Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias forestales
Print version ISSN 2007-1132
Rev. mex. de cienc. forestales vol.12 n.66 México Jul./Aug. 2021 Epub Oct 04, 2021
https://doi.org/10.29298/rmcf.v12i66.689
Scientific article
Seed fungi of Pinus montezumae Lamb. and Pinus greggii Engelm. ex Parl. stored under two relative humidities
¹ Universidad Autónoma Agraria Antonio Narro. Centro de Capacitación y Desarrollo en Tecnología de Semillas. México.
² Universidad Autónoma Agraria Antonio Narro. Departamento de Fitomejoramiento. México.
Fungi develop upon pine seeds during storage, and deteriorate their quality. The objective of this study was to identify the fungal genera that are associated with the seeds of Pinus montezumae and P. greggii in two relative humidities (HR). They were stored at 60 and 80 % RH at 5 °C. The samplings were made for 180 days. Seed moisture content (HS), fungal free seeds (SLH) were assessed and fungal genera (GH) were identified by colony morphology. In HS and SLH, a completely randomized experimental design was applied and a comparison of means was made. All sources of variation were significant. The mean HS per species was 9.59 and 12.37 % and SLH of 64.52 and 69.28 % for P. greggii and P. montezumae, respectively. In the P. greggii seed stored at 60 HR, HS was 7.97 %, with 66.19 % in SLH and at 80 HR, 11.21 % and 62.85 % in SLH. For P. montezumae at 60 HR, the HS was 10.21 % and 71.42 % in SLH; at 80 HR, HS was 14.53 % and 67.14 % in SLH. The GH identified promote the deterioration of the seeds; they were: Alternaria sp, in P. greggii at 60 HR it was not observed, Penicillium sp. increased and predominated after 120 days, Fusarium sp. was constant; Aspergillus sp. and Rizhopus sp., appeared sporadically
Key words: Storage; quality; fungi; relative humidity; Pinus spp.; seed
Las semillas de pinos durante su almacenamiento pueden presentar hongos que deterioran su calidad. El objetivo de la presente investigación consistió en identificar los géneros fúngicos que se asocian a las semillas de Pinus montezumae y P. greggii en dos humedades relativas (HR). Se les almacenó a 60 y 80 % de HR a 5 °C. Los muestreos se hicieron durante 180 días. Se evaluó el contenido de humedad de semilla (HS), las semillas libres de hongos (SLH); y se identificaron los géneros de los hongos (GH) por la morfología de sus colonias. En HS y SLH se aplicó un diseño experimental completamente al azar y se hizo una comparación de medias. Todas las fuentes de variación fueron significativas. La HS media por especie fue de 9.59 y de 12.37 %; la SLH de 64.52 y 69.28 % para P. greggii y P. montezumae, respectivamente. En la semilla de P. greggii almacenada a 60 HR, la HS fue de 7.97 %, con 66.19 % en SLH y a 80 HR, de 11.21 % y 62.85 % en SLH. Para P. montezumae a 60 HR, la HS fue de 10.21 % y de 71.42 % en SLH; a 80 HR, la HS fue de 14.53 % y 67.14 % en SLH. Los GH identificados que promueven el deterioro de las semillas fueron: Alternaria sp, que en P. greggii a 60 HR no se observó; Penicillium sp incrementó y predominó a partir de los 120 días; Fusarium sp. fue constante; de forma esporádica se presentaron Aspergillus sp y Rizhopus sp.
Palabras clave: Almacenamiento; calidad; hongos; humedad relativa; Pinus spp.; semilla
Introduction
Restoring degraded forests and agricultural lands has become a global conservation main aspect (Christin et al., 2016). Several countries develop methods for preserving the seeds of forest species, in order to improve their adaptation, growth and quality (Skrøppa and Fjellstad, 2017). Pinus montezumae Lamb. and Pinus greggii Engelm. ex Parl. are priority species of coniferous forests in Mexico because they have great economic, ecological and social importance (Conafor-FAO, 2011). In addition, the second is an endemic species of North America and is classified in vulnerability status according to the International Union for the Conservation of Nature (Conabio, 2018).
For the Pinaceae family, seed is the major propagation resource. However, during storage, various factors cause its deterioration: its moisture content, relative humidity and environmental temperature (Wang and Beardmore, 2004). Other agents that favor damage to these structures are living organisms (Arguedas, 1997) such as: fungi, bacteria, viruses, nematodes and arthropods, of which fungi are the group of pathogens with the greatest transmission, as they develop on their surface, internal parts or both and through different forms of propagation such as spores and sclerotia (Neegaard, 1977).
Information on the pathogens associated with stored forest seeds is scarce, and fungi are of interest because the damage they cause affects their subsequent establishment. Based on the above, the objective was to identify and quantify the fungal genera associated with the stored seeds of Pinus montezumae Lamb. and Pinus greggii Engelm. under two conditions of relative humidity.
Materials and Methods
The research was carried out at the Center for Training and Development in Seed Technology of the Antonio Narro Autonomous Agrarian University, in Saltillo, Coahuila State, since June 2018. The seeds of Black pine (Pinus greggii) and Royal pine (Pinus montezumae) collected in the municipalities of Metepec and Calimaya respectively, in the State of Mexico, were provided by the National Forestry Commission (Conafor).
A representative sample of one kilogram was taken from each lot , for each species four replications of 50 seeds were used for each relative humidity (60 and 80 %) and for each sampling, which were carried out at 0, 30, 60 , 90, 120, 150 and 180 days. The seeds were placed in a mesh bag and suspended on a pallet in plastic containers, so the seeds in the storage time did not directly touch the solutions. Both relative humidity (RH) were obtained with salts according to Winston and Bates (1960); for 60 % RH, 1.5 kg of grain salt was placed for samplings two (30 days) and three (60 days), while for samplings four to seven (90 to 180 days), it was 2.0 kg. For 80 % RH, 0.5 kg plus 1 L of water was added for the first two samplings and 1.1 kg plus 2 L of water for samplings four to seven.
The material thus treated was stored in a Torrey model CV-32 cooling chamber at a constant temperature of 5 °C. The assessed variables were: seed moisture content (HS), which was calculated based on ISTA (2004) with 15 seeds on average for each replication; the seeds were placed in previously weighed aluminum boxes with a lid, then the boxes with the wet seed were weighed and placed in a SHEL-LAB FX14-2 model drying oven, at 103 °C for 16 + 1 hours; the boxes were subsequently weighed after drying. An OHAUS AV264 model analytical balance was used for all measurements. HS was calculated by weight difference and expressed as a percentage.
The identification and quantification of fungal genera (GH) and fungal-free seeds (SLH) was carried out using the growth technique in Malta Sal Agar culture medium (Moreno, 1988). 10 seeds were handled with four repetitions per sampling, which were disinfested with NaClO at 0.5 % for P. greggii and at 1.0 % for P. montezumae, for 1 minute for both species, the differences in the concentration of NaClO is due to the fact that the integument of P. greggii is thinner and more sensitive to sodium hypochlorite than P. montezumae. Subsequently, the sowing was done inside an ALDER brand laminar flow hood, with 10 seeds per Petri dish; Subsequently, they were placed in a SHEL-LAB model FX14-2 brand incubation chamber at a temperature of 28-30 °C for 7 days. GH were identified by colony morphology with the taxonomic keys by Barnett and Hunter (1998) and by Moreno (1988). For SLH, the seeds that did not develop mycelium were quantified and recorded in percentage. The incidence (I) of GH for the two pine species was calculated using the following formula (Abdullah and Al-Mosawi, 2010):
A completely randomized experimental design was applied for seed moisture and free seeds, and a comparison of means according to Tukey (P <0.05), with the statistical SAS (2002) package.
Results
The analysis of variance showed that for the sources of variation analyzed, such as species, relative humidity and time, there was significance at 0.01. For HS by species, P. montezumae reached a mean of 12.37 %, which was statistically different from P. greggii with 9.59 %; in SLH, P. montezumae obtained the highest values with 69.28 %, compared to P. greggii (64.52 %). The lowest HS was determined under the 60 HR condition, P. montezumae presented 10.21 % and P. greggii 7.97 %; for 80 HR, it increased for the first species (14.53 %) and for the second, 11.21 % of seed moisture. In regard to the fungus-free seeds. in the 60 HR condition, P. montezumae registered 71.42 % and P. greggii 62.85 % and for the 80 HR environment, the first species, 67.14 % and the second, 66.19 %.
During the storage time, the HS was similar for both species. In P. montezumae under the 60 % relative humidity condition, the lowest HS was observed at 0 days (8.72 %); at 80 HR, the highest HS was at 180 days (16.42 %). Regarding SLH, the highest percentage was identified at 30 days under conditions of 60 HR and, at 80 HR, at 60 and 90 days (76.66 and 73.33 %). The behavior of P. greggii was similar to that of the other species of interest since at zero days under 60 HR the lowest HS value was obtained (7.58 %); for SLH it was at 120 days at 80 HR with the highest value (75.6 %), while at 60 HR the most prominent values were recorded at 150 and 180 days with 73.72 %. For both species, the highest HS was reached at 180 days in both RH humidity conditions: P. greggii 9.08 and P. montezumae 13.06 %, and finally for SLH there were no significant differences between the two relative humidity conditions (Figure 1).
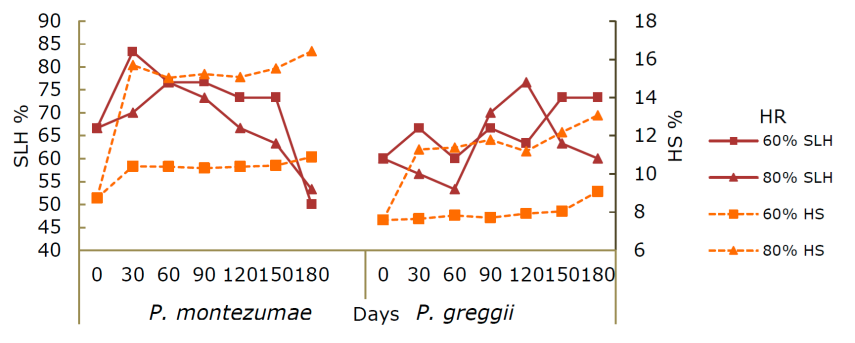
Figure 1 Response of Pinus montezumae Lamb. and Pinus greggii Engelm. ex Parl. seed moisture (HS) and fungus-free seeds (SLH) during 180 days of storage at 60 and 80 % relative humidity (RH).
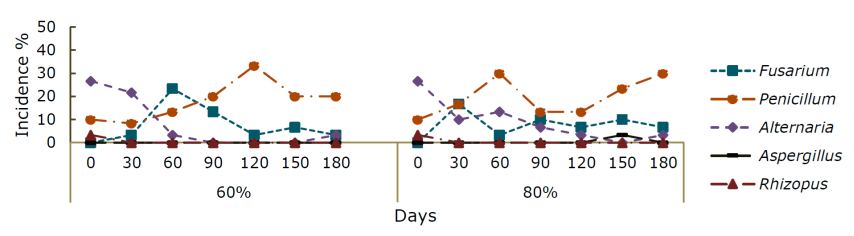
The fungal genera present in both species and humidities during storage were Fusarium sp., Penicillium sp., Alternaria sp., Rizhopus sp. and Aspergillus sp.; however, this last genus was not manifested in P. greggii at 60 % RH. At the beginning of storage, the quantity and variability of genera was higher, but, at the end of the samplings, Penicillium sp. was predominant.
In P. montezumae at 60 % RH, Penicillium sp. recorded incidences of 30 % at 180 days; the maximum incidence of Fusarium sp. was at 90 days (16.7 %). Alternaria sp. and Aspergillus sp. decreased to 0 %, whereas Rizhopus sp. presemt up to 180 days (Figure 2 a). At 80 % RH, Penicillium sp. was dominant with an incidence of 8.33 to 43.3 %; on the other hand, Fusarium sp. was found until the last sampling, while Aspergillus sp. at day 0 occurred with 10 % and Rizhopus sp with 2.33 % at 0 days (Figure 2).
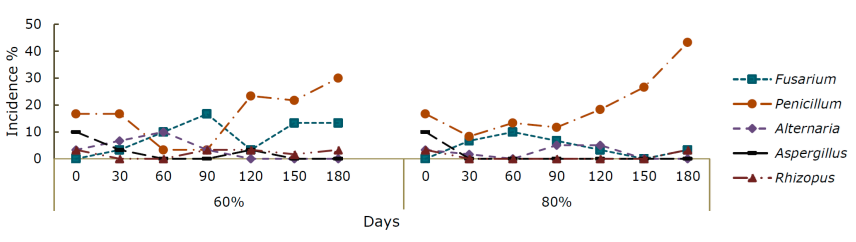
Figure 2 Incidence percentage of different fungi genera (GH) in Pinus montezumae Lamb. seeds for 180 days of storage at 60 and 80 % relative humidity.
The incidence of fungi in P. greggii was similar to that of P. montezumae, since at 0 days Alternaria sp. dominated with 27 % (Figure 3), but decreased over time. Fusarium sp. was present throughout storage. At 60 % RH, its maximum incidence was at 60 days (23.3 %) and at the 80 % RH of at 30 days it was 16.7 %; Rizhopus sp. was only present at the beginning of the experiment. In the 60 % RH environment, Aspergillus sp. was not detected, and the incidence of Penicillium sp. was greater than 120 days with 33 %. In the 80 % RH environment, the greatest increase of Penicillium sp. it was 30 % at 60 and 180 days, while the genus Aspergillus sp. it was only observed at 150 days with 3.33 %.
Discussion
HS is a direct function of relative humidity and environmental temperature. By having the ability to lose or gain moisture according to environmental conditions (Delouche, 1972), P. montezumae and P. greggii reached equilibrium humidity at 30 days and maximum values of 16.42 and 13.06 % respectively. However, Conafor (2017a b) recommends that the most appropriate storage humidity for P. montezumae is 8-10 % and for P. greggii, 6-7 %; during the storage of these species, HS turned out to be a determining factor to keep seed quality (SNICS, 2018). These percentages of seed moisture favor the presence of fungi and thus, the deterioration process (Engels and Visser, 2007).
In regard to HR, Ortiz-Catón et al. (2011) established that the biological activity of some fungi strains increases when they are in a range of 81-92 %. In Brassica sp., Suma et al. (2013) concluded that the viability of the seeds and the vigor parameters of the seedlings were reduced when they were subjected to a high RH (75 %), which coincide with the results obtained with the present study, in which it was observed that at 80 % RH the incidence of fungi was higher than at 60 % RH in the two species of interest.
Ceballos-Freire and López-Ríos (2007) established that in seeds of Alnus acuminata Kunth (alder), Guarea guidonia (L.) Sleumer (cedrillo), Juglans neotropica Diels (black cedar), Retrophyllum rospigliosii (Pilg.) C. N. Page (chaquiro) and Cordia gerascanthus L. (solera) the high relative humidity (90n%) caused an increase in the moisture of the stored seed and, as a consequence, a severe attack of fungi of the Penicillium and Aspergillus genera occurred, which affected 50 and 70 % of the preserved material, respectively.
The SLH trend for P. montezumae decreased dramatically in the two HRs after 90 days; in P. greggii at 80 %, the trend decreased after 120 days, which coincides with investigations on seeds of Pinus spp. than losing viability due to increased physiological damage (Hilli et al., 2003; Du Hyun and Sim, 2018) during long-term storage.
The genera determined in both pine species subjected to environments of 60 and 80 % RH during the 180 days coincide with the information provided by Guerra et al. (2004) and Campo-Aranda et al. (2014) on the mycobiota in seeds of Pinus spp. According to Moreno (1988), field fungi such as Alternaria sp. and Fusarium sp., develop in humidities between 90-100 %. In this way, Alternaria sp. was present in the seeds at the beginning of the samplings and its incidence decreased as time passed. Fusarium sp. which behaved in the same way, was higher at 60 % RH than at 80 % in both species. When this genus develops in the seed, it causes the quality of the plant to become lower, since it damages the embryo before germinating and causes necrosis of the hypocotyl and the cotyledons (Peterson, 2008; Solano-Bonilla and Brenes-Chacón, 2012).
In this study, fungi of the genus Penicillium sp., Aspergillus sp. and Rhizopus sp., cataloged as warehouse fungi with growth in a range between 65 and 90 % RH. Penicillium sp. its incidence increased in all environments and in both species after 120 days, as well as Aspergillus sp. it was identified more frequently in P. montezumae but in P. greggii it was only identified at 80 % RH. Rhizopus sp. it was observed sporadically in P. montezumae. These genera decrease the phytosanitary quality of seedlings by causing rotting, growth reduction and seedling death (Borges and Urdaneta, 2010; Arguedas, 2011; Lee, 2011).
A large part of these fungi genera are considered saprophytes, some do not always cause direct damage to the seed, but it is recognized that when the incidence is very high, the vigor and viability of the seeds tend to decrease (Mittal et al., 1990 ).
Conclusions
The storage conditions of P. montezumae and P. greggii seeds with high relative humidity increase the fungal incidence of genera such as: Fusarium sp., Aspergillus sp., Alternaria sp., Rhizopus sp. and Penicillium sp., genera that have been reported as causing deterioration.
Cold (5 °C) and dry (60 % RH) environments can be safe for the storage of P. montezumae and P. greggii seeds, as these are the conditions where the lowest percentage of seed moisture was reached and consequently obtained better incidence of fungi that cause deterioration.
Acknowledgements
To the Universidad Autónoma Agraria Antonio Narro (UAAAN) for the support with the equipment and laboratories during seed storage. To the Consejo Nacional de Ciencia y Tecnología (Conacyt) for the financial support to carry out the research, and to the Comisión Nacional Forestal (Conafor) for the provision of seed.
REFERENCES
Abdullah, S. K. and K. A. Al-Mosawi 2010. Fungi associated with seeds of sunflower (Helianthus annuus) cultivars grown in Iraq. Phytopathologia. 57:11-20. https://www.cabi.org/ISC/FullTextPDF/2011/20113179658.pdf (20 de junio de 2019). [ Links ]
Arguedas, M. 1997. Plagas de semillas forestales en América Central y el Caribe. CATIE. Turrialba, Costa Rica. 120 p. [ Links ]
Arguedas, M. 2011. Problemas fitosanitarios en teca (Tectona grandis L.f.) en América Central. In: Chavarriaga, D. M. (ed.). Protección fitosanitaria forestal. ICA. Medellín, Colombia. pp: 147-160. [ Links ]
Barnett, H. L. and B. B. Hunter. 1998. Ilustrated genera of imperfect fungi. American Phytopathological Society Press. St. Paul, MN, USA. 218 p. [ Links ]
Borges J., A. y J. Urdaneta. 2010. Efecto de Fusarium sp. en la germinación, fenología y supervivencia de plántulas de Leucaena leucocephala (lam.) de Wit. Agronomía Tropical 60(2): 155-160. http://ve.scielo.org/scielo.php?pid=S0002-192X2010000200004&script=sci_abstract (13 de enero de 2019). [ Links ]
Campo-Aarana, R. O., N. Urango-Esquivel y M. Espitia-Camacho. 2014. Hongos asociados a la semilla de seis forestales nativos, cultivados en el departamento de Córdoba. Fitopatología Colombiana 38(2):27-31. https://www.researchgate.net/publication/298809860_Hongos_asociados_a_la_semilla_de_seis_forestales_nativos_cultivados_en_el_departamento_de_Cordoba (20 de noviembre de 2018). [ Links ]
Ceballos-Freire, A. J. y J. A. López-Ríos. 2007. Conservación de la calidad de semillas forestales nativas en almacenamiento. Cenicafé 58(4): 265-292. https://www.cenicafe.org/es/publications/arc058%2804%29265-292.pdf (24 de enero de 2019). [ Links ]
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio). 2018. Pinus prieto (Pinus greggii) Naturalista. https://www.naturalista.mx/taxa/135782-Pinus-greggii (6 de noviembre de 2018). [ Links ]
Comisión Nacional Forestal (Conafor) - Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). 2011. Situación de los recursos genéticos forestales en México. http://www.fao.org/3/a-be793s.pdf (15 de octubre de 2018). [ Links ]
Comisión Nacional Forestal (Conafor). 2017a. Paquete tecnológico Pinus greggii Englem. http://www.conafor.gob.mx:8080/documentos/docs/13/961Pinus%20greggii.pdf (10 de octubre de 2018). [ Links ]
Comisión Nacional Forestal (Conafor). 2017b. Paquete tecnológico Pinus greggii Englem. Pinus montezumae Lamb. http://www.conafor.gob.mx:8080/documentos/docs/13/971Pinus%20montezumae.pdf (10 de octubre de 2018). [ Links ]
Christin, Z. L., K. Bagstad and M. Verdone, 2016. A decision framework for identifying models to estimate forest ecosystem services gains from restoration. Forest Ecosystems 3:3. Doi: 10.1186/s40663-016-0062-y. [ Links ]
Delouche, J. C. 1972. Harvesting, handling and storage of soybean seed. In: Proceedings of Short Course for Seedsmen. Mississippi State University. Mississippi, MS, USA. pp. 97-122. [ Links ]
Du Hyun, K. and H. H. Sim. 2018. Seed coat and aging conditions affect germination and physiological changes of aging Korean pine seeds. Journal of Forest Research. 23: 372-379. Doi: 10.1080/13416979.2018.1531478. [ Links ]
Engels, J. y L. Visser. 2007. Guía para el manejo eficaz de un banco de germoplasma. Manuales para Bancos de Germoplasma No. 6. Bioversity International. Roma, Italia. 209p. [ Links ]
Guerra, C., H. Cruz, I. Vila, A. Duarte y O. M. López. 2004. Principales hongos que afectan a Pinus tropicalis Morelet en Cuba. Fitosanidad 8: 9-12. http://www.fitosanidad.cu/index.php/fitosanidad/article/view/373 (6 de febrero de 2019). [ Links ]
Hilli, A., S. Tillman, E. and A. Kauppi, 2003. Germination of pretreated Scots pine seeds after long-term storage. Canadian Journal of Forest Research. 33(1): 47-53. Doi:10.1139/x02-155. [ Links ]
International Seed Testing Association (ISTA) 2004. International rules for seed testing. Supplement Rules. Seed Science and Technology. Vol. 24. Zürichstr, Bassersdorf, Switzerland. 335 p. [ Links ]
Lee, S. S. 2011. Diseases of acacias in South-Eas Asia. In: Chavarriaga, D. M. (ed.). Protección fitosanitaria forestal. ICA. Medellín, Colombia. pp. 69-76. [ Links ]
Moreno M., E. 1988. Manual para la identificación de los hongos en granos y sus derivados. Universidad Nacional Autónoma de México. México, D. F. México. 109 p. [ Links ]
Mittal, R. K., R. L. Anderson and S. B. Mathur. 1990. Microorganisms associated with tree seeds. World Checklist. Patawawa National Forestry Institute. Ontario, Canada. 57 p. [ Links ]
Neegaard, P. 1977. Seed pathology. MacMillan Press Ltd. London, UK. 829 p. [ Links ]
Peterson, M. 2008. Fusarium species, a British Columbia perspective in forest seedling production. In: Dumroese, R. K. and L. E. Riley (eds.). Proceedings of the Forest and Conservation Nursery Associations Meetting 2007. Sidney, 17-19 September 2007. USDA Forest Service. Fort Collins, CO, USA pp. 109-125. [ Links ]
Sistema Nacional Inspección y Certificación de Semillas (SNICS). 2018. ¿El contenido de humedad afecta la calidad de las semillas? Gobierno de México. https://www.gob.mx/snics/articulos/el-contenido-de-humedad-afecta-la-calidad-de-la-semilla?idiom=es (20 de septiembre de 2018). [ Links ]
Skrøppa, T. and K. B. Fjellstad. 2017. Conservation of forest genetic resources in Norway in a climate change perspective. In: Ahuja, M. and S. Jain (eds.). Biodiversity and conservation of woody plants. Sustainable Development and Biodiversity. 17:129-153. Doi: 10.1007/978-3-319-66426-2_5. [ Links ]
Solano-Bonilla, M. y D. Brenes-Chacón. 2012. Evaluación de métodos de curación de sustratos para la prevención del mal de talluelo. Revista Forestal Mesoamericana. Kurú 9: 63-65. Doi: 10.18845/rfmk.v9i22.365. [ Links ]
Statical Analysis System (SAS). 2002. (Version 9.0). Statical Analysis System Institute Inc. Cary, NC, USA. n/p. [ Links ]
Suma, A., K. Sreenivasan, K. Singh and J. Radhamani. 2013. Role of relative humidity in processing and storage of seeds and assessment of variability in storage behaviour in Brassica spp. and Eruca sativa. The Scientific World Journal Vol. 2013: 1-9. Doi: 10.1155/2013/504141. [ Links ]
Wang, B. S. y T. Beardmore. 2004. Almacenamiento y manejo de germoplasma. ln: Vargas, H., J. Jesús, B. Bermejo y F. T. Ledig (eds.). Manejo de recursos genéticos forestales. Segunda edición. Colegio de Posgraduados, Montecillo, México y Comisión Nacional Forestal. Zapopan, Jal., México. pp. 107-140. [ Links ]
Winston, P. W. and D. H. Bates. 1960. Saturated solutions for the control of humidity in biological research. Ecology 41: 232-237. Doi: 10.2307/1931961. [ Links ]
Received: November 19, 2020; Accepted: May 05, 2021











 text in
text in