Introduction
Ponds are defined as small (1 m2 to about 5 ha), man-made or natural shallow waterbodies, which permanently or temporarily hold water (Meester et al., 2005). In water-deficient areas, ponds are very common. For a long time, ponds have been ignored by freshwater biologists (Céréghino et al., 2008). Recently, there is growing evidence that ponds are species-rich (Williams et al., 2003) and important surrogate habitats for aquatic organisms (Peltzer et al., 2006) in water-deficient areas. With the rapid growth of the economy, the water quantity of rivers is strongly reduced as a result of water resources development for agricultural, industrial and domestic use. Many rivers are dried out for most of the year. Consequently, rivers are changed into some ponds with extremely low velocity. Such process can be observed in many water-deficient regions of the world (Céréghino et al., 2008; Hale et al., 2015).
Managing river system often includes many river habitat modifications (RHM), such as flood protection works, water intake engineering, and ecological restoration projects (Pedersen, 2009; Szoszkiewicz et al., 2006). A number of habitat modification projects are done in river-changed ponds (Liu et al., 2009; Men et al., 2010) and obviously, these modifications have direct impacts on aquatic organisms (Albertson et al., 2011). Meanwhile, the alteration exerts on habitat quality features, such as water quality and bottom stability, have more effects in structuring aquatic communities (Petkovska & Urbanič, 2015). Benthic invertebrates play an important role (Duan et al., 2011) and changes of benthic invertebrate communities provide valuable information about the current status of aquatic systems (Mehler et al., 2015). Therefore, it is important to establish more precise links of invertebrate communities and environmental factors under the effect of RHM for river management.
Generally, the distribution of invertebrates in rivers appears to be mainly dependent on river discharge (Death & Zimmermann, 2005), water quality (Guilpart et al., 2012), and bottom stability (Duan et al., 2011). Most of these environmental drivers are associated with water flowing. In contrast, invertebrate assemblages seem to be primarily affected by various aspects of vegetation in lentic environments (Trigal-Dominguez et al. 2009). However, river-changed ponds in water-deficient areas are different from running rivers and totally lentic lakes. Few studies compared invertebrate communities in different types of ponds and tried to identify the main environmental drivers. Hale et al. (2015) compared the invertebrate community composition in natural stream ponds and man-made stock ponds watering domesticated animals and found that 81% of the taxa were exclusive to either stock ponds or natural ponds. Jurado et al. (2009) showed that the most important factors responsible for the differences in invertebrate community structures between natural stream ponds and wastewater treatment ponds were pH, vegetation structure and pollution levels. On the other hand, Oertli et al. (2008) suggested that connectivity was highly important in structuring the invertebrate assemblages in alpine ponds. Some other factors, such as the presence of bullfrog, are also discussed (Hale et al., 2015). Despite the important ecological role of river-changed ponds in the water-deficient area, there is a paucity of knowledge regarding whether there are differences for invertebrate communities in different types of river-changed ponds, and the factors determining such differences, including anthropogenic habitat modifications.
In this study, the main aims are to (i) compare community composition, abundance and biodiversity of benthic invertebrate communities in different types of river-changed ponds, (ii) investigate the effect of RHM and habitat quality on invertebrate communities, and (iii) quantify the effect of RHM on habitat quality features and identify the main factors. The results will provide an important basic for enhancing freshwater biodiversity in river-changed ponds and be benefit for river management and rehabilitation.
Methods
Study Area
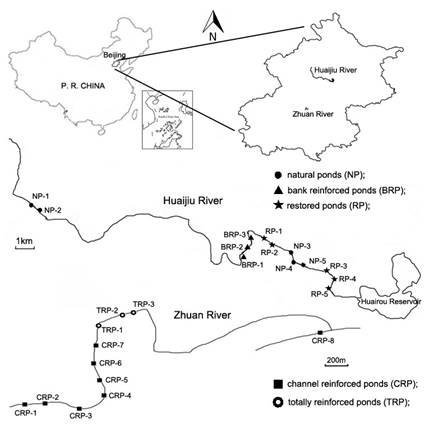
As composed by different types of ponds and have relatively good water quality in Beijing of China, Huaijiu River and Zhuan River are selected (Figure 1). Huaijiu River, with an area of 347.2 km2, is located in the Huairou district which is one of the most important water sources in Beijing. With relatively less anthropogenic disturbance, it contains several ponds without any water projects. But segments across villages are embanked with nearly no floodplain. Some ponds are ecologically restored, including rehabilitation of natural riparian and hydrophytic vegetation. In contrast, Zhuan River, with a length of 3.7 km, is located in the central urban area and all of the channels are reinforced by concrete. But for some ponds, shallow waters with hydrophytes are created within the marginal area. During August 2014, a field investigation was conducted in Huaijiu River and Zhuan River.
Survey Design
Five types of ponds are defined here. Totally reinforced ponds (TRP) refer to the ponds with reinforced channel and vertical cement revetment (Figure 2a). Channel reinforced ponds (CRP) refer to the ponds with concrete channel underwater, but the vertical cement revetment above water are removed and shallow waters with hydrophytes are built in the marginal area (Figure 2b). Bank reinforced ponds (BRP) refer to the ponds with natural streambed, but vertical cement revetment and severely encroached flood plain (Figure 2c). Natural ponds (NP) refer to the fragmented habitats in rivers, without any artificial water utilization or restoration projects (Figure 2d). Cement revetment with slop in some ponds are covered with soils and ecologically restored by the rehabilitation of riparian and hydrophytic vegetation. Thus these ponds are defined as restored ponds (RP, Figure 2e).

Figure 2 Sketch of five types of ponds in this research: totally reinforced ponds (a), channel reinforced ponds (b), bank reinforced ponds (c), natural ponds (d), and restored ponds (e).
A total of 24 sampling sites were chosen from the two rivers in Beijing, including 5 sites in NP, 3 sites in BRP, 5 sites in RP, 8 sites in CRP and 3 in TRP (Figure 1). All of the ponds contain water year round, with velocity lower than 0.3 m/s for most of the year.
Benthic Invertebrate Data Collecting
A D-frame dip net was used to sample along the substrates and in plant clusters. Replicate samples for each site were combined to form a composite sample, amounting to at least a minimum area of 1 m2 (Duan et al., 2011). The samples were rinsed vigorously through a 300-μm sieve and the invertebrates were collected in plastic sample containers with 95% ethanol in the field. All invertebrates were identified and counted under a stereoscopic microscope in the laboratory. Invertebrates were identified to the finest taxonomic unit as possible, mostly to genus or species. For each sample, the total species number (S), individual density (N), and biomass density (W) were calculated or weighed.
Environmental Data Collecting
For each site, environmental data including bottom stability (BS), water quality (WQ), vegetation (Veg) and RHM were obtained, and the specific environmental variables are listed in Table 1. Some environmental parameters including the water temperature, pH, dissolved oxygen (DO) were measured in situ using portable multiparameter water quality analyzer (YSI 6600). A water sample of approximately 1L was taken at each site to measure chemical oxygen demand (CODMn), five day’s biochemical oxygen demand (BOD5), total nitrogen (TN), ammonium (NH3-N), and total phosphorus (TP) in laboratory, according to China’s National Environmental Quality Standards for Surface Water (Chinese Research Academy of Environmental Sciences, 2003).
Table 1 Summary of environmental variables for the 24 sampling sites
| Abbreviation | unit | Variable group | Median (min-max) | |
|---|---|---|---|---|
| pH | pH | - | WQ | 7.79 (7.45-8.64) |
| dissolved oxygen | DO | mg/L | WQ | 5.84 (4.14-13.44) |
| total nitrogen | TN | mg/L | WQ | 1.45 (0.20-3.93) |
| ammonium | NH3-N | mg/L | WQ | 0.17 (0.03-1.60) |
| total phosphorus | TP | mg/L | WQ | 0.11 (0.02-0.84) |
| chemical oxygen demand | CODMn | mg/L | WQ | 2.30 (1.09-17.98) |
| five day’s biochemical oxygen demand | BOD5 | mg/L | WQ | 1.60 (0.36-16.92) |
| Rock angularity | Ra | Score a | BS | 3 (1-4) |
| Brightness | Br | Score a | BS | 1 (1-4) |
| Consolidation of particles | Cp | Score a | BS | 4 (2-8) |
| Bottom size distribution | Bsd | Score a | BS | 8 (4-12) |
| Scouring and deposition | Sd | Score a | BS | 12 (6-24) |
| Aquatic vegetation | Av | Score a | BS | 2 (1-4) |
| banktop vegetation structure | Btvs | Score b | Veg | 0 (0-3) |
| banksurface vegetation structure | Bsvs | Score b | Veg | 2 (0-3) |
| channel vegetation types | Cvt | Score b | Veg | 11.0 (0.0-16.5) |
| Artificial bank material | Abm | Score b | RHM | 0 (0-6) |
| bank modification | Bm | Score b | RHM | 0 (0-7) |
| Artificial channel material | Acm | Score b | RHM | 0 (0-6) |
| channel modification | Cm | Score b | RHM | 0 (0-4) |
| Artificial bank profile | Abp | Score b | RHM | 1.5 (0.0-6.5) |
| Bridge | Bri | Score b | RHM | 1.5 (0.0-3.0) |
| Ford | For | Score b | RHM | 0 (0-2) |
| Weir/sluice c | Ws | Score b | RHM | 0 (0-3) |
| Outfalls | Ouf | Score b | RHM | 0 (0-2) |
| water impoundment | Wi | Score b | RHM | 0 (0-2) |
a score of individual variable is calculated according to the bottom component of Pfankuch Stability Index (Pfankuch, 1975 ).
b score of individual variable is calculated according to the SIHM method (Tavzes & Urbanič, 2009).
c variables excluded from further analysis according to occurrence frequency lower than 10%.
Bed stability has significant effects on the composition of benthic invertebrate communities (Townsend et al., 1997). There are different approaches and techniques to quantify bed stability, such as the distance travelled by in-situ-marked tracer stones (Death, 2005), and the percentage of substrate that would move at bankfull discharge (Duncan et al., 1999). Schwendel et al. (2011) compared these methods and found that the Pfankuch Index of bottom component was one of the most suitable measures for researching invertebrate communities. The Pfankuch Stability Index is a method for visual evaluation of streambed and bank stability (Pfankuch, 1975), and the bottom component of Pfankuch Stability Index (BCP) is used here to assess the bottom stability (Schwendel et al., 2011).
SIHM is a method for evaluating different categories of river features regarding their influence on benthic invertebrate communities, including the parts of Veg and RHM (Tavzes & Urbanič, 2009). Previous studies (Petkovska & Urbanič, 2015; Urbanič, 2014) showed a good explanatory power of SIHM linking to benthic invertebrates. Therefore, variables of Veg and RHM are used here and their scores are calculated according to the SIHM method (Tavzes & Urbanič, 2009).
Multivariate Data Analysis
For each invertebrate sample, the Margalef index (D), Pielou index (J) and Shannon-Wiener index (H) are calculated to determine the species richness (Margalef, 1958), evenness (Pielou, 1966) and diversity (Shannon & Wiener, 1949). Mean species number (S), individual density (N), biomass density (W), D, J, H, the percentages of phylum Mollusca (Mol), Annelida (Ann), and Arthropoda (Atr) individuals are compared among different types of ponds by using Mann-Whitney U test.
Ordination techniques are applied based on CANOCO 4.5. Benthic invertebrate data are ln(x+1) transformed to reduce the weights of rare taxa in all cases. Detrended Correspondence Analysis (DCA) on invertebrate data is used to analyze the relationship between invertebrate communities and environmental variables. Since the largest gradient length is 5.38, unimodal species responses are assumed, and thus the Canonical Correspondence Analysis (CCA) is applied. Firstly, CCA with forward selection on RHM are applied to identify the effect of RHM on invertebrate assemblages. Then the importance of the three habitat quality groups (WQ, BS and Veg) in explaining variability among benthic invertebrate assemblages is tested by CCA. Furthermore, DCA with forward selection is used to investigate the significance of RHM in explaining variations of habitat quality groups (WQ, BS and Veg) separately.
Results and Discussion
Invertebrate Communities in Different Types of Ponds
As shown in the appendix, a total of 82 invertebrate taxa (21 identified to species, 51 identified to genus, 10 identified to family) are identified, including 15 Mollusca, 9 Annelida and 58 Arthropoda. In Table 2, there are several statistically significant differences (P < 0.05) in invertebrate community variables between pairs of pond types through the Mann-Whitney U test. TRP have significant smaller species numbers (1~4) than CRP (4~18, P = 0.018), NP (6~22, P = 0.025) and RP (10~17, P = 0.024). TRP also have significant smaller individual density (6~94 ind./m2) than NP (104~348 ind./m2, P = 0.025) and RP (172~392 ind./m2, P = 0.025). However, the biomass densities in TRP (11.20~79.79 g/m2) are significantly higher than that in CRP (0.46~9.01 g/m2, P = 0.014). The percentages of Mollusca in TRP (100%) are significantly higher (P = 0.013 to 0.037) than that in other ponds while the percentages of Arthropoda are significantly lower (P = 0.013 to 0.025).
Table 2 P values of Mann-Whitney U test between every two types of ponds.
| Compared ponds | S | N | W | Mol | Ann | Art | D | J | H |
|---|---|---|---|---|---|---|---|---|---|
| TRP-CRP | 0.018 | 0.014 | 0.013 | 0.013 | 0.028 | 0.025 | 0.014 | ||
| TRP-SP | 0.025 | 0.025 | 0.022 | 0.022 | 0.025 | 0.025 | |||
| TRP-RSP | 0.024 | 0.025 | 0.022 | 0.022 | 0.025 | 0.025 | |||
| TRP-BRP | 0.037 | 0.037 | 0.037 | ||||||
| CRP-SP | 0.013 | 0.013 | 0.028 | ||||||
| CRP-RSP | 0.013 | 0.013 | |||||||
| CRP-BRP | 0.025 | 0.041 | 0.024 | 0.014 | 0.041 | ||||
| SP-RSP | 0.016 | ||||||||
| RSP-BRP | 0.025 |
The Shannon-Wiener index is the most recognizable variable for different types of ponds. It is significantly highest in RP (2.36~3.66) and CRP (1.89~3.51), significantly lower in NP (1.96~2.41), and significantly lowest in BRP (1.11~2.27) and TRP (0.00~1.50). According to comparing TRP and RP, there is no significant difference (P = 0.770) in the Shannon-Wiener index, but significant differences (P = 0.013) in percentages of Mollusca and Arthropoda. Since all of these variables have no significant differences between NP and BRP, the results are not included in Table 2.
Although BRP and RP are modified by anthropogenic activities, they have similar community composition with NP. Such modified and river-changed ponds provide important surrogate habitat for some aquatic invertebrate taxa (Hale et al., 2015) in Beijing area. However, the species richness is rather low in TRP (only mollusk). Obviously, the channelization has given rise to strong changes of the habitat structure and reduced the integrity and complexity of the living environment, which is necessary to support diverse aquatic biota (Blann et al., 2009). On the other hand, the higher biomass in TRP is mainly caused by the existence of the large-sized gastropods (Pan et al., 2012).
A comparison between TRP and BRP reveals that there is no significant difference in species numbers, biodiversity, and biomass densities. However, the invertebrate assemblages in TRP are composed mainly of mollusks, while arthropods are observed in BRP. Since the two pond types have similar bank profiles but different bottom materials, the differences in the composition may be mainly owing to the substrate material and its stability (Duan et al., 2011).
For CRP, the vertical concrete bank above water has been removed, and slop bank with complex vegetation and riparian vegetation have been rebuilt, but the reinforced channel underwater is retained. Compared to TRP, CRP shows a significantly higher species richness and biodiversity, suggesting the importance of bank form and riparian vegetation (Demars et al., 2012). The relatively lower biodiversity in BRP confirms this point from the opposite angle.
The invertebrate fauna of urban rivers is often highly restricted when compared with non-urban watercourses in the same region (Davies & Hawkes, 1981). However, compared to NP in the suburban area, CRP in the urban area has significantly higher biodiversity and a similar taxonomic composition, which prove the modification successful.
Comparing the Effects of RHM and Habitat Quality on Invertebrate Assemblages
The total variance in species data is 4.561, including 24 sampling sites and 82 invertebrate taxa. After forward selection only Abp (P = 0.004) and Out (P = 0.018) are selected. The total explained variance of RHM is 0.63 (13.9%), and the explanatory powers of Abp and Ouf were 7.4% and 6.5% (Figure 3).
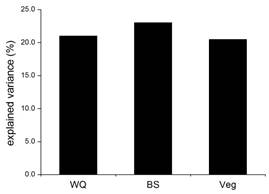
For detecting the effects of habitat quality on invertebrate communities, pH (P = 0.030), TP (P = 0.002), Br (P = 0.002), Av (P = 0.038), and Cvt (P = 0.002) are selected after forward selection. Testing the explanatory power of each variable group individually, each group comprises two or three variables after forward selection. From Figure 4, the highest explanatory power of variable groups is shown in bottom stability (23.0%), followed by the water quality (21.0%) and vegetation (20.5%).
The RHM explains the least variation among the four variable groups. Thus the habitat quality has relatively more significant or direct impacts on invertebrate communities for river-changed ponds. That conforms to the conclusion that benthic invertebrate assemblages respond less to the physical alteration itself but more to the effect that the alteration exerts on habitat quality features (Petkovska & Urbanič, 2015).
Each habitat quality variable group is tested for its significance in structuring benthic invertebrate assemblages. Generally, approximate explanatory powers are shown among the WQ, BS and Veg. The importance of WQ, BS, and Veg to invertebrate assemblages has been reported from the previous researches (Duan et al., 2011; Demars et al., 2012), but studies on quantificationally comparing their effects are rare. Jurado et al. (2009) identified the main environmental factors structuring invertebrate community in natural and wastewater treatment ponds, and the results showed that WQ and the instream vegetation accounted for 26.53% and 17.69% of the explained variance. On the other hand, Petkovska & Urbanič (2015) found that the slope (20%) and the predominant flow (12%) were the most important in explaining invertebrate assemblage variances, while the Btvs (2%), Bsvs (1%) and Cvt (3%) were much less important. Moreover, Demars et al. (2012) found that the marginal and instream vegetation accounted for 60% of the total explained genus composition variance within the river and 26% between rivers. In fact, the natural substrate characteristics are the consequences of the interaction between the flowing water and the channels (Duan et al., 2011). Therefore the explanatory power of BS partly merges the influence of water flow on invertebrate assemblages. Compared to natural streams, all of the ponds in this study have similar low velocity and flow regimes. Consequently, the similarity reduces the importance of bottom stability and enhanced the importance of vegetation. Compared to totally lentic lakes, water in these ponds can flow and be connected in flood period or by human manipulation. That may be why WQ, BS, and Veg revealed approximate significances in structuring benthic invertebrate assemblages of river-changed ponds in water-deficient areas.
Effects of RHM on Habitat Quality
DCA on WQ, BS, and Veg are performed, and the largest gradient lengths (0.37~1.48) are small. Thus RDA with forward selection is applied to detect the relationship between RHM and the other habitat quality variable groups (Table 3). RHM explains 43.59% of the variance in WQ with 3 statistically significant variables after forward selection, 60.85% of the variance in BS with 2 statistically significant variables, and 52.46% of the Veg variance with 2 statistically significant variables. The most explanatory RHM variable is the artificial bank profile (Abp), explaining 14.6%, 49.9% and 43.1% of the variance in WQ, BS and Veg, respectively. In addition, the artificial bank material (Abm) explained most of the variance in water quality (19.5%).
Table 3 Results of RDA with forward selection.
| RHM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abp | Bm | Wi | Abm | For | total | ||||||
| ƛ | P | ƛ | P | ƛ | P | ƛ | P | ƛ | P | ƛ | |
| WQ | 14.6 | 0.012 | 9.5 | 0.010 | 19.5 | 0.002 | 43.59 | ||||
| BS | 49.9 | 0.002 | 10.9 | 0.002 | 60.85 | ||||||
| Veg | 43.1 | 0.002 | 9.3 | 0.014 | 52.46 | ||||||
Many changes in WQ, BS and Veg of the hydrosystems are directly or indirectly caused by anthropogenic habitat modification (Schlosser & Karr, 1981; Wyżga et al., 2012). Since rivers in Beijing area are highly disturbed by human activities, RHM explains a considerable amount (43.59% ~ 60.85%) of variations in habitat quality. RHM has relatively higher explanatory power for BS and Veg which are changed directly in many projects such as channelization works, alteration of the channel and bank materials. In contrast, water quality variance is relatively less explained because it is indirectly affected by channel materials, vegetation, water impoundment and so on (Marzin et al., 2012).
Variables for bank alteration (Abp, Bm, Abm) affect WQ (34.1%), BS (49.9%) and Veg (52.4%) significantly. Bank modification is also a good explanatory variable for invertebrate assemblages in other studies (Stefania et al., 2006). It can be inferred that the bank system play an important role in maintaining the habitat quality and biodiversity for river-changed ponds. Thus the natural bank profile, material and vegetation should be modified carefully.
Conclusions
This study has proved that the river-changed ponds are important habitats for benthic invertebrates in Beijing for some of these modified ponds even supported higher biodiversity than natural stream pools. RHM can affect the benthic invertebrate community through changing the habitat quality, including WQ, BS, and Veg. Modification of river banks is the main human pressure affecting habitat quality in river-changed ponds. Moreover, the most important habitat quality variables for structuring benthic invertebrate assemblages are the bottom brightness, total phosphorus and vegetation types. Finally, we suggest that vegetation replanting in channels and riparian areas might be a useful way to improve invertebrate biodiversity in Beijing.











 text new page (beta)
text new page (beta)