Introduction
Parasites play a key role in the life of the host and often exert a significant selection pressure on them (Hart 1990; Zhang et al. 2010). By restraining resource investment in their host, parasites may affect its growth, survival or reproduction (Hart 1990; Mooring et al. 2002; ter Hofstede and Fenton 2005; Zhang et al. 2010).
The knowledge about external parasites (ectoparasites) of bats provides key information for understanding the biology, systematics and phylogeny of the host (Fritz 1983). In addition, this allows clarifying epidemiological aspects of the transmission of some diseases among bats and is essential for gaining a deeper insight on the ecology and behavior of bats in their shelters (Aguiar et al. 2006; Aguiar and Antonini 2011). A total of 687 bat ectoparasite insect species are known from bats, belonging to orders Dermaptera, Lepidoptera, Diptera, and Siphonaptera (Marshall 1982; Schuh and Slater 1995). Six families of these orders are unique to bats (Berlota et al. 2005), of which Nycteribiidae, Streblidae and Polyctenidae are those most studied and best known worldwide.
The family Nycteribiidae, which contains approximately 275 species distributed in 12 genera, includes ectoparasites that are exclusive of bats. These are highly-specialized obligated blood-feeding true flies that are wingless and show a spider-like appearance; their legs and small head protrude above the thoracic dorsal surface (Theodor 1967; Whitaker et al. 2009). This family is divided into three subfamilies: Archinycteribiinae (1 genus, 3 species); Cyclopodiinae (4 genera, 2 species), which are unique to the eastern hemisphere and are associated with bats of the taxa Pteropodidae; and Nycteribiinae (7 genera, 191 species), which are cosmopolitan. The family Streblidae is distributed worldwide and includes five subfamilies, 32 genera and 227 species described. The majority of streblid flies have wings, but in some genera these are vestigial, while some fully winged forms are poor flyers. Streblids are cosmopolitan flies with a well-established specificity for a host, and the largest number of species thrive in the western hemisphere (Kunz 1976; ter Hofstede et al. 2004; Dick and Gettinger 2005; Whitaker et al. 2009).
The family Polyctenidae includes bat bugs. These are rarely collected permanent ectoparasites of bats in tropical and subtropical regions around the world (Maa 1964; Marshall 1991; Esberard et al. 2005). Little is known about the taxonomy of this family, in which 32 species in five genera have been described (Maa 1964; Marshall 1991). The genus Hesperoctenes (16 species) is restricted to the western hemisphere, while the four remaining genera are unique to the Old World, which is apparently the center of origin of this group (Ueshima 1972; Esberard et al. 2005). Polyctenid bugs are exclusive parasites of microchiropterans, feeding on their blood (Maa 1964; Ueshima 1972; Marshall 1991), and are highly host-specific (Ryckman and Casdin 1977; Whitaker et al. 2009).
In Colombia, between the decades of 1940 and 1970, some descriptions and inventories of parasite species and their hosts were carried out (Boshell and Kerr 1942; Marinkelle 1967; Clark 1967; Machado-Allison and Antequera 1969; Marinkelle and Grose 1981). Tasmitt and Fox (1970) elaborated a list of ectoparasites of bats for Colombia and Puerto Rico, recording 28 species parasitizing 18 bat species for Colombia. The study of ectoparasites of bats in the country was subsequently abandoned. In 2012, Calonge-Camargo evaluated the ectoparasites of bats in fragments of tropical dry forest subjected to conventional livestock and silvopastoral management in the Department of Córdoba, and observed changes in the species composition of ectoparasites and their degree of specificity. Herrera-Sepúlveda (2013) compared the ectoparasite load in harems and mixed groups of a Carollia perspicillata population in Cueva Macaregua (Santander), finding higher diversity and richness of ectoparasites in harems versus mixed groups. Tarquino-Carbonell et al. (2015) elaborated an inventory of ectoparasites of bats in a gallery forest in Vereda El Chorrillo (Tolima), and reported 24 species of ectoparasites associated with 14 bat species. Dick et al. (2016) developed a catalog of parasites of the family Streblidae in Colombia, including 73 species distributed in 19 genera and 3 subfamilies. Duran et al. (2017) recorded 17 species belonging to 8 genera of Streblidae that parasitize 19 bat species of five families in the Department of Sucre. Despite the recent interest in the study of bat ectoparasites in Colombia, no studies are currently available for environments subjected to extensive livestock farming in the Caribbean zone of Colombia. This paper lists the ectoparasites of bats found in fragments of tropical dry forest (BST) in the Caribbean region of Colombia (Cordoba, Colombia).
Materials and Methods
Study area. Sampling was conducted in four BST fragments located in the Department of Cordoba, northern Colombia, between 9° 26’ 16” - 7° 22’ 05” N and 74°47’43” -76°30’01” W. The main vegetation types are tropical dry forest (BST) in the low zone, and tropical moist forest (BHT) in higher elevations (Racero-Casarrubia et al. 2015). The climate is tropical warm humid, with mean temperature of 28 °C and mean annual precipitation of 1200 mm with unimodal distribution, showing a dry season from December to March, and a rainy season from April to November (Racero-Casarrubia et al. 2017). Two of the fragments were located in extensive livestock farms subjected to silvopastoral management (tree planting for timber production; Las Palmeras: fragment area ~45 ha, and San Lorenzo: fragment area ~90 ha), and two in extensive livestock farms subjected to conventional management (pastures with scarce shrub or tree cover; Guacamayas: fragment area ~34 ha, and Chimborazo: fragment area ~55 ha; Figure 1).
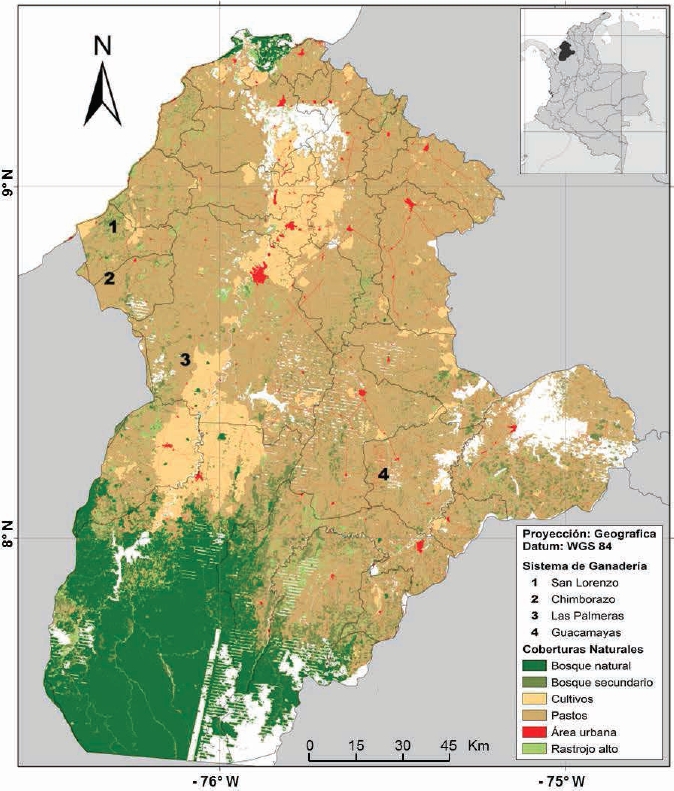
Figure 1 Map of natural cover and localities in the study area: San Lorenzo (Los Córdobas), Chimborazo (Canalete), Las Palmeras (Montería) and Guacamayas (Buenavista), in the Department of Córdoba, Colombia (modified from Ballesteros-Correa, 2015).
Collection of Bats and Ectoparasites. Sampling was carried out from August 2011 to January 2012. Bats were captured using 14 mist nets. Ten nets were placed inside of each fragment; in order to broaden the sampling coverage in each production system, four nets were placed in the matrix adjacent to each fragment, at a distance of 20 meters maximum. For three consecutive nights, nets were opened from 6:00 p.m. to 6:00 a.m., reviewed at half-hour intervals, and relocated occasionally within the forest fragment. Seven field trips were conducted to each farm, for a total of 21 sampling nights/farm. Cumulative species curves were drawn and fitted to the Clench model to obtain an estimate of the expected number of bat and ectoparasite species (Figure 2), taking the number of specimens collected as a unit of effort (Figure 3).
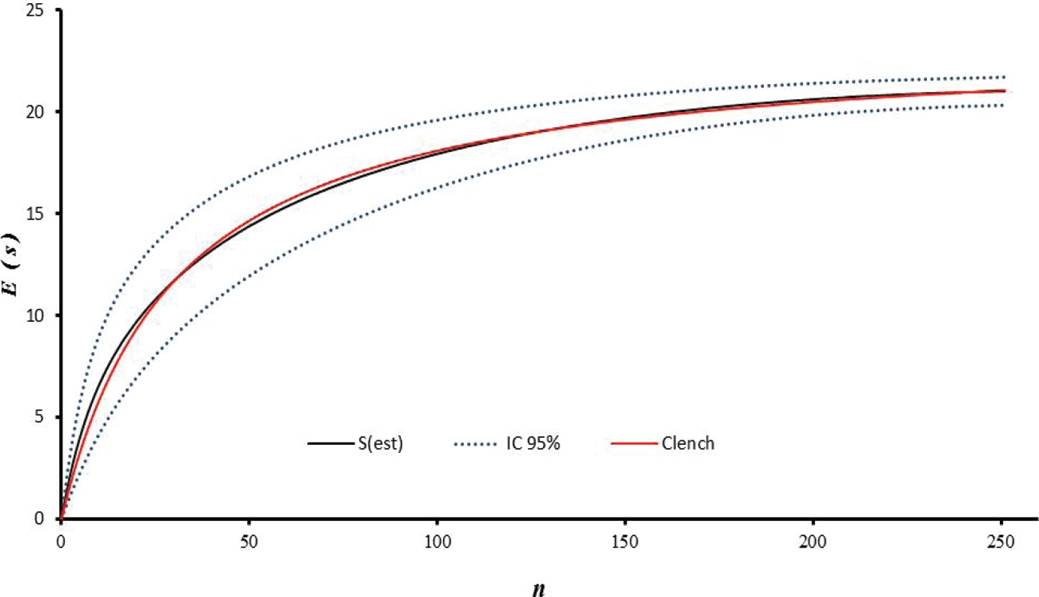
Figure 3 Species accumulation curve S(est) for bats, fitted to the Clench model (red line) for species representativeness. The blue dotted line represents the 95% confidence interval. E(S) = Expected Number of species. n = Number of specimens.
Bats were killed directly on the mist net by craniocervical dislocation using forceps (Sikes 2016), and were deposited in double plastic bags, avoiding direct contact to minimize the loss of ectoparasites (Whitaker et al. 2009). Subsequently, bats were injected with 70 % alcohol (through the bag) in the abdomen to prevent decomposition. The preserved specimens were transported to the Laboratory of Functional Ecology at Pontificia Universidad Javeriana, where each individual specimen was examined under a Krüss stereomicroscope (240V AC 12V/20W) To this end, specimens were placed on a white sheet and examined using fine tweezers and a dissecting needle to sort ectoparasites into separate containers. Each bat specimen was reviewed twice to maximize the detection of the ectoparasites. Ectoparasites collected were preserved in 70 % alcohol (Wenzel et al. 1966; Whitaker et al. 2009). Bats and ectoparasites were deposited in the collection of mammals of the Museo Javeriano de Historia Natural (MPUJ-MAMM:1911-2162) at Pontificia Universidad Javeriana, Bogotá.
Identification of Parasites and Bats. Dipterans and hemipterans were preserved in alcohol and some were cleared with glycerin for identification. Specimens were identified using the keys of Wenzel (1976), Guerrero (1993), and Graciolli and Carvalho (2001), with the assistance of Dr. Ricardo Guerrero (Universidad Central de Venezuela) (Guerrero 1997). Bats were identified using the keys of Fernández et al. (1988), Emmons and Feer (1997), Tim and LaVal (1998), Linares (1998), Laval and Rodríguez-H. (2002), and Gardner (2007).
Data Analysis. For the purposes of this work, bat parasitism patterns were evaluated at the regional level for the BST in the Colombian Caribbean, so data from the four farms were pooled together. The parasite-host association was evaluated by estimating the rates of prevalence and mean intensity (Bush et al. 1997). Prevalence was calculated as the number of bats parasitized with one or more individuals of a given species of parasite, divided by the number of hosts examined for that parasite species, then multiplying the resulting figure by 100 (Bush et al. 1997). Intensity was calculated as the number of parasites per bat (Christie et al. 2003), and mean intensity as the mean intensity of a parasite species relative to the total number of infected individuals of a particular bat species (Bush et al. 1997).
Results
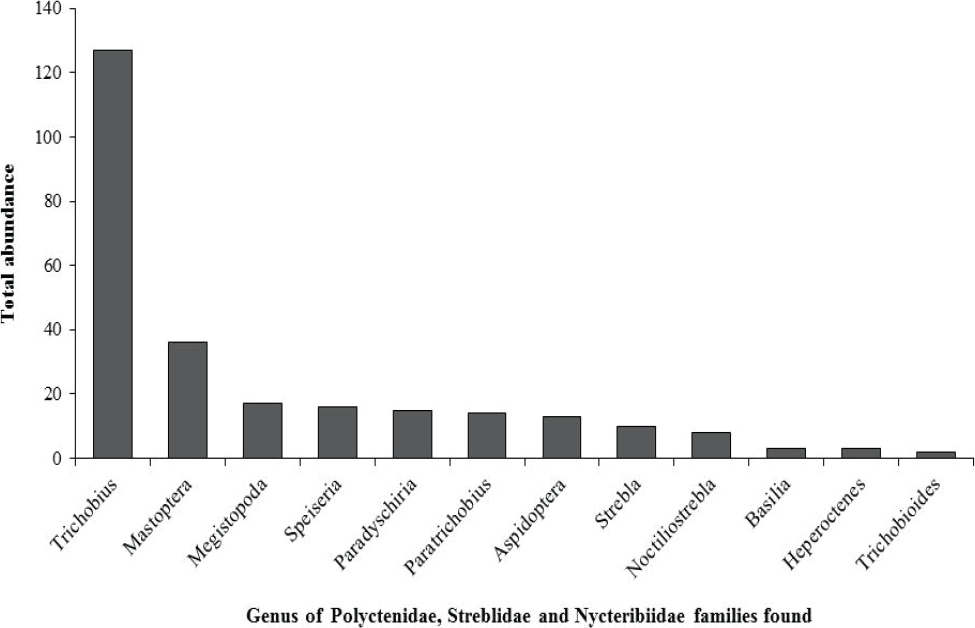
A total of 251 bats were caught, belonging to 21 species and five families. The most abundant family was Phyllostomidae (94 % of total catch), followed by Emballonuridae (2.3 %), Noctilionidae (0.8 %), Vespertilionidae (0.8 %), and Molossidae (0.8 %). Carollia perspicillata was the most abundant species (n = 51), followed by Artibeus planirostris (n = 41), and Uroderma bilobatum (n = 35; Table 1). The least abundant species were mainly insectivorous bats. Correspondingly, 12 genera of ectoparasites were recorded, distributed in three families. The family Streblidae showed the highest taxonomic richness (10 genera) and the highest abundance. Trichobius was the most abundant genus (49 %), followed by Mastoptera (14 %), Megistopoda (7 %), and Speiseria (6 %) (Figure 2). The six remaining genera, i.e. Noctiliostrebla, Paradyschiria, Trichobioides, Paratrichobius Strebla, and Aspidoptera, accounted for 24 % of total abundance (Table 1; Figure 2). The sampling representativeness for bats, fitted to the Clench model, was 91 % (Figure 3); for ectoparasites, an 85 % sampling representativeness was estimated (Figure 4).
Table 1 List of bats and ectoparasites inhabiting four fragments of BST in the Caribbean region of Colombia. Abbreviations: n = Sample size, Nm = Number of parasitized bats, Ne = Number of bats carrying each ectoparasite species, P = Prevalence, Mi = Mean intensity.
| Bat | Ectoparasite | |||||||
|---|---|---|---|---|---|---|---|---|
| Family and species | n = 251 | Nm | Ne | Species | n | P(%) | Mi | |
| Emballonuridae | ||||||||
| Saccopteryx leptura | 6 | 0 | 0 | |||||
| Noctilionidae | ||||||||
| Noctilio albiventris | 2 | 2 | 2 | Noctiliostrebla maai | 8 | 100 | 4.0 | |
| 2 | Paradyschiria parvuloides | 15 | 100 | 7.5 | ||||
| Phyllostomidae | ||||||||
| Carollia brevicauda | 13 | 10 | 3 | Speiseria ambigua | 5 | 23 | 0.5 | |
| 2 | Strebla guajiro | 2 | 15 | 0.2 | ||||
| 7 | Trichobius joblingi | 11 | 53 | 1.1 | ||||
| 3 | Trichobius persimilis | 4 | 23 | 0.4 | ||||
| Carollia castanea | 3 | 1 | 1 | Trichobius joblingi | 1 | 33 | 1.0 | |
| Carollia perspicillata | 51 | 31 | 9 | Speiseria ambigua | 11 | 17 | 0.3 | |
| 5 | Strebla guajiro | 7 | 9 | 0.2 | ||||
| 28 | Trichobius joblingi | 63 | 55 | 2.0 | ||||
| Desmodus rotundus | 9 | 4 | 4 | Trichobius parasiticus | 6 | 44 | 1.5 | |
| Glossophaga soricina | 8 | 1 | 1 | Trichobius dugesii | 1 | 12 | 1.0 | |
| Lophostoma brasiliense | 3 | 3 | 3 | Mastoptera minuta | 11 | 100 | 3.6 | |
| 2 | Trichobius silvicolae | 7 | 66 | 2.3 | ||||
| Lophostoma silvicolum | 6 | 3 | 3 | Mastoptera minuta | 4 | 50 | 1.3 | |
| Mimon crenulatum | 3 | 2 | 2 | Basilia sp. | 3 | 66 | 1.5 | |
| Phyllostomus discolor | 2 | 2 | 1 | Strebla hertigi | 1 | 50 | 0.5 | |
| 1 | Trichobioides perspicillatum | 1 | 50 | 0.5 | ||||
| 1 | Trichobius costalimai | 6 | 50 | 3.0 | ||||
| 1 | Trichobius longipes | 4 | 50 | 2.0 | ||||
| Phyllostomus cf. elongatus | 1 | 1 | 1 | Trichobius dugesioides | 3 | 100 | 3.0 | |
| Phyllostomus hastatus | 2 | 2 | 2 | Mastoptera guimaraesi | 21 | 100 | 10.5 | |
| 2 | Trichobius longipes | 21 | 100 | 10.5 | ||||
| Artibeus lituratus | 26 | 2 | 2 | Megistopoda aranea | 1 | 8 | 0.5 | |
| Artibeus planirostris | 41 | 5 | 5 | Megistopoda aranea | 6 | 12 | 1.2 | |
| 1 | Paratrichobius longicrus | 1 | 2 | 0.2 | ||||
| Dermanura phaeotis | 6 | 0 | 0 | |||||
| Platyrrhinus helleri | 12 | 0 | 0 | |||||
| Sturnira lilium | 17 | 9 | 6 | Aspidoptera delatorrei | 13 | 35 | 1.4 | |
| 6 | Megistopoda próxima | 10 | 35 | 1.1 | ||||
| Uroderma bilobatum | 35 | 11 | 2 | Paratrichobius dunni | 4 | 6 | 0.3 | |
| 7 | Paratrichobius salvini | 7 | 20 | 0.6 | ||||
| 2 | Paratrichobius sp. | 2 | 6 | 0.1 | ||||
| Vespertilionidae | ||||||||
| Rhogeessa io | 2 | 0 | 0 | |||||
| Molossidae | ||||||||
| Molossus molossus | 3 | 1 | 1 | Hesperoctenes sp. | 3 | 33 | 3.0 | |
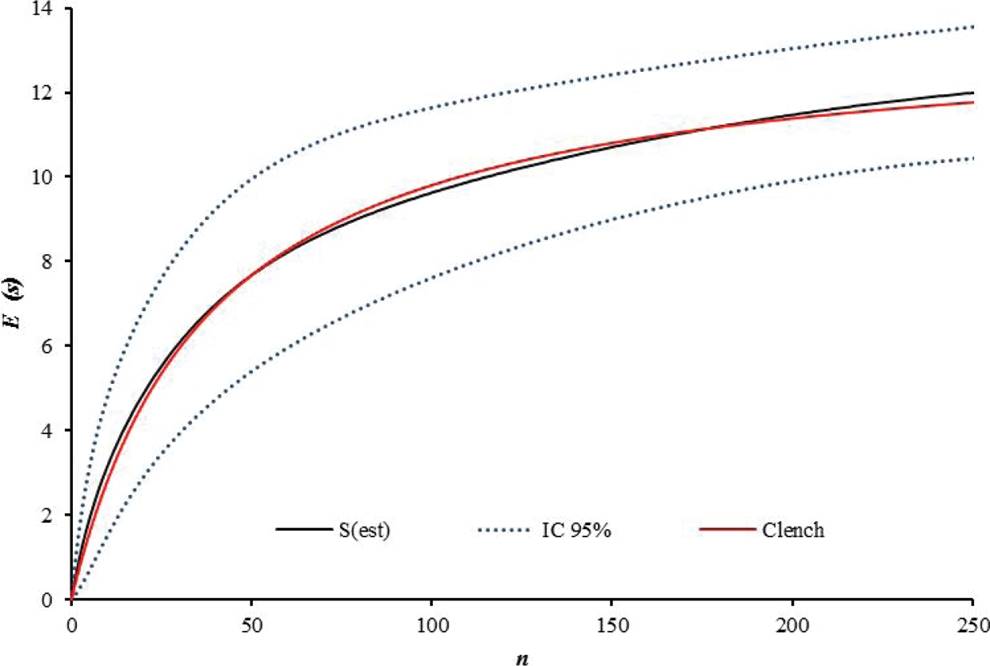
Figure 4 Cumulative curve for ectoparasites S(est) fitted to the Clench model (red line) for species representativeness. The blue dotted line represents the 95% confidence interval. E(S )= Expected Number of species. n = Number of specimens.
We found three individuals each of the genera Basilia (Nycteribiidae) and Herperoctenes (Polyctenidae; Table 1). The bat species Carollia brevicauda and Phyllostomus discolor showed the largest number of associated ectoparasite species (four species each; C. brevicauda: Speiseria ambigua, Strebla guajiro, Trichobius joblingi, and Trichobius persimilis; P. discolor: Strebla hertigi, Trichobioides perspicillatum, Trichobius costalimai and Trichobius longipes), followed by U. bilobatum (Paratrichobius dunni, P. salvini and P. sp.) and C. perspicillata (Speiseria ambiguous, S. guajiro and T. joblingi), with three species each (Table 1).
The total number of individuals with ectoparasites was 90, representing a total prevalence of 36 %. The prevalence of the ectoparasites Noctiliostrebla maai, Paradischiria parvuloides, Mastoptera minuta, Trichobius dugesioides, Mastoptera guimaraesi, and T. longipes in their respective specific hosts was 100 % (Table 1). In contrast, Strebla guajiro, Speiseria ambigua, Trichobius dugesii, Megistopoda aranea, Paratrichobius longicrus, and P. dunni showed a prevalence lower than 20 % in specific hosts (Table 1).
The mean intensity of each ectoparasite species in specific hosts ranged between 0.1 and 10.5. Mastoptera guimaraesi and Trichobius longipes showed higher values, with a mean intensity of 10.5, while the genus Paratrichobius sp. showed the lowest values (Table 1).
Discussion
The bat species with the lowest ectoparasite abundance were mostly insectivorous; this finding is hardly surprising when using mist nets only, as these nets tend to be readily detected by bats and many fly above the canopy (Bergallo et al. 2003). Thirty six percent of the bats studied had at least one ectoparasite. In the Department of Tolima, Tarquino et al. (2015) found that only 8 (38%) of the 21 bat species captured had dipterans. This study, carried out in fragments of tropical dry forest in Cordoba, found that 17 (81%) of 21 bat species had dipterans. This difference may be due to the sample processing methods used, since dipterans tend to escape readily (Whitaker et al. 2009) when the host is manipulated. This indirect disturbance, named “transfer of disturbance” by Wenzel et al. (1966), is common among Streblidae parasites of bats captured with mist nets.
Molossus molossus was the only species found with hemipteran ectoparasites of the family Polyctenidae. It has already been reported that the members of this family of ectoparasites are unique to the family Molossidae in the Neotropics (Marshall 1982).
Eighty seven dipterans belonging to three species were found in the 51 specimens of C. perspicillata examined. This bat species has been reported to show high parasitism levels, mainly of flies (Komeno and Linhares 1999), although ter Hofstede et al. (2004) reported low ectoparasitism levels in this bat species in Belize. The high abundance of ectoparasites typically found in this species could be due to its distinctive social structure (McCracken and Wilkinson 2000). It has been reported that C. perspicillata usually shows a harem-type social structure (McCracken and Wilkinson 2000); it has been suggested that harems promote the exchange of ectoparasites between individuals that perch together (McCracken and Wilkinson 2000). In a separate study, Herrera-Sepulveda (2013) found in Macaregua cave (Santander, Colombia) that ectoparasitism was higher (richness and diversity) in individuals living in harems, which show a closer physical contact, relative to individuals living in mixed groups where the extent of physical contact is lower. In addition, species that tend to group together in perching sites may bear a variable number of ectoparasites, depending on the extent of allogrooming within the group (Kerth 2008; Altringham 1996). Separately, Wenzel et al. (1966) explained that the high diversity of ectoparasites in C. perspicillata results from its high ecological flexibility, that is, from the wide range of ecological conditions where it thrives.
No pattern was evident regarding the bat species showing the greatest infestation level by ectoparasites in Neotropical regions. Some studies have reported that the majority of dipteran species are associated with the genus Artibeus (Anderson and Filho 2006; Azevedo and Linardi 2002; Camilotti et al. 2010). In Brazil, Berlota et al. (2005) and Graciolli et al. (2006) reported that Sturnira lilium is the species with the highest number of etoparasite individuals and species. In Paraguay, Dick and Gettinger (2005) found that the bat species with the highest number of flies were S. lilium, Noctilio albiventris and Desmodus rotundus.
In this work, C. brevicauda showed the highest richness of ectoparasites, with four species. In the work carried out by Duran et al. (2017) in a tropical dry forest in Sucre, Colombia, D. rotundus and A. planirostris showed the greatest richness of ectoparasites, with seven species each. In this study, P. hastatus, N. albiventris, S. lilum, and L. brasiliense, respectively, followed C. perspicillata in terms of the amount of dipterans (number of individuals). The variation in ectoparasite richness per species may be due to differences in the composition and structure of the local host assemblages studied. According to Rui and Graciolli (2005), differences in ectoparasite assemblages in a given host species may also be attributed to regional differences in the species composition of bats, the biogeographic history of the area, as well as the lack of specificity of ectoparasites. On the other hand, the local environmental conditions, perching behavior of each species, as well as its social structure, may all influence these differences. The feeding habits and foraging pattern may also affect the degree of ectoparasitism, since the blood chemistry changes according to the type of food consumed by the host (Emerson and Roark 2007). Further studies should be conducted to analyze this relationship, since given that blood chemistry is sensitive to food type, seasonal variations in the diet may also lead to temporary changes in blood chemistry, thus leading to temporal changes in the type, extent, or prevalence of ectoparasites in bats.
At the regional level, the richness and abundance of ectoparasite species are governed by various factors. Species richness can be explained based on the structure of vegetation, abundance of host shelters, and abundance of host species and individuals (Santos et al. 2013). Correspondingly, the abundance of ectoparasites may be related to the characteristics of the host, such as body size, sex, age, and social organization, among others (Moore and Wilson 2002, Dick et al. 2003, Morand et al. 2004, ter Hofstede and Fenton 2005, Patterson et al. 2007). The extent to which each factor determines the abundance of ectoparasites is specific to each parasite-host system (Presley and Willig 2008).
The degree of parasitism by flies per individual was found to be higher in P. hastatus. We suggest that this may be due to the size of host individuals (which in turn determines the surface area available to accommodate ectoparasites), or also to socializing in harems (potential increase in ectoparasite transfer through physical contact). These bats often use termite mounds, caves and hollow trees as perching sites (Ochoa 1985; Norman 1970). Each harem may comprise 10 to 100 females and a single male, which would facilitate the exchange of parasites among them (Santos et al. 2004). Sex ratio, as well as social structure, are factors that contribute to explain the abundance of parasites in a given species. From a work conducted in Paraguay, Presley and Willig (2008) indicated that since harems are common in the Neotropics, adult females are vectors for host-to-host infection (transferring parasites to offspring), hence skewing the infestation preference toward females vs. males.
The parasitism prevalence and mean intensity values reported in this study contrast the findings reported by Tarquino et al. (2015) also in BST fragments in the Andean Region, likely because that study captured different host species and recorded different abundance levels per species. It should also be considered that environmental conditions, the configuration of the landscape, sampling techniques, and climate season differed between our work and that of Tarquino et al. (2015). Similar to the findings of Tarquino et al. (2015), this work recorded a 100 % prevalence for the association between P. hastatus and T. longipes, and a 50 % prevalence between C. brevicauda and T. joblingi, although with a very small sample size (n = 2) in this case; therefore, sample size should be increased for these species. Separately, C. perspicillata showed a mean intensity of 2 for T. joblinji in both studies, while S. lilium had a mean intensity of 1 for Megistopoda proxima.
In conclusion, the present work recorded ectoparasites of families Streblidae, Nycteribidae, aad Polyctenidae. The bat species C. brevicauda, P. discolor, U. bilobatum, and C. perspicillata showed the largest number of associated ectoparasite species. The species of bats with the highest abundance of ectoparasites (C. perspicillata, P. hastatus, N. albiventris, S. lilum, and L. brasiliense) showed no evident overall characteristic that would explain this finding.











 text new page (beta)
text new page (beta)