evolutionary histories. Probably, it could be linked with differential environmental conditions in the northern Andes along the Neogene that could promote the spatial structuring. Judged as a whole, the range of Neomicroxus shows an important gap in southern Colombia (Figure 2b). If this “lagoon,” which turns sharply allopatric both species, is artefactual or real is debatable. Colombian southernmost portions to the Ecuadorian border have been largely controlled by armed forces, turning mammalogical surveys an almost impossible task. Since N. latebricola is recorded in Ecuador very close to this border, and taking into account the habitat continuity (Curay 2019), its occurrence in Colombia is highly expected. The Andean geography in southern Colombia is very complex involving, towards north of Nudo de los Pastos, the occurrence of three main chains (cordilleras) instead of the two branches characterizing the Ecuadorian Andes. We could assume that contact between populations of N. latebricola and N. bogotensis has been limited by factors associated with this complexity. However, the finding of a single specimen from Nariño, Pasto Municipality, (Ramírez-Chaves and Noguera-Urbano 2010), which was erroneously identified as N. latebricola (Appendix 6), evidences that the distribution of bogotensis extends to the south of Colombia, and supports our hypothesis of reduced sampling in the region.
Neomicroxus bogotensis spatial structure and taxonomic implications. Of the two species currently considered in Neomicroxus, N. bogotensis, the smallest in body size, is the most poorly known. Almost a century after its shallow original description (Thomas 1895), N. bogotensis received some attention. Reig (1987:360) concluded, after the inspection of its holotype, that bogotensis belongs “… neither to Akodon nor to Abrothrix and that is a distinctive genus of Akodontini.” In addition, provided an informal diagnosis of Microxus, the genus where he placed this form, and distinguished bogotensis by their unique diploid complement (2n = 35-37, FN = 48; Barros and Reig 1979), and the lacking of paired ventral prostates (shared with Thaptomys, a finding conducted by Voss and Linzey 1981). The most recent descriptions of the species (Alvarado-Serrano and D’Elía 2015:98; Pardiñas and Brito 2017:409) considered this taxon as monotypic, despite previous indications in opposite way (see below).
Although stated as “rare” (see Linares 1998:272; Alvarado-Serrano and D’Elía 2015:98), N. bogotensis is an abundant cricetid in Andean highlands, at elevations between 2,400 and 3,900 masl, which corresponds to the cloud forest and páramo ecosystems (Cuatrecasas 1958; López-Arévalo et al. 1993; Rangel 2001). Ecological and systematic studies report it as an easy species to found in evergreen ombrophile montane forest and shrubby upland meadows (e. g.,Reig 1986; López-Arévalo et al. 1993; Soriano et al. 1999; Ventura et al. 2000; Vianchá et al. 2012).
Originally described for the “Plains of Bogota” (Thomas 1895:369), Cundinamarca, it has also been collected in others departments associated with the Cordillera Oriental in Colombia as Boyacá, Santander, and Norte de Santander (Saénz-Jiménez 2010; Vianchá et al. 2012). Some databases of mammalian collections also list specimens, not reviewed in this contribution, from the departments of César, Tolima, and Huila (i. e., American Museum of Natural History, The Field Museum of Natural History). The record of Ramírez-Chaves and Noguera-Urbano (2010) from the Nariño department is a significant data about the extension of the N. bogotensis geographic range towards southern Colombia. The range for the species is completed by its occurrence in the Cordillera de Mérida and Páramo de Tamá, in the Venezuelan states of Táchira and Mérida (Alvarado-Serrano 2005), plus an unconfirmed mention from Trujillo (Soriano et al. 1999).
Our analyses revealed a clear geographic structure in N. bogotensis, separating with strong support the specimens of Norte de Santander and Santander from that of Cundinamarca. Although our study has only a sequence of Cundinamarca, the high genetic distance values (> 6%) suggest the specific distinction of the populations from northern Colombia. This also warns about the restricted gene flow between northern departments and Cundinamarca.
It is interesting to note that so far, no studies have evaluated populations in northern Colombia and Venezuela as a whole. Soriano et al. (1999) highlight the need to examine the taxonomic identity of the populations of N. bogotensis in Venezuelan Andes. According to these authors, “it is convenient to examine the taxonomic identity of the populations of the latter Neomicroxus bogotensis, in the light of the parapatric or gradient speciation model, as has been referred to byPatton et al. (1990). Thus, given its high Andean distribution pattern, we expect that the morphotype of the Cordillera de Mérida, by virtue of its possible geographical isolation, could be distinguishable from the rest of the Andean populations. In the same way, we think that the identity of the Venezuelan populations of T.homasomys laniger and Chilomys instans would have to be examined” (Soriano et al. 1999:22).
The Andes in northern Colombia and Venezuela have great geographical complexity characterized by some depressions (e. g., Táchira and Barquisimeto Depression) that separate the mountains and generate significant breaks that lead to isolation and formation of so-called “montane sky islands” (Reig 1986; Anderson et al. 2012). In this sense, the Táchira Depression, characterized by a dry subtropical climate, has been regarded as a biogeographical barrier to the dispersal of Andean species from both cordilleras (Cordillera Oriental de Colombia and Cordillera de Mérida, Soriano et al. 1999; Soriano et al. 2005). Species with lower vagility and strictly restricted to the cloud forest and páramo, would be virtually absent today, but probably had a wider and continuous distribution during glacial periods as suggested for Heteromys australis (Anderson and Soriano 1999) and Marmosa waterhousei (Gutiérrez et al. 2011), both species distributed in a lower altitudinal range than Neomicroxus. Based on the morphometrical results, the individuals from Venezuela are smaller (Figure 3, Table 1), clearly distinguishable from the Colombian specimens. It seems unlikely that the absence of N. bogotensis in the Táchira Depression is just an artifact of inadequate sampling. Probably, the current climatic conditions of this geographical barrier are too dry and would be an inadequate habitat for a typical species of cloud forest and páramo environments, which would restrict gene flow between Colombian and Venezuelan populations. In this way, a study with greater geographical coverage could favor the predictions of Soriano et al. (1999).
Our preliminary data from populations of the Norte de Santander and Santander add diversity to the current concept of N. bogotensis in Colombia. The high divergence level suggests a deep break between specimens from northern Colombia and Cundinamarca (the department where the type locality is placed; Thomas 1895). An important geographic barrier of this area is the Chicamocha canyon produced by the erosion of the tributary of Chicamocha river through the Boyacá and Santander departments, and it has been referred to as responsible for the allopatric speciation in some small vertebrates (e. g.,Guarnizo et al. 2015; Cárdenas 2017). Unpublished data from one of the authors (JCP) also suggests a high divergence degree on cytb sequences in Cryptotis thomasi, Thomasomys niveipes and Notosciurus granatensis from both sides of this barrier.
Although N. bogotensis has only been formally mentioned for Cundinamarca, Boyacá and Santander departments (Saénz-Jiménez 2010; Vianchá et al. 2012), is very probably that the species occurs in the Cordillera Oriental and extends its distribution southward. In this sense, the record of Ramírez-Chaves and Noguera-Urbano (2010) from Nariño, support this assumption and suggest that the museum specimens from Tolima, Huila and Cauca would correspond to N. bogotensis. So, the gap that we observe in the distribution of this species is probably due to insufficient sampling and both Neomicroxus species would not be allopatric.
Our findings expose key points to consider in future studies: i) the diversity of N. bogotensis seems greater than that reflected in its current concept, ii) topographic and climatic complexity are playing an important role in the diversification of small Andean mammals being probably responsible for the observed genetic discontinuities, especially in the northern Andes of Colombia, iii) as a way of clarifying the gap observed towards southern Colombia and bordering Ecuador, is necessary to focus the sampling efforts towards regions still unexplored, iv) the review of specimens deposited in collections that were not evaluated in this work is imperative, and v) the importance of future studies that evaluate the Colombian and Venezuelan populations as a whole.
Neomicroxus latebricola spatial structure and taxonomic implications. N. latebricola was originally described from a single specimen from Tungurahua province in central Ecuador (Anthony 1924). A few additional studies have extended its distribution to include Napo, Pichincha, Imbambura, and Carchi provinces as well as revealed findings like a shallow geographical structure (Curay 2019; Voss 2003; Alvarado-Serrano 2005; Brito 2013). Additionally, Muñoz et al. (2018) determined a chromosomal number of 2n = 44, FN = 42, for specimens from Pirámides de Cochasqui, Cantón Pedro Moncayo, Pichincha province. Similar to N. bogotensis, N. latebricola is a common and abundant species easy to capture in Polylepis forests and páramo between 2,420 and 3,950 masl (Brito 2013; Curay 2019).
Our molecular phylogenetic analysis confirms the structure observed with the morphometric dataset, which shows a partial overlapping between specimens from along both cordilleras in Ecuador. This overlapping is reflected in the genealogical relationships between individuals from Carchi and a specimen from Napo province, which could be evidence of an area of primary or secondary contact. A primary contact zone implies the differentiation of the population in situ, while the secondary is produced by the contact of previously allopatric populations (e. g., Schneider 1996; Bertl et al. 2018). For now, our data are insufficient to distinguish between these two scenarios.
The variation observed within N. latebricola allows to highlight two important points: i) the measurements of the specimens from Cordillera Oriental fit into the metric variation recorded by Anthony (1924) and later authors (Moreno and Albuja 2005; Alvarado-Serrano and D’Elía 2013), and ii) the potential distinction of a new subspecies for the Cordillera Occidental populations, distinguished from the nominotypic form by a summatory of craniodental traits and coloration. In fact, Curay (2019) notes variations in the dorsoventral coloration of the body and the forefoot and hindfoot, which could be related to the habitats characteristics (e. g., topography, climate, vegetation) in both cordilleras. Our geographic coverage allows us to state that N. latebricola in the Cordillera Occidental is a frequent species, strictly associated to forests with shrubs and trees where Polylepis incana is the dominant plant (Brito 2013). By the contrary, N. latebricola in the Cordillera Oriental occurs in the ecotonal zone between the páramo and forest whose typical vegetation is the wiry bunch grass to 1 m high and other larger species frequent of wooded environments (Voss 2003). The intraspecific color variation in rodents has been associated with the sex, age, seasonality, and habitat (e. g., Camargo et al. 2016; Ríos and Álvarez-Castañeda 2012; Sandoval et al. 2016). In this regard, the coloration pattern in N. latebricola seems linked with the soil and vegetation color and the exposure to be detected by predators. It varies from darker in the open habitat from the Cordillera Oriental to light brownish in the habitat with more vegetation coverage in the Cordillera Occidental.
Related to the molecular data, the shallow topology (Figure 2a), shared haplotypes, and low genetic distance values reveal the existence of current genetic flow among its populations suggesting there are no apparent geographical barriers that limit it. Contrary to what we have inferred for N. bogotensis populations, N. latebricola has experienced recent demographic expansion. These results imply that the geographic complexity of the Ecuadorian Andes is not a determining factor in the differentiation of these populations.
The presumptive existence of a new infraspecific taxon within N. latebricola implies raising the wide debate over the importance and utility of the subspecies (e. g., Wilson and Brown 1953; Endler 1977; Fitzpatrick 2010). The traditional concept involves geographic discontinuities on some morphological traits within a species as the result of ecological and historical factors, but the constant search for agreement between morphological and molecular data has led to an incorrect interpretation of what subspecies would be. Based mainly on DNA data, many authors have equated obtaining geographic structure and reciprocal monophyly, used to delineate species, as useful and appropriate ways to identify or to reject subspecies. However, this goes against the gene flow that exists between the populations of a species and that maintains them as a clear taxonomic unit. The geographic variation recovered in N. latebricola reminds us of the statement of Patton and Conroy (2019:1019) about the subspecies “… are genealogical networks of populations, often without cladistics structure…” instead the species are considered “…hierarchical units with a dichotomous branching history.” This conceptual distinction is key to improve the understanding that species and subspecies are not equivalent, and that this misunderstanding has caused us to ignore or obscure the infraspecific diversity of taxa. In this case, our data clearly support the existence of a new subspecies for N. latebricola such a typical inhabitant of the forests of Polylepis.
Conflicts between molecular data and morphological evidence, especially the necessity to find data congruence and monophyly, and the attempt to delineate molecular clades with phenotypical features, triggered the progressive discard of infraspecific treatments and its biological value. Paradigmatic examples are abundant among Patagonian sigmodontines with prolific nominal contents (e. g., Abrothrix, Loxodontomys, Oligoryzomys, Paynomys; see Palma et al. 2010; Cañón et al. 2010; Alarcón et al. 2011; Palma and Rodríguez-Serrano 2017). Clearly, we need to reevaluate large series of specimens, looking for diagnosable patterns of size and color in accordance with geography, in a refoundational effort to recover the value of geographic races among South American cricetids.
Finally, despite the verifiable progress during last decades there is a remarkable lack of basic knowledge affecting many Andean sigmodontines (e. g., Aepeomys lugens, Chilomys instans, several Thomasomys). Neomicroxus is a crystal example for which many aspects of its natural history, ecology, biogeography and alpha taxonomy still remain unknown. Our contribution set a preliminary base for future studies evaluating the variation within the genus, as well as that of other small non-volant mammals with shared distributions. On the other hand, it exposes the importance of the subspecies concept such as nonhierarchical, nonreciprocal monophyletic, closely interbreed, and geographically structured groups.











 text new page (beta)
text new page (beta)