Introduction
Polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) are a class of brominated flame retardants (BFRs). They are widely applied in various domestic products, such as carpet pads, castings, foam cushions in chairs, and couches (De Wit, 2002; Alaee, Arias, Sjodin, & Bergman, 2003). Because of their lack of chemical binding and migratory aptitudes, PBDEs and PBBs may enter the environment during production and use as well as dismantling of these products (Osako, Kim, & Sakai, 2004; Wang, Ma, Lin, Na, & Yao, 2009). Accordingly, PBDEs and PBBs have been extensively detected in water as well as in sediment (Law et al., 2014; Wang et al., 2015; Zhang et al., 2015; Yu et al., 2016). Owing to their lipophilicity and bioaccumulative properties, they had been detected in various aquatic species. Because of their biotoxicity, some congeners of PBDEs (tetra-, penta-, hexa-, and hepta-bromodiphenyl ether) and PBBs (hexabromobiphenyl) were labeled as persistent organic pollutants (POPs) at the fifth meeting of the Conference of the Parties to the Stockholm Convention on POPs. Although some of these compounds were phased out in developed regions, their use has been increasing in some developing countries. In China, the production of deca-BDE increased 1.5 times in 2004 relative to 2000 (Xiang et al., 2007).
The Yangtze River, the longest river in China and the third longest in the world, descends 7,500 m from the Tibetan Plateau to the East China Sea with a journey of 6,300 km. The Three Gorges Dam (TGD), which is the largest dam in the world, has a length of 2,335 m and a height of 185 m. The Three Gorges Reservoir (TGR) located at the portion of the Yangtze river basin between Chongqing and Yichang, has an area of 1,080 km2 and is the source of fresh drinking water and favorite fish for 16 million people (Wang et al., 2017). Some researchers investigated the level and distribution of PBDEs and PBBs in surface water and sediment in TGR (Zhao et al., 2013; Wang et al., 2017). Wang et al. (2017) detected some low molecular weight PBDEs in water and large molecular weight PBDEs in sediment. The concentration PBDEs was up to 811 pg g-1 lipid (dissolved bioavailable PBDEs) in water and 52,843 pg g-1 by dry weight (dw) in sediment. Zhao et al. (2013) measured the concentrations of PBDEs and PBBs as 22.41 and 35.24 pg g-1 dw, respectively. Given their high bioaccumulative abilities, PBDEs and PBBs are likely accumulated in fish in the TGR. However, no studies on BFRs in edible fishes from the TGR have been published to date and the accumulation of chemicals in edible fish has rarely been known by the public. Diet is the most common pathway for human exposure to PBDEs or PBBs. Since TGR is the main source of edible fish for local residents, it is crucial to investigate the levels as well as the distribution of PBDEs and PBBs in fishes in this area.
In this study, environmental concentrations of 49 BFRs in 24 fish specimens from 12 species were collected at the TGR. All of the sampling sites were located at a tributary of the Yangtze River. The monitored BFRs included 27 PBDE congeners and 22 PBB congeners. Twelve fish species included carp (Cyprinus carpio), grass carp (Ctenopharyngodon idella), brass gudgeon (Coreius heterodon), trout (Squaliobarbus curriculus), topmouth culter (Erythroculter ilishaeformis), catfish (Silurus asotus), yellow catfish (Pelteobagrus fulvidraco), silver carp (Hypophthalmichehys molitrix), mandarin fish (Siniperca kneri Garman), rock carp (Spinibarbus sinensis), yellowcheek carp (Elopichthys bambusa), and basilewsky (Hemicculter leuciclus). The main aim of this study was to examine the levels and distribution of PBDEs and PBBs in different fish species from the TGR and to evaluate the bioaccumulation of individual PBDE and PBB congeners in 12 fish species.
Materials and Methods
Sample Collection and Preparation
Twenty-four freshwater fish samples of 12 species were collected at the sampling locations shown in Fig. 1. The fish lengths were measured, fishes were dissected, and the muscle tissue was maintained at -20°C before use.
The fish samples were frozen, dried, ground into powder in a mortar, and passed through a 100-mesh sieve for follow-up analysis. Five grams of each sample was spiked with 10 ng of 2,4,5,6-tetrachloro-m-xylene, pentachloro-nitrobenzene and PCB209 as surrogate congener standards and placed into extraction cells together with 2 g activated copper powder and kieselguhr. The cells were extracted using ASE 300 (accelerated solvent extractor) (Dionex, CA, USA). The extracted solvents were n-hexane and methylene chloride (1:1, v/v).
Extracts were combined and concentrated to 6.0 mL under a nitrogen stream and 2.5 mL of each concentrated extract was cleaned by Gel Permeation Chromatography (GPC). The mobile phase for GPC was ethyl acetate and hexamethylene (1:1, v/v). The portion including the analyzed BFRs was collected between 8 and 16 min (total of 37.6 mL of eluent). The extract was concentrated to 1 mL by rotary evaporation (400 mbar, 40°C). The concentrated extract was further cleaned using a multilayer silica gel column that consisted of 4 g deactivated silica (3.3% organic-free reagent water w/w), 2 g anhydrous sodium sulfate, 4 g deactivated silica (3.3% organic-free reagent water w/w), 2 g anhydrous sodium sulfate, and 8 g acidic silica (44% concentrated sulfuric acid w/w). To protect the samples from debromination caused by UV light, the silica gel column was wrapped in aluminum foil and then pre-eluted with 80 mL hexane before use. To collect PBB and PBDE congeners, 100 mL n-hexane and 10% methylene chloride in 80 mL n-hexane were used (EPA, 1996). The eluents were concentrated to 1 mL individually through rotary evaporation and the solvent from each sample was evaporated to dryness at 25°C under a gentle nitrogen stream and finally re-dissolved in 100 µL n-hexane.
Instrumental Analysis
Target compounds were analyzed by Varian CP3800/300 triple-quadrupole system (GC-MS/MS) (Varian, USA). The capillary was VF-5-MS (Varian, USA). The temperature of the column oven was increased from 90°C (initial time: 1 min) to 250°C at a rate of 4°C/min, then increased at a rate of 25°C/min from 250°C to 300°C and it was maintained at 300°C for 5 min. The temperature of the GC injector, the MS ion source, and the transfer line was kept at 260°C, 230°C, and 250°C, respectively. Helium was the carrier gas with a flow rate of 1.5 mL/min and the injection volume of 1 μL. For the analysis of PBBs and PBDEs, the mass spectrometer was run in the negative chemical ionization (NCI) mode.
Quality Control and Statistical Analysis
To avoid contamination, a solvent blank and a procedural blank were operated for every ten samples. Limits of detection (LOD) for the analytes in the present study were defined as three times the signal-to-noise ratio (S/N) and were 0.1-0.6 pg g-1 ww for PBDEs, 0.1-0.3 pg g-1 ww for PBBs, and 0.8-1.0 pg g-1 ww for PBB209 and PBDE209, respectively. Spike-recoveries for 13C12-labeled PBDEs (at 1 ng), PCNB, TMX, and PCB209 were 73.5-94.6%, 80.4-105.3%, 70.2-91.7%, and 90.5-111.2%, respectively. Standard calibration curves were established for quantification and they fitted the data well (r 2 > 0.99).
Results
The values of ∑PBDEs and ∑PBBs refer to the sum of all targeted PBDE and PBB congeners, respectively. Information on the levels of ∑PBDEs and ∑PBBs in fish samples is summarized in Tables 1 and 2.
Table 1 Biometric information and concentrations of PBDEs (pg g-1 ww) in 12 fish species.
| Carp | Grass carp | Brass gudgeon | Trout | Top mouth culter | Cat-fish | Yellow catfish | Silver carp | Mandarin fish | Rock carp | Yellow-cheek carp | Basile-wsky | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 2 | 3 | 1 | 1 | 2 | 2 | 1 | 4 | 3 | 2 | 2 | 1 |
| aWeight(kg) | 2.4 | 2.7 | 1.2 | 0.4 | 0.44 | 0.2 | 0.33 | 1.51 | 0.25 | 0.53 | 0.22 | 0.2 |
| aLength(cm) | 36.8 | 41.5 | 24.5 | 21.6 | 31.15 | 30.35 | 29.2 | 40.05 | 24.7 | 26.42 | 33.25 | 17.7 |
| BDE3 | b - | 8.21 | - | - | - | - | - | - | - | - | - | - |
| BDE7 | - | - | - | - | - | - | - | 0.16 | 2.41 | - | - | 0.08 |
| BDE15 | 4.78 | 2.27 | - | - | 3.58 | - | - | - | 4.65 | - | - | - |
| BDE17 | - | - | - | 1.46 | - | 2.17 | 0.66 | 1.23 | 0.29 | 1.05 | - | 1.16 |
| BDE28 | 4.21 | 2.92 | 1.67 | 13.47 | 7.77 | 18.21 | 14.67 | 14.79 | 9.69 | 15.38 | 1.89 | 22.02 |
| BDE49 | 4.32 | 1.90 | 1.00 | 4.85 | 7.50 | 10.99 | 16.48 | 13.53 | 3.97 | 9.29 | 1.00 | 18.60 |
| BDE71 | 2.76 | 1.98 | 1.15 | 4.72 | 7.21 | 10.44 | 15.68 | 12.79 | 4.49 | 8.81 | 1.09 | 18.61 |
| BDE47 | 8.36 | 3.80 | 6.60 | 6.87 | 22.25 | 31.28 | 42.48 | 3.59 | 13.04 | 8.65 | 4.23 | 13.27 |
| BDE66 | 0.21 | 0.55 | 1.27 | 1.24 | 1.17 | 0.57 | 6.19 | - | 1.57 | 0.83 | - | - |
| BDE77 | - | 0.55 | - | - | 1.21 | - | - | - | 0.63 | - | - | - |
| BDE100 | 0.13 | 0.19 | 0.28 | - | 2.79 | 4.01 | 4.33 | - | 0.61 | - | - | - |
| BDE119 | 0.55 | 0.37 | 0.20 | - | 2.59 | 3.99 | 4.46 | 1.62 | 0.47 | 0.23 | 0.62 | 1.38 |
| BDE99 | 2.61 | 1.22 | 2.63 | 0.91 | 3.84 | 6.83 | 21.04 | 1.49 | 2.93 | 0.77 | 1.95 | 5.82 |
| BDE85 | 0.89 | 1.23 | - | 0.92 | 5.03 | 7.51 | 6.07 | - | 1.07 | - | - | - |
| BDE126 | - | - | 8.28 | - | - | 1.10 | - | - | 1.82 | - | 1.97 | 4.55 |
| BDE154 | 1.25 | 0.53 | 1.59 | 0.66 | 8.23 | 11.55 | 11.36 | 0.85 | 4.00 | 2.07 | 0.71 | 0.65 |
| BDE153 | - | 1.19 | 1.44 | 2.29 | 5.88 | 5.85 | 10.69 | 1.87 | 2.14 | 1.42 | 0.51 | 2.38 |
| BDE138 | - | - | - | - | - | - | - | - | - | - | - | - |
| BDE156 | - | - | - | - | 2.95 | 2.92 | - | - | - | - | - | - |
| BDE184 | - | - | - | - | - | - | - | - | - | - | - | - |
| BDE183 | - | 4.46 | - | 8.60 | 7.99 | - | 4.83 | 4.78 | - | - | - | - |
| BDE191 | - | 4.82 | - | - | - | - | - | 6.84 | - | - | - | - |
| BDE197 | - | - | - | - | - | - | - | - | - | - | - | - |
| BDE196 | - | - | - | - | - | - | - | - | - | - | - | - |
| BDE207 | - | - | - | - | - | - | - | - | - | - | - | - |
| BDE206 | - | - | - | - | - | - | - | 3.85 | 3.36 | - | - | - |
| BDE209 | 36.63 | 6.12 | - | - | - | - | 58.80 | 81.89 | 8.71 | 9.80 | 8.65 | 65.96 |
| c∑PBDEs | 66.71 | 42.33 | 26.10 | 45.98 | 89.97 | 117.41 | 217.75 | 149.28 | 65.86 | 58.30 | 22.62 | 154.48 |
a average concentrations;
b “-”: not detected;
c geometric mean.
Table 2 Biometric information and concentrations of PBBs (pg g-1 ww) in 12 fish species.
| Carp | Grass carp | Brass gudgeon | Trout | Top mouth culter | Catfish | Yellow catfish | Silver carp | Mandarin fish | Rock carp | Yellow-cheek carp | Basilewsky | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 2 | 3 | 1 | 1 | 2 | 2 | 1 | 4 | 3 | 2 | 2 | 1 |
| a Weight(kg) | 2.4 | 2.7 | 1.2 | 0.4 | 0.44 | 0.2 | 0.33 | 1.51 | 0.25 | 0.53 | 0.22 | 0.2 |
| a Length(cm) | 36.8 | 41.5 | 24.5 | 21.6 | 31.15 | 30.35 | 29.2 | 40.05 | 24.7 | 26.42 | 33.25 | 17.7 |
| PBB1 | 1.25 | 3.05 | 1.49 | 1.33 | 2.67 | 0.80 | 2.32 | 0.81 | 2.36 | 3.18 | - | - |
| PBB2 | 0.11 | 0.81 | 5.74 | - | 0.58 | 4.69 | - | 0.16 | 3.01 | 0.70 | 11.75 | 24.37 |
| PBB3 | - | - | 2.54 | - | - | 2.09 | - | - | - | - | - | - |
| PBB10 | - | - | - | - | - | - | - | - | - | - | - | - |
| PBB4 | - | - | - | - | - | - | - | - | - | - | - | - |
| PBB9 | - | - | - | - | - | - | - | - | - | - | - | - |
| PBB7 | 2.98 | 1.13 | - | - | 2.56 | - | - | - | 7.07 | - | - | - |
| PBB15 | - | - | - | - | - | - | - | - | 0.57 | - | - | - |
| PBB30 | - | - | - | - | - | - | - | - | 0.40 | 0.94 | - | - |
| PBB18 | - | - | - | - | - | - | - | - | - | - | - | - |
| PBB29 | - | - | - | - | - | - | - | - | 1.68 | - | - | - |
| PBB26 | - | - | - | 2.15 | - | - | - | - | - | - | - | - |
| PBB31 | 1.96 | 1.23 | 1.02 | 5.10 | 2.60 | 9.41 | 4.45 | 8.21 | 4.73 | 11.27 | 3.40 | 16.20 |
| PBB53 | - | - | - | - | - | - | - | - | 8.90 | - | - | 45.91 |
| PBB38 | 0.61 | 0.34 | - | 1.72 | 0.45 | 2.68 | 1.84 | 0.45 | 1.28 | 1.29 | 1.09 | 4.63 |
| PBB52 | - | - | - | - | - | - | - | - | 0.40 | - | - | - |
| PBB49 | 10.13 | 4.62 | 7.51 | 7.62 | 24.35 | 40.91 | 47.10 | 6.41 | 12.84 | 9.60 | 8.61 | 25.19 |
| PBB103 | - | - | - | - | - | - | - | - | - | - | - | - |
| PBB101 | - | 0.51 | - | - | 1.42 | - | 2.82 | - | 0.84 | 3.96 | - | - |
| PBB155 | 0.44 | 1.13 | 1.12 | 0.51 | 3.79 | - | 6.01 | 1.09 | 0.45 | - | - | - |
| PBB153 | 2.43 | 1.29 | 3.24 | 1.12 | 12.17 | 18.04 | 14.10 | 2.52 | 4.87 | 3.86 | 0.99 | 3.59 |
| PBB209 | 2.98 | 37.25 | 4.05 | 78.83 | 80.95 | 19.37 | 1.29 | 1.75 | 4.29 | 2.44 | 3.72 | 15.67 |
| c∑PBBs | 33.02 | 56.08 | 34.30 | 106.06 | 155.75 | 138.51 | 126.64 | 27.83 | 59.40 | 46.83 | 38.19 | 123.98 |
a average values; b “-”: not detected; c geometric mean.
The levels of PBDEs in muscle ranged from 22.62 (yellowcheek carp) to 217.75 (yellow catfish) pg g-1 ww (Table 1). BDE209 was the dominant BDE congener (contribution to PBDEs ranged from 26.97% to 54.59%) in five species (carp, yellow catfish, silver carp, yellowcheek carp, basilewsky); meanwhile, the level of BDE209 in these species ranged from 8.65 to 81.89 pg g-1 ww (Figure 2). BDE47 was the dominant congener in topmouth culter (22.25 pg g-1 ww, 24.74% of PBDEs), catfish (31.28 pg g-1 ww, 26.51% of PBDEs), and mandarin fish (13.04 pg g-1 ww, 19.76% of PBDEs). BDE28 was the main congener in trout (13.47 pg g-1 ww) and rock carp (15.38 pg g-1 ww) and the ratios of BDE28-to-PBDEs in these two species were 29.29% and 26.52%, respectively. The dominant BDE congener in grass carp was BDE3 (41.07 pg g-1 ww), with a BDE3/PBDEs ratio of 19.56%. The dominant BDE congener in brass gudgeon was BDE126 (41.38 pg g-1 ww), with a BDE126/PBDEs ratio of 31.83%. Furthermore, the sum of BDE47 and BDE209 in 11 fishes (except trout) accounted for 23.62% to 68.16% of total PBDEs.
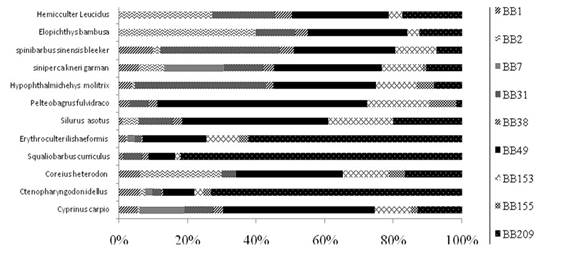
For all samples, the levels of ∑PBBs in muscle ranged from 27.83 pg g-1 ww in silver carp to 155.75 pg g-1 ww in topmouth culter (Table 2). BB209 was the main congener in grass carp (186.26 pg g-1 ww), Spualiobarbus curriculus (394.13 pg g-1 ww), and topmouth culter (404.76 pg g-1 ww), with the BB209/PBBs ratios of 71.64%, 80.43%, and 61.33%, respectively (Figure 3). BB49 was the dominant BB congener (contribution to PBBs ranged from 25.71% to 58.87%) in six species (Cyprinus carpio, brass gudgeon, catfish, Pelteobagrus fulvidraco, Siniperca kneri Garman, basilewsky) with concentrations ranging from 37.54 to 235.49 pg g-1 ww. In addition, the dominant BB congener in the chub and Spinibarbus sinensis was BB31 (41.03 pg g-1 ww and 56.37 pg g-1 ww), which accounted for 37.30% and 29.67% of PBBs, respectively. The dominant BB congener in yellowcheek carp was BB2 (58.76 pg g-1 ww), with a ratio of 39.18% to PBDEs.
Discussion
Concentrations of PBDEs in Fish
PBDE levels in muscle (22.62-217.75 pg g-1 ww) varied with the species type because of the bioaccumulative characteristics of different species at different age groups. The concentrations of PBDEs in yellow catfish were higher (up to 217.75 pg g-1 ww) than those in other species (about 22.62 pg g-1 ww). The most frequently analyzed congener, BDE47, was used for comparison with other studies listed in Table 3. In the present study, mean PBDE concentrations in muscle were close to those from the Eastern China coastline (1.11 to 5.08 ng g-1 lipid weight) (Xia et al., 2011) and Bo Sea (0.31 to 2.73 ng g-1 dw), but lower than those from Europe (119 ng g-1 lipid weight) (Hayward, Wong, & Krynitsky, 2007), as well as from Taiwan (107 ng g-1 lipid weight) (Peng, Huang, Weng, & Yak, 2007).
Table 3 Levels of PBDEs and PBBs (ng g-1 ww) in fishes from other studies.
| Location | Year | Units | PBDEs | BDE47 | PBBs | BB153 | References |
|---|---|---|---|---|---|---|---|
| TGRa | 2010 | Wet weight | 0.11-1.09 | 0.02-0.21 | 0.14-0.78 | 0.01-0.09 | Present study |
| Laurentian Great Lakes, Canada | 1997 | Wet weight | 27-95 | 16-58 | 0.25-3.10 | 0.19-2.08 | Luross et al. (2002) |
| Guangzhou, China b | 2003-2004 | Lipid weight | 46.3 | 27.4 | 9.86 | 0.21 | Jiang et al. (2005) |
| Zhoushan, China c | 2003-2004 | Lipid weight | 6.72 | 2.87 | 2.09 | 0.02 | Jiang et al. (2005) |
| Control site, Zhejiang, China | 2007 | Lipid weight | 35.9 | 13.3 | 20.3 | NA | Zhao et al. (2009) |
| Disassembly site, Zhejiang, China | 2007 | Lipid weight | 43.3 | 17.2 | 79.9 | 5.2 | Zhao et al. (2009) |
| Pearl River Estuary, China d | 2004 | Lipid weight | 67.28-181.20 | 42.9-99.7 | - | - | Xiang et al. (2007) |
| Yangtze River, China e | 2006 | Lipid weight | NA-1100 | 8.3-160 | - | - | Xian et al. (2008) |
| Sir Dam Lake, Kahramanmaras, Turkey f | 2003 | Lipid weight | 0.08-0.67 | 0.07-0.54 | - | - | Perugini et al. (2004) |
| The Ross Sea, South of Italy g | 2001-2002 | Wet weight | 0.09-0.44 | - | - | - | Borghesi et al. (2009) |
| Mediterranean Sea h | 2003 | Wet weight | 15.1 | - | - | - | Borghesi et al. (2009) |
| Wild caught, USA i | 2004 | Wet weight | 0.04-38 | - | - | - | Hayward, Wong, & Krynitsky (2007) |
| Farm-raised, USA i | 2004 | Wet weight | 0.5-1.7 | - | - | - | Hayward, Wong, & Krynitsky (2007) |
| Northeastern Pacific Ocean j | 1993-1996 | Lipid weight | 203-1015 | - | 3.0-31 | - | Rayne et al. (2004) |
| Ellasjøen and Øyangen k | 1999 and 2001 | Wet weight | - | <0.15-13.98 | - | <0.09-0.59 | Evenset, Christensen, & Kallenborn (2005) |
a Including carp, grass carp, brass gudgeon, trout, topmouth culter, catfish, yellow catfish, silver carp, mandarin fish, rock carp, yellowcheek carp, and basilewsky
b White mouth croaker, belt fish, Japanese mackerel, silvery pomfret, and small yellow croaker
c Small yellow croaker, cinnamon flounder, white mouth croaker, conger pike, silvery pomfret
d Including silvery pomfret, robust tonguefish, large yellow croaker, and flathead fish
e Including carp, bream, perch
f Including native flounder, eel, native gray mullet, native horse mackerel, cultured flounder, native red sea bream, native sea bass, cultured yellowtail, and cultured red sea bream
g Including thynnus
h Including chinook, sockeye, and coho salmon
i Including Atlantic and Icelandic salmon fillets
j Including killer whales
k Including zooplankton, chironomid larvae, small Arctic char, and tadpole shrimps
“-” means not studied
PBDEs Congener Profiles
BDE209 was the dominant BDE congener in all samples, similar to the results from the Pearl River, Bo Sea, Dongguan, and Shunde (Zhang, Ni, Guan, & Zeng, 2010). Such a finding is not surprising because the annual global consumption of deca-BDE (i.e., PBDE209) is 28,000 ton, which accounts for as much as 70% of the total amount of PBDEs. These findings are in agreement with a previous study on PBDEs in sediments from the TGR (Zhao et al., 2013).
BDE47 was the main congener in three fish species, including topmouth culter, catfish, and mandarin fish, and the ratios of BDE47 to total PBDEs in these species were 24.74%, 26.51%, and 19.76%, respectively. This result is consistent with a previous study conducted in the Mediterranean Sea (Borghesi et al., 2009) and with the studies of different species in other parts of the world (Brown, Winkler, Visita, Dhaliwal, & Petreas, 2006; Aksoy et al., 2011; Liu et al., 2011). BDE47 and 99 were considered the dominant isomers in commercial penta-BDE products, accounting for 38.2% and 48.6% of the total product (DE-71) Evenset et al. (2005). The increasing use of the penta-BDE mixture in China may also lead to unwanted emissions of BDE47. The reasons for the abundance of BDE47 in fishes include source emissions and the debromination of PBDEs to BDE47 in organisms (Bezares-Cruz, Jafvert, & Hua, 2004; Stapleton, Letcher, & Baker, 2004; Zheng et al., 2016).
BDE28 was the main congener in trout (29.29%, 13.47 pg g-1 ww) and rock carp (26.52%, 15.38 pg g-1 ww). This result is on agreement with the findings for the Yangtse River (Xian et al., 2008) and Pearl River deltas (Guo et al., 2008). Past research demonstrated that BDE28 was the predominant photoproduct of BDE47 in surfactant micelles (Erdogrul, Covaci, & Schepens, 2005). Zhao et al. (2013) also detected high BDE28 concentrations in the study area. Therefore, the origin of high concentrations of BDE28 in fishes is the BDE28 in the environment.
The dominant BDE congener in grass carp and brass gudgeon was BDE3 (19.56%, 41.07 pg g-1 ww) and BDE126 (31.83%, 41.38 pg g-1 ww). The sum of BDE47 and BDE209 in 11 fish species (except trout) in the present study accounted for 23.62-68.16% of total PBDEs. BDE47 and BDE209 originated from penta-BDE and deca-BDE mixtures used in electrical or electronic products are the likely sources of BDE47 and BDE209 in fishes, respectively. The differences in species and sampling locations result in the differences in dominant BDE congeners (BDE47 or BDE209) in fish.
Ratio of BDE99/BDE100
The ratio of PBDE congeners is significant for exploring the mechanisms of bioaccumulation in organisms (Borghesi et al., 2009). In the present study, the ratio of BDE99 to BDE100 was different among species (varied from 42:58 to 5:95). It was 5:95 in carp, 13:87 in grass carp, 10:90 in brass gudgeon, 42:58 in topmouth culter, 37:63 in catfish, 17:83 in yellow catfish and mandarin fish (ratios were not calculated for trout, silver carp, rock carp, yellowcheek carp, and basilewsky because BDE100 or BDE99 was not detected in those samples). The average ratio of these congeners in fish was 30:70 (w/w) (Harris, Kiparississ, & Metcalfe, 1994) because BDE100 has a higher bioavailability or a lower biodegradability than BDE99.
The BDE99/BDE100 ratios in this study were less than 84:16 (ratio of BDE99 to BDE100 in the industrial product Bromkal70-5DE), indicating that the species used in this study had the ability to metabolize BDE99 or that BDE100 can pass through biota membranes more easily than BDE99 (Luross et al., 2002).
A relevant study reported the debromination of BDE99 to BDE47 in carp (Harris et al., 1994), which is considered a high metabolizer due to the lowest ratio of BDE99 to BDE100 (5:95). Both topmouth culter and catfish had higher BDE99/BDE100 ratios (42:58 and 37:63, respectively) and higher PBDE concentrations (89.97 and 117.14 pg g-1 ww, respectively). Although the BDE99/BDE100 ratio in carp was the lowest (5:95), the concentration of PBDE (66.71 pg g-1 ww) was higher. The differences in BDE99/BDE100 ratios and PBDE concentrations in different species suggested that, besides their living and feeding habits, the metabolization ability was important for the accumulation of PBDEs in biota. These results confirmed that the BDE99/BDE100 ratio depended on the species type as well as the location (Perugini et al., 2004).
Concentrations of PBBs in Fish
In all the samples, ∑PBBs ranged from 27.83 pg g-1 ww in silver carp to 155.75 pg g-1 ww in topmouth culter. These concentration levels were comparable to the results from other regions, such as Laurentian Great Lakes (Luross et al., 2002), Guangzhou, and Zhoushan (Jiang et al., 2005), but lower than those in fish samples collected from e-waste disassembly sites in Zhejiang (Zhao et al., 2009). This comparison might be confounding because of the differences in fish species and the amount of analyzed PBB congeners.
PBBs Congener Profiles
The relative abundance of each PBB congener in fish are shown in Fig. 3. BB49 and BB209 were the main congeners and detected in all fishes; however, the concentration of BB49 (4.62-47.10 pg g-1 ww) was similar to that in lake trout (6.8-125 pg g-1 ww) (Luross et al., 2002). In the present study, high concentrations (1.29-80.95 pg g-1 ww) and ratios (ratio of 100%) of BB209 were detected in most fishes, different from those detected in fish samples from Zhejiang (Zhao et al., 2009). The high proportion of BB209 in this study might be caused by the use of specific technical mixtures of PBBs in this area. Zhao et al. (2013) studied polyhalogenated aromatic hydrocarbons in surface sediments of the TGR and measured high concentrations of BB209. However, BB209 was not an important PBB congener reported in other studies. In other studies, BB153 was the most abundant congener in fishes (Rayne et al., 2004), while this study detected lower concentrations (1.29-18.04 pg g-1 ww) and smaller ratios of BB153-to-∑PBBs (2.30%-13.02%) (Table 2). These differences are likely caused by the differences in the study area and fish species. In addition, continual emissions of the hexa-BB mixture to the environment during the past few decades may lead to the high abundance of BB153 in the area (100%). Anaerobic debromination of commercial octa- and deca-BB mixtures is another factor that plays a role in the results.
PBDEs and PBBs in Different Fish Species
Topmouth culter, catfish, mandarin fish, and yellowcheek carp are carnivorous; grass carp that often feeds on phytoplankton, zooplankton, and bacteria is herbivorous; and carp, brass gudgeon, silver carp, rock carp, trout, yellow catfish, and basilewsky are omnivorous fish species. The concentration of ∑PBDEs was the highest in basilewsky and yellow catfish and the concentration of ∑PBBs was the highest in topmouth culter and catfish (Table 2). The levels of PBDEs and PBBs in carnivorous fish showed no significant difference from the levels in omnivorous or herbivorous fish (P > 0.05).
Relation of PBDE and PBB Concentrations with Fish Characteristics
We analyzed the relationship between PBDE and PBB concentrations and specific characteristics of fish. No significant correlation with the weight or length of the fish was observed in the TGR. This result is consistent with those of the previous studies conducted in the Hudson River, New York (Xia et al., 2008) and the Mediterranean Sea (Corsolini, Guerranti, Perra, & Focardi, 2008). However, some studies reported positive correlations between the concentrations of hydrophobic substances with fish length (Bordajandi, Martin, Abad, Rivera, & Gonzalez, 2006; Malakhova & Voronov, 2008).
Conclusion
This study is the first to investigate the concentrations and the distribution of PBBs and PBDEs in 12 edible fishes from the TGR. The results show that the levels of PBDEs and PBBs were, in general, low in fishes collected from the TGR. The relatively high levels of BDE209 and BB209 in fishes indicate that China is one of the major consumers of industrial compounds such as deca-BDE and deca-BB mixtures in Asia. The predominance of lower brominated PBDE and PBB congeners including BDE47 and BB49 in samples suggests that the debromination may play a key role in the fate of BFRs in the environment and more research should be conducted in the transformation mechanisms of BFRs in biota. Furthermore, the levels and distribution of PBBs and PBDEs are related to the feeding habits of fishes.











 text new page (beta)
text new page (beta)