Introduction
The use of space is a key component of the ecological niche for all animal species (Lopez-Darias et al. 2012). One of the essential parameters to quantify the use of space is the home range (Börger et al. 2008). The size and the internal dynamics of the home range (HR) are influenced by the environmental, biological, and social factors of each species. In the case of primates, key factors as group biomass (Campos et al. 2014), group size (Shaffer 2014), habitat quality (Campera et al. 2014), vegetation type (Pebsworth et al. 2012), seasonality (Pyritz et al. 2011), and population density (Glessner and Britt 2005).
Unlike a territory, where individuals must invest time and energy to protect it (Kodric-Brown 1978; Amsler and Brown 2010), the HR can be shared either partially or totally. In this sense, it is argued that the level of HR overlap in various primate species depends on both the conditions of the habitat and the physical characteristics of the species (Pearce et al. 2013). In the Neotropics, the howler monkey (Alouatta spp.) is considered a non-territorial species (Milton 1980; Hopkins 2013) because the home range frequently overlaps. Hopkins (2013) has pointed out that the separation between groups of howler monkeys seem to be influenced by the hierarchical status of neighboring groups. However, he makes no reference to the influence of habitat quality, access to resources and their availability.
The objective of this work was to determine the size of the home range and the extent of the spatial interaction between two groups of howler monkeys, based on the overlap of their respective HR. We explored whether the tree species richness and diversity, and the size of the groups of monkeys, are associated with the use of space by two neighboring groups of howler monkeys that inhabit a fragmented landscape in Los Tuxtlas Biosphere Reserve, Veracruz, Mexico.
Materials and Methods
Study area and subjects. The study area is located in the region of Los Tuxtlas, Veracruz, Mexico. This area is fragmented and disturbed, with patches of primary vegetation within a matrix of agricultural and livestock areas (Mendoza et al. 2005; Solórzano-García et al. 2012). The patch inhabited by monkeys is an irregular-shaped polygon encompassing 100 ha approximately, with connections that enable the displacement of groups to other forested areas of similar size. It is located near the community of Montepio (18.63306° to 18.64778° N and -95.09556 to -95.07861°° W; Figure 1), within Los Tuxtlas Biosphere Reserve, Veracruz, Mexico. The vegetation is evergreen forest with various degrees of disturbance. The canopy species include Lonchocarpus cruentus, Dussia mexicana, Nectandra ambigens, Brosimum alicastrum, Ficus yoponensis, Ficus tecolutensis, Pouteria sapota, Spondias radlkoferi and Bursera simaruba, among others (Castillo-Campos and Laborde 2004). The climate is warm and humid, with a dry season from March to May and a rainy season from June to February. Rainfall ranges from 3,500 to 4,000 mm per year (60 mm in the driest month), and the mean annual temperature is above 22 ºC (Soto 2004). Whithin this patch, Cristobal-Azkarate and Arroyo-Rodríguez (2007) estimated the presence of seven groups of Alouatta palliata mexicana; for this work, two neighboring and potentially interacting groups were selected, to obtain behavioral records. At the start of the study, the groups selected were the following: group 1 (G1), 10 individuals (2 adult males, four adult females and four infants); group 2 (G2), 15 individuals (six adult males, five adult females, a young and three infants). The number of adults did not vary throughout the recording period; however, new infants were born, so that at the end of the recording period, G1 and G2 had four and three infants, respectively. The sex and age of individuals was determined based on the classifications of Clarke (1990) and Domingo-Balcells and Veà-Varo (2009).

Figure 1 Location of the study area (box) where various groups of the howler monkey, Alouatta palliata, coexist in the region of Los Tuxtlas, Veracruz, Mexico, on the Gulf of Mexico coast.
Location of groups and behavioral records. The location of each group was recorded between March 2002 and June 2003 using a portable Global Positioning System (Garmin model V plus). Groups were recorded through sightings in the morning (07:00 to 13:00 h) and evening (13:00 to 18:00 h) which were alternated during 55 and 67 days (G1 and G2, respectively). The difference in the recording days between the two groups was due to the fact that G1 stabilized its HR in February 2003, while G2 did it in March; hence, the latter required a greater number of days of follow-up and a larger number of locations for the estimates. On average, five days of location records were considered for each group per month.
After recording the location and identity of the group found, the daily activity pattern was recorded by a continuous record over one hour in a focal animal sampling (Altmann 1974; Martin and Bateson 1993) of adult individuals of both sexes. The groups were compared in terms of the proportion of episodes dedicated to each behavior, using a Mann-Whitney U test to compare the percentage of episodes of each behavior per month as the dependent variable. The behaviors observed were: rest, when the individual maintained a given position, either sitting, lying or hanging, without performing any other activity. Feeding, when the animal consumed or manipulated a food item (particularly leaves, flowers or fruits). Locomotion, when the animal moved at least a length equivalent to its body size along varying distances not beyond the tree or set of trees where the group was seated. Trip, when the animal moved over a distance that exceeded the set of trees where the rest of the group was located. Social behavior, when one individual interacted with others within the group. This category included aggressive behaviors such as biting, shoving, slapping, showing teeth, chasing, fighting; affiliative behaviors, when subjects interacted closely with another individual displaying a non-aggressive behavior; or sexual behaviors, including sexual intercourse and courtship.
Calculation of the home range (HR). The HR of each group was estimated using three methods, so that the size of the HR could be comparable with other studies: 1) the Minimum Convex Polygon (PMC) method, at 100 % (Kernohan et al. 2001); 2) the grid method (100 x 100 m); and 3) the adaptive and fixed 95 % Kernel method (Estrada 1984; Worton 1989; Dunn et al. 2009). The data for calculating the HR were the coordinates of each group recorded at the time of finding, recognizing and identifying each group within the five-day period mentioned above. The home range was calculated through the Home Range extension (Rodgers and Carr 1998) of ArcView 3.2 (ESRI 1999). In order to determine the representativeness of the sample size, the cumulative area used was plotted versus time for each group (Laver and Kelly 2008). It was assessed whether the home range size was significantly related to the group factor, using monthly data (HR) for each group and applying a linear mixed model (the data were previously tested for normality). To this end, monthly replicates were set as a random factor and the group as a fixed factor. The analysis of variance of the linear mixed model was run with SPSS 20.0 (IBM 2011), with a significance of P < 0.05.
Estimate of tree species diversity. Food resources were estimated through the diversity of tree species. The data were obtained in 10 random plots measuring 2 x 50 m within the home range of each group. All trees with a diameter at breast height greater than or equal to 10 cm were identified. Specimens were taxonomically identified with the assistance of a botanical specialist (Santiago Sinaca-Colin) and by consulting specimens deposited in the Xal herbarium at the Institute of Ecology, Xalapa, and the Xalu herbarium at the Universidad Veracruzana. From these data, we evaluated the tree species richness in the HR of each group. In addition, we calculated the following indices: order 1 diversity index (inverse of the Shannon index); order 2 diversity (inverse of the Simpson index), and similarity indices based on incidence and abundance, using the Spade program (Chao and Shen 2010). Order 1 diversity is adjusted for species abundance, while order 2 diversity is based on the most abundant species in the community (Moreno et al. 2011).
On the other hand, since the species of the genus Ficus (family Moraceae) have been frequently considered as a major food item in the diet of the howler monkey (Milton 1984; Serio-Silva et al. 2002; Días and Rangel-Negrin 2015), the number of individuals and the Impartance Value (IV, Coroi et al. 2004) of these species within the HR of the two groups was analyzed.
Results
Daily activity pattern and HR. The sampling effort was 330 observation hours for G1 and 402 hours for G2, for obtaining location records for both groups as well as for sampling the habitat (210 hours for G1 and 182 for G2); also, behavioral records were obtained (120 hours for G1 and 220 for G2, with 120 and 220 focal, respectively), resulting in an average of 20 hours per adult specimen.
The home range of neighboring groups in a patch of approximately 100 ha varied according to the estimation method used. G1 had an average home range of 8.5 ha (7.0 to 10.0); G2, of 21.0 ha (19.7 to 23.3 ha; Table 1). The average HR size deffered between both groups (F1, 20 = 14.45, P = 0.001). The monthly home range size stabilized after 12 months for G1 and at 13 months for G2 (February and March 2003, respectively; Figure 2). An overlap between home range of both groups was detected (Figure 3) using the fixed Kernel method (4.4 %), and 100 x 100 m grids (25 %).
Table 1 Estimates of the home range of two groups (G1 and G2) of the howling monkey, Alouatta palliata, in Veracruz, Mexico, based on various methods and indicators of overlap between groups.
| Method | G1 | G2 | Overlap Percent home range shared by G1 and G2 (average) |
|---|---|---|---|
| (Home range ha) | |||
| Minimum Convex Polygon | 9.4 | 19.7 | 0.0 |
| 100 m grids | 10.0 | 21.0 | 25.0 |
| Kernel, adapted | 7.8 | 23.3 | 0.0 |
| Kernel, fixed | 7.0 | 20.0 | 4.4 |
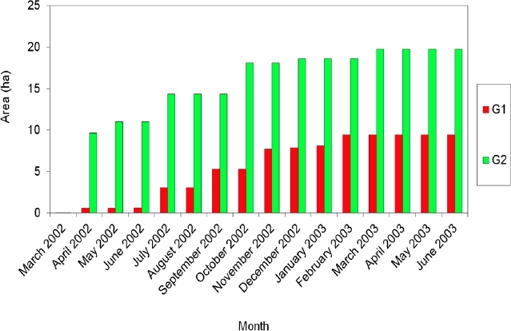
Figure 2 Cumulative area of the home range in the period March 2002 to June 2003 for two groups (G1 and G2) of the howler monkey, Alouatta palliata. The increase in the size of the area used through time reached an asymptotic behavior for both groups, in February 2003 for G1 and March 2003 for G2.
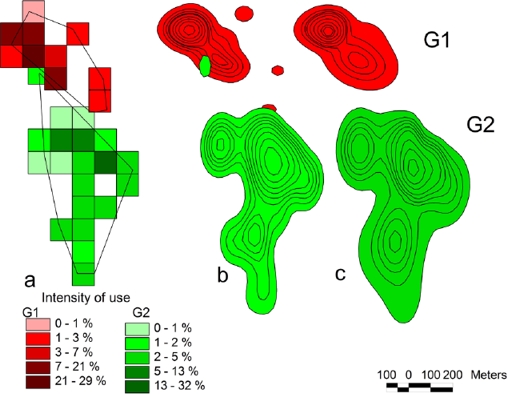
Figure 3 Shape and size of the home ranges of each group (G1 and G2) of the howler monkey, Alouatta palliata. 3a) Minimum convex polygon and 100 x 100 m grids; 3b) Fixed Kernel method; 3c) Adapted Kernel method. Overlap was detected between the groups using the 100 x 100 m grid method (25 %) and the fixed Kernel method (4.4 %). The color intensity in the grid method corresponds to the relative frequency of occupation calculated using the extension of ArcView 3.2.
As regards the daily activity pattern, G1 recorded significantly more locomotion episodes than G2 (U = 38, P < 0.05; Figure 4). In the case of trip, feeding, rest, and social behaviors, no significant differences were found (P > 0.05).
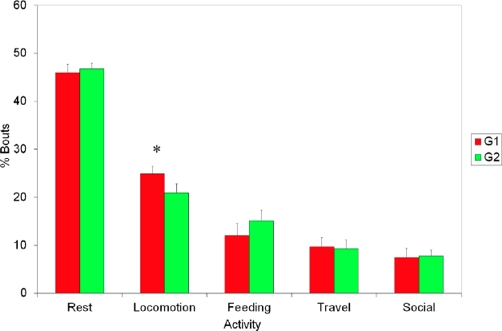
Figure 4 Comparison of behaviors observed in each group (G1 and G2) of the howler monkey, Alouatta palliata. Only the locomotion behavior showed significant differences between the two groups.
Tree species diversity and availability of Ficus spp. The total number of tree species in the habitat was 39 for the two groups. The number of tree species was higher within the HR of G2 (31 species) relative to G1 (20 species). Of the species found, G1 had eight unique tree species, while G2 had 19. The indicators of true diversity, represented by the exponential of the Shannon index (G1: 5.3; G2: 18.7) and the inverse of the Simpson index (G1: 2.45; G2: 12.2; order-1 and order-2 diversity, respectively) were also higher for G2. The number of species shared by both groups was 12 (nearly 30 % of all species). In this sense, the similarity of tree species between groups was low; the Jaccard index was 0.29 (Table 2).
Table 2 Indicators of tree diversity in the home range of two groups (G1 and G2) of the howler monkey, Alouatta palliata, in Veracruz, Mexico.
| Estimator | G1 | G2 | Comparison | |
|---|---|---|---|---|
| Exclusive Species | 8 | 19 | Total Exclusive Species | 27 |
| Shared Species | 12 | |||
| Order 1 Diversity | ||||
| Exponential of the Shannon index (MLE). | 5.31 ± 0.91 | 18.75 ± 2.06 | Similarity Jaccard index (according to incidence) | 0.29 ± 0.05 |
| Order 2 Diversity | ||||
| Inverse Simpson Index | 2.45 ± 0.31 | 12.22 ± 0.23 | ||
| Species of the family Moraceae | 2 | 3 | ||
| Importance Value Index for species of the family Moracea | 14.1 | 32.2 |
The record of the species of the family Moraceae showed two species of the genus Ficus in the HR of G1 (F. maxima Mill. and F. yoponensis Desv.), and three species in the HR of G2 (F. colubrinae Standl., F. petenencis Lundell and F. yoponensis Desv.). Only one species is shared in the HR of the two groups (F. yoponensis). The importance value index of these Ficus species (in relation to the tree species found in each HR separately) was as follows: G1, F. maxima Mill: 17.2, F. yoponensis Desv: 10.9; G2, F. colubrinae Standl: 49.0, F. petenencis Lundell: 27.4, and F. yoponensis Desv: 20.3. Average Importance Value Index values for the family Moraceae in each HR were 14.1 for G1 and 32.2 for G2.
Discussion
Home Range estimates are within the range reported for A. palliata by Dunn et al. (2009); these authors obtained variations of HR between 5.8 and 89.5 ha (in habitats with areas of 7.2 ha and 244 ha, respectively). On the other hand, the grid method (100 x 100 m) estimated a maximum HR of 21 ha, that is, one third of the area estimated by Estrada (1984) also in the Los Tuxtlas region and using the same method (60 ha). The greatest similarity in the comparison of methods with other works conducted in the same area occurred with the Minimum Convex Polygon method (PMC), which yielded 9.4 ha for G1 and 19.7 ha for G2. These data are equivalent to those reported for A. palliata by Cristobal-Azkarate and Arroyo-Rodríguez (2007), who estimated 14.7 ha. On the other hand, Colias and Southwick (1952) in a continuous habitat in Barro Colorado, Panama, and Williams-Guillen et al. (2006) in a fragmented habitat in Nicaragua, estimated the HR for A. palliata using PMC; their estimates did not exceed 35 ha for groups of 15 individuals.
There are multiple factors that influence home range size. Our results support the hypothesis proposed by Seth and Seth (1986), who suggest that a large HR generally involves higher richness and diversity of both plant species and food resources. Among the explanations of the variation in the home range of A. palliata, HR size is suggested to be associated with the amount of habitat available (Cristobal-Azkarate and Arroyo-Rodríguez 2007). In the present study, the largest group that displayed the largest number of adult males and females had a larger HR coupled with the highest richness of tree species. The increase in group size also involves higher costs. In this regard, Chapman et al. (1995) have suggested that a larger group of monkeys leads to a faster depletion of resources and, therefore, results in the need to move to other sites in search for food. In that sense, the largest group of monkeys was G2, which corresponds to the largest HR having the highest richness of tree species. However, its locomotion episodes were fewer than those of G1, which displayed smaller group size and HR. It has been mentioned that the cost associated with search for food increases along with the increase in group size (Chapman and Chapman 2000; Chapman and Pavelka 2005; Robbins et al. 2009); the greater locomotion recorded in G1 is likely related to the lower habitat richness, which promotes a more intense search for food resources. In this regard, Dunn et al. (2009) consider that some changes in the locomotion of the howler monkey result from the lower availability of trees and fruits. In addition, Rodríguez-Luna et al. (2003) reported a relationship between larger displacement and lower time spent foraging; in addition, they point to the incorporation of new species to the diet when the density of howler monkeys increased considerably.
In the present study, the larger group size coupled with the larger number of males in G2 resulted in the appropriation of patches with the greatest availability of food resources. In this regard, Van-Schaik and Kappeler (1993) point out that the more richness and abundance of food resources there is, the greater the size of the group. As a result, males will be more efficient in competing and defending these resources against a neighboring group. In this work, the habitat of G2 had an Importance Value Index of more than twice the figure obtained for the habitat of G1 regarding the species of Ficus sp., as well as a higher percentage of trees (26.1 % for G2 versus 14.2 % for G1). The species in this family are considered as key in the diet of the howler monkey; the time spent by A. palliata mexicana foraging on Ficus trees ranges between 14 % and 48 % (Estrada 1984; Serio-Silva et al. 2002; Rodríguez-Luna et al. 2003; Asencio et al. 2007; Cristobal-Azkarate and Arroyo-Rodríguez 2007). In this regard, the presence of trees of the Moraceae family suggests a favorable aspect for the two groups. In an adjacent area covered by a continuous forest, Estrada (1984) reported a relative density of 32.4 trees/ha belonging to that family. However, although a density of only 1.4 trees/ha was reported for Ficus spp., the A. palliata individuals observed devoted a greater percentage of their time feeding on Ficus trees. Therefore, the opportunities of G2 to feed on Ficus trees were nearly twice as large as those of G1. This suggests that the groups studied use sites where, in proportion to their size, there are sufficient resources for both groups without the need to invade areas outside their home ranges.
This could explain the reduced HR overlap and the absence of agonistic behavior observed between G1 and G2. Theoretically, under these circumstances, there is an indirect exploitation competition, i. e., a low intergroup tolerance coupled with a differential use of resources; hence, most of the time there is no aggressive competition between the groups (Nicholson 1954; Wrangham et al. 1993). Although the species A. palliata shows wounds and scars from injuries associated with agonistic behaviors (Cristobal-Azkarate et al. 2004), it has been found that the groups of this species remain relatively separated from one another (Hopkins 2013), which reduces the possibility of intergroup aggressions.
Of all the methods used to measure HR, only the 100-m grid and the Kernel methods suggested some degree of overlap, 25 % and 4.4 %, respectively. These figures are similar to those by Mittermeier (1973), who reported an average 8.5 % overlap between neighboring A. palliata groups; and those by Williams-Guillen et al. (2006), who recorded a maximum overlap of 7 % between three groups; this figure was estimated with the PMC method. A. caraya is characterized by a high overlap between groups (94 % overlap), which contrasts with our findings (Baldwin and Baldwin 1972).
The results revealed information consistent with the ecological restriction model proposed by Chapman et al. (1995). It was recorded that the largest group inhabits the site with the greatest tree species richness within the largest HR. The intergroup dynamics of neighboring howler monkey groups in a fragmented habitat shows a small HR overlap. Although competition takes place, it seems to be of the indirect type, where the most important food resources (Ficus trees) show differences in abundance within the home ranges of the two groups; no direct aggression of any sort was observed. It is essential to advance the investigations on intergroup dynamics in the genus Alouatta to characterize the coexistence strategies between neighboring groups in sites subjected to different degrees of disturbance and different areas of activity. This will contribute to better understand the variability in the use of space by howler monkeys.











 text new page (beta)
text new page (beta)


