Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias forestales
Print version ISSN 2007-1132
Rev. mex. de cienc. forestales vol.9 n.50 México Nov./Dec. 2018
https://doi.org/10.29298/rmcf.v9i50.225
Articles
Diversity of spiders in forest ecosystems as elevation and disturbance indicators
1Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. México.
2Instituto de Ecología Aplicada, Universidad Autónoma de Tamaulipas. México.
Spiders are ideal predators for studies of environmental variation and disturbance due to their small size and ease of collection. In two major mountains of northeastern Mexico: Cerro El Potosí (in Southern Nuevo León) and Peña Nevada (in Southern Tamaulipas), 45 spider communities were studied. Human activity, vegetation type, disturbance, livestock, and land degradation were measured. As a hypothesis, it was anticipated finding low spider diversity in highly degraded sites. 541 individuals from 23 families were found. The most abundant families were Lycosidae, Anyphaenidae and Gnaphosidae. Spider species distribution was highly associated with presence of leaf-litter. Spider diversity was unrelated to elevation or disturbance. Pardosa sp. was the most abundant and dominant at well-preserved sites. Lycosidae, Thomisidae and Pholcidae were more abundant in areas with greater human intervention. This study in two important forest zones in northeastern Mexico will be a guide for future research on biodiversity on forest ecosystems and influence of environmental variation and disturbance.
Key words: Spiders; biodiversity; mountain ecosystems; ecological gradient; Lycosidae; Pardosa sp
Las arañas son organismos depredadores que por ser pequeños y fáciles de detectar resultan ideales para la realización de estudios de variación ambiental y disturbio. Se estudiaron 45 comunidades de arañas en dos grandes montañas del noreste de México: el cerro El Potosí, en el sur de Nuevo León; y Peña Nevada, en el sur de Tamaulipas. Se determinó el tipo de vegetación, la actividad humana, la ganadería, y la degradación de suelo. Se definió un índice de disturbio. La hipótesis planteada se refiere a la presencia de una menor diversidad de arañas en los sitios con más disturbio. Se obtuvieron 541 individuos, agrupados en 23 familias; de ellas, las más abundantes fueron: Lycosidae, Anyphaenidae y Gnaphosidae. La distribución de las especies se asoció con la presencia de hojarasca. No se detectó relación entre la diversidad de arañas y la altitud o el disturbio. Pardosa sp. fue la más abundante en sitios conservados. Las familias Lycosidae, Thomisidae y Pholcidae fueron las mejor representadas en zonas con mayor intervención humana. Este estudio en dos zonas forestales importantes del noreste de México servirá de pauta para investigaciones posteriores de biodiversidad en ecosistemas forestales y la influencia de la variación ambiental y el disturbio.
Palabras clave: Arácnidos; biodiversidad; ecosistemas de montaña; gradiente ecológico; Lycosidae; Pardosa sp
Introduction
The edaphic macrofauna consists of invertebrates larger than 2 mm in diameter (Cabrera, 2012). Of these, spiders are a group of ecologically important predators (Eggs and Sanders, 2013); they are located in the highest part of the food chain of invertebrates (Uma and Weiss, 2012); they influence the density and activity of detritivorous and fungivorous fauna, and they also indirectly affect the decomposition processes (Willett, 2001; Ávalos et al., 2007).
Spiders are a regular component of any ecosystem where they live (Deza and Andía, 2009). Despite being considered among the most numerous entomophages, little is known about their role as predators, their diversity and abundance in temperate forests (Ruíz and Coronado, 2002; Ávalos et al., 2007, Gómez-Rodríguez and Salazar, 2015). Because their diversity and density respond to changes in ecosystems, they have been considered as indicators of habitat quality (Willett, 2001; Ávalos et al., 2007). Among the features that justify them as such, the following can be pointed out: the advantage of their ecological diversification, their continuous presence throughout the year, and the possibility of their being manipulated and identified, as well as the short period that elapses between their generations (Willett, 2001).
Spiders have been little investigated in Mexico; however, there are very good studies in natural environments such as tropical forests (Rivera-Quiroz et al., 2016), coniferous forest and eucalyptus plantations (Corcuera et al., 2016), cloud forest (Campuzano et al., 2016) and in urban conditions (Desales-Lara et al., 2013; Rodríguez-Rodríguez et al., 2015). The objective of this study is to identify and establish variations in spider communities, depending on the disturbance in two forest ecosystems of northeastern Mexico. In addition, it is hypothesized that its diversity is lower in the sites with more disturbance.
Materials and Methods
The investigation was carried out in the Cerro El Potosí, in Galeana municipality, Nuevo León state and in the Sierra Peña Nevada, which includes Zaragoza municipality, in Nuevo León and Miquihuana municipality, in Tamaulipas state in northeastern Mexico. The two places are promontories that are part of the Gran Sierra Plegada, within the Sierra Madre Oriental province; they are characterized by having temperate ecosystems and reaching the highest altitudinal range in the region (Cantú et al., 2013).
Sierra de Peña Nevada is located in the Sierra Madre Oriental and is part of the priority land region (RTP) No. 86, San Antonio-Peña Nevada (Arriaga et al., 2000). It has a height of 3 500 masl and an area of 60 500 hectares. Its extreme coordinates are 23°33'18'' to 23°52'28'' N and 99°38'55'' to 99°56'45'' W.
Cerro El Potosí has an area of 989.38 hectares; is located 15 km west of Galeana, Nuevo León, between 24°50'35'' and 24°53'16'' N and 100°13'9'' and 100°15'12'' W It has a maximum height of 3 700 masl.
Samplings were carried out throughout the year, in such a way that an attempt was made to incorporate the greatest seasonal variation. As far as possible, the field work dates were stratified, so that the two sampling sites and the altitudes were homogeneously distributed in the seasons of the year, to avoid biases when sampling. Other studies have determined that the density, but not the diversity and activity of the spiders varies according to the time of collection (Campuzano et al., 2016, Rivera-Quiroz et al., 2016).
Sampling was carried out between 2013 and 2015. The sites were visited only once and were selected based on an altitudinal distribution that included 10 levels, with an increase of 150 m, started at 2 100 m and finished at 3 450 m; in each one, two collections were conducted in geographically registered sites, by means of a satellite geolocator (Garmin eTrex 10). A transect of 100 m was established in each site, in which five alternating points were placed, marked with flags. The capture was direct, when the spiders were found visually and were taken from the ground above and under the litter, this action was executed around noon, to decrease variables in the activity of the same, with a sampling effort of 75 minutes per site; that is, 15 minutes per meter.
The specimens were captured with the help of entomological tweezers and with a brush moistened in alcohol, they were placed in a pet bottle, labeled and kept fixed in ethyl alcohol at 70 % (v / v). Individuals were initially identified as morphospecies, and subsequently as a family, genus or species, using taxonomic keys (Levi, 1991; Ubick et al., 2005), databases (Gómez-Rodríguez et al., 2014; World Spider Catalog, 2018) and with the help of specialists. The specimens were deposited in the invertebrate collection of the Laboratorio de Ecología of the Instituto Tecnológico of Ciudad Victoria, Tamaulipas.
The number of sites sampled was limited by access to the locations and each point corresponds to a replication by altitude or disturbance condition. These were quantified independently of the spider sampling; for this, a quadrant of 50 m2 was established where the sources of disturbance were counted, according to the method of Martorell and Peters (2005). This allowed us to obtain a disturbance index based on the analysis of 13 parameters, in three categories: 1) human activities, 2) cattle breeding and 3) state of soil degradation. The compaction of soil was not considered, because it consumed a lot of time, nor severely modified surfaces, as it was not the case in the study areas.
The index of each site was determined with the formula proposed by Martorell and Peters (2005) that considers values from 0 (without disturbance) to 100 (highly disturbed). A logarithmic base 10 scale was used and the 45 sites sampled were categorized into: High Disturbance: (1.4 -1.8), Medium (1- 1.4) and Low (0.6 - 1).
A matrix was constructed from the perturbation variables, in which each of the sites obtained a disturbance index. After the designation of the disturbance indexes, the highest value variables were extracted (component 1), which together with the litter, altitude and disturbance were considered as dependent variables in the Principal Component Analysis.
The Shannon diversity index was calculated for each sampling point; Spearman correlation analyzes were also made between the altitude and the disturbance index; the diversity of spiders (Shannon index) and disturbance, as well as between the diversity of spiders and altitude. To determine differences in diversity in relation to the perturbation gradient, a one-way analysis of variance was used. An analysis of Kruskal-Wallis was used as well, whose dependent variable was the disturbance index and the altitude as the independent one (2 000-2 400 low, 2 500-2 900 average and 3 000-3 500 high).
The composition of the species with respect to environmental factors; that is, the variables that make up the main components of greater importance (with values of 0.654 to 0.775), was analyzed by means of a canonical correspondence (ACC) of two matrixes, one with abundance data by species (species matrix) and another with the environmental factors (ecological-environmental matrix), both with classification of the 45 sites sampled. The elements of the latter were the presence of feces of bovines and horses; number of plants in the quadrant with parts or branches extracted for fuel; surface of the trails within the quadrant (surface of the intersection); percentage of the area of the quadrant used for some type of activity (foraging, logging and agriculture), stoniness, altitude and litter.
A one-way ANOVA was applied; when the data did not meet the theoretical assumptions, nonparametric tests were used; all analyzes were done with the IBM SPSS Statistics ver. 22 program, except for the canonical correspondence analysis (Canoco for Windows 4.5 Software) that was used.
Results and Discussion
541 spiders were obtained which belong to 71 morphospecies, 42 genera and 23 families; from them, the most abundant were Lycosidae (56 %), Agelenidae (10 %) and Anyphaenidae (9 %) (Table 1). It is probable that the number of morphospecies overestimates that of taxonomic species, due to the risk of incorrectly identifying juvenile individuals. The total of morphospecies is comparable with that cited for Mexico in other studies; thus, 91 taxa were recorded in a remnant fragment of tropical forest (Rivera-Quiróz, 2016), 63 in the urban area of Chilpancingo (Rodríguez-Rodríguez et al., 2015) and 41 taxa in Toluca (Desales-Lara , 2013).
Table 1 Spiders in Cerro El Potosí and Sierra Peña Nevada in Nuevo León and Tamaulipas, as well as characteristics of the zones.
| State | Ecosystem | Altitude elevation | Site state | Family | Species |
| Nuevo León | Pine forest | 2800-3400 | Preserved | Agelenidae | Melpomene sp. |
| Thomisidae | s/i | ||||
| Lycosidae | Pardosa sp. | ||||
| Salticidae | Mexigonus sp. | ||||
| Tamaulipas | Pine forest Pine forest | 2100-3300 | Preserved | Lycosidae | Pardosa sp. |
| Lycosidae | Rabidosa rabida | ||||
| Lycosidae | Hogna sp. | ||||
| Caponiidae | Orthonops lapanus | ||||
| Agelenidae | Melpomene coahuilana | ||||
| Anyphaenidae | Clubiona sp. | ||||
| Nuevo León | Pine forest | 2850-3850 | Disturbed | Agelenidae | s/i |
| Anyphaenidae | Anyphaena sp. | ||||
| Corinnidae | Castianeira sp. | ||||
| Dictynidae | s/i | ||||
| Gnaphosidae | Zelotes sp. | ||||
| Gnaphosidae | s/i | ||||
| Hahniidae | s/i | ||||
| Linyphiidae | s/i | ||||
| Lycosidae | Melocosa sp. | ||||
| Lycosidae | Pardosa sp. | ||||
| Salticidae | Hasarius sp. | ||||
| Salticidae | s/i | ||||
| Theridiidae | s/i | ||||
| Thomisidae | s/i | ||||
| Tamaulipas | Pine forest | 2850-3300 | Disturbed | Agelenidae | s/i |
| Agelenidae | Melpomene sp. | ||||
| Filistatidae | Kukulcania hibernalis | ||||
| Linyphiidae | Agyneta sp. | ||||
| Linyphiidae | s/i | ||||
| Linyphiidae | Frontinella sp. | ||||
| Lycosidae | Pardosa sp. | ||||
| Lycosidae | s/i | ||||
| Phrurolithidae | Piabuna sp. | ||||
| Salticidae | s/i | ||||
| Tetragnathidae | Chrysometa sp. | ||||
| Theridiidae | s/i | ||||
| Nuevo León | Conifer forest | 3000-3150 | Preserved | Agelenidae | s/i |
| Gnaphosidae | Zelotes sp. | ||||
| Lycosidae | s/i | ||||
| Lycosidae | Pardosa sp. | ||||
| Tamaulipas | Conifer forest | 3150-3450 | Preserved | Agelenidae | s/i |
| Anyphaenidae | Anyphaena sp. | ||||
| Anyphaenidae | s/i | ||||
| Gnaphosidae | s/i | ||||
| Linyphiidae | Agyneta sp. | ||||
| Lycosidae | Pardosa sp. | ||||
| Lycosidae | s/i | ||||
| Tetragnathidae | s/i | ||||
| Tetragnathidae | Chrysometa sp. | ||||
| Thomisidae | s/i | ||||
| Agelenidae | s/i | ||||
| Anyphaenidae | Anyphaena sp. | ||||
| Nuevo León | Oak forest | 2500-2550 | Disturbed | Eutichuridae | Strotarchus sp. |
| Gnaphosidae | Zelotes sp. | ||||
| Linyphiidae | s/i | ||||
| Salticidae | Mexigonus sp. | ||||
| Tetragnathidae | Leucauge sp. | ||||
| Theridiidae | Theridion sp. | ||||
| Theridiidae | s/i | ||||
| Tamaulipas | Oak forest | 2700-3000 | Preserved | Agelenidae | s/i |
| Anyphaenidae | Anyphaena sp. | ||||
| Dipluridae | s/i | ||||
| Linyphiidae | s/i | ||||
| Lycosidae | s/i | ||||
| Salticidae | s/i | ||||
| Nuevo León | Pine-oak forest | 2100-3000 | Disturbed | Agelenidae | Agelenopsis sp. |
| Gnaphosidae | s/i | ||||
| Linyphiidae | s/i | ||||
| Lycosidae | s/i | ||||
| Lycosidae | Pardosa sp. | ||||
| Pholcidae | s/i | ||||
| Salticidae | s/i | ||||
| Theridiidae | s/i | ||||
| Tamaulipas | Pine-oak forest | 2500-2550 | Preserved | Agelenidae | s/i |
| Araneidae | s/i | ||||
| Linyphiidae | Frontinella sp. | ||||
| Lycosidae | Pardosa sp. | ||||
| Lycosidae | s/i | ||||
| Nuevo León | Scrubland | 2100-2700 | Disturbed | Lycosidae | Pardosa sp. |
| Oxyopidae | s/i | ||||
| Diguetidae | Diguetia sp. | ||||
| Agelenidae | s/i | ||||
| Lycosidae | s/i | ||||
| Nuevo León | Scrubland | 2100-2700 | Preserved | Corinnidae | Castianeira sp. |
| Agelenidae | s/i | ||||
| Tamaulipas | Scrubland | 2500-2700 | Preserved | Agelenidae | Melpomene sp. |
| Anyphaenidae | Anyphaena sp. | ||||
| Araneidae | Metepeira sp. | ||||
| Araneidae | s/i | ||||
| Gnaphosidae | s/i | ||||
| Lycosidae | Pardosa sp. | ||||
| Salticidae | Habronattus sp. | ||||
| Salticidae | s/i | ||||
| Theridiidae | s/i | ||||
| Zoropsidae | Zorocrates unicolor | ||||
| Tamaulipas | Desert Rosetophyllous scrub | 2100-2250 | Preserved | Agelenidae | Novalena sp. |
| Anyphaenidae | s/i | ||||
| Araneidae | Neoscona sp. | ||||
| Araneidae | s/i | ||||
| Linyphiidae | Frontinella sp. | ||||
| Lycosidae | s/i | ||||
| Tamaulipas | Desert Rosetophyllous scrub | 3000-3300 | Disturbed | Agelenidae | s/i |
| Lycosidae | Pardosa sp. | ||||
| Linyphiidae | s/i | ||||
| Lycosidae | s/i |
Unidentified (s / i). The nomenclature follows the World Spider Catalog (2018).
Three components explained 62.40 % of the variance of the correlation of sources of disturbance. Component 1 corresponded to 32.67 % which refers to the presence and frequency of feces of cattle and horses, number of plants in the quadrant with parts or branches extracted for fuel, number of paths in the quadrant used by people, surface of the trails within the quadrant (surface of the intersection) and percentage of the area of the quadrant for foraging, logging, agriculture, and so on. Component 2 explained 10.71 % of the variables.
A negative correlation was obtained for the proximity to nuclei of human activities (number of activities that are located up to 200 m), evidence of signs of fire, percentage of soil erosion and exposed soil. Component 3 explained 9.65% of the total variables, is associated with the proximity of human settlements in kilometers.
The analysis of canonical correspondence between groups showed an impact of the disturbance in the assemblages of spiders present in the areas sampled under some degree of disturbance. The most important factor regarding axis 1 corresponded to the leaf litter variable; in axis 2 the relevant independent variables were perturbation and non-perturbation (Figure 1). The diversity index did not differ with the disturbance index in the ANOVA (p <0.05).

(19.9 %); Axis 2 (34.6 %); (Hecesvac = Frequency of stools found at the site; Trails = Small trails used by animals; Firewood = Frequency of plants used for the extraction of fuel (wood, firewood); Hojarasc = Level of litter per sampling site; Elevation = Altitude elevation; Ramoneo = Number of plants browsed; Tamasen = Diameter of the intersection between the paths and paths within the quadrant; NoPerturb = Sites with disturbance index under 0.6-1.2; Disturbance = Sites with high disturbance index 1.2-1.8).
Figure 1 Canonical correspondence analysis that indicates the dynamics of spider groups with respect to environmental and disturbance factors.
The leaf litter was determinant in the distribution and assembly of the spiders, since almost half of them were associated with the presence of this factor. Hogna sp., Orthonops lapanus, Melpomene sp. and Rabidosa rabida were associated to that variable in pine forests. Novalena sp., Neoscona sp. and some species of the Corinnidae family are located in the opposite direction of the leaf litter vector, because they are distributed in areas of desert scrub devoid of litter and with a low level of disturbance. Kukulcania hibernalis and Castianeira sp., were recorded in areas devoid of litter and in the least disturbed. The first is located, mainly, under rocks and trunks. The influence of leaf litter and the structure of the understory are determining factors in the density of hunting spiders in Mexican coniferous forests (Corcuera et al., 2016).
Axis 2 defines the disturbance as a determining factor in the assembly of the spiders. Leucauge sp., Mexigonus sp. and Strotarchus sp. were present in sites with a high degree of disturbance and never in undisturbed sites. This condition is also related to the browsing and size of paths variables; together they include the families Salticidae, Dipluridae and Linyphiidae.
For the non-disturbance variable, the relationship with Mexigonus sp., Agyneta sp. and some species of Agelenidae and Lycosidaeis well defined; which in turn have a close association with the trails variable, most likely because they were present in virtually all areas.
Piabuna sp. and certain taxa of the Gnaphosidae family were correlated with leaf litter and altitude. Chrysometa sp. was associated with the altitude and the areas where there was wood extraction, they were only observed in pine forests. Pardosa sp. was located in the center of the disturbance and leaf litter vectors mainly in forested areas (Figure 1).
Table 2 presents the information of the sampling sites, the number of spider morphospecies and the Shannon diversity index. No correlation was detected between altitude and disturbance index (Rho = 0.18, P = 0.10), nor between diversity and disturbance (Rho = 0.92, P = 0.39). Neither between diversity and altitude (Table 2) (Rho = 0.18, P = 0.23). The sampling sites along the mountains did not show a relationship between disturbance index and diversity (Rho = 0.002, P = 0.98).
Table 2 Characteristics of the sampling points and number of morphospecies in the Cerro El Potosí (EP) and Sierra Peña Nevada (PN) in Nuevo León and Tamaulipas.
| Area | Type of vegetation | Sampling dates | Disturbance index | State of the site | Altitude masl | Morphospecies | Diversity (Shannon) |
|---|---|---|---|---|---|---|---|
| PN | Pine forest | 12/04/13 | 1.467 | Disturbed | 2 850 | 12 | 0.59 |
| PN | Scrubland | 09/06/13 | 1.485 | Disturbed | 2 565 | 10 | 1.38 |
| PN | Oak forest | 19/06/13 | 1.024 | Undisturbed | 2 686 | 17 | 1.26 |
| PN | Pine forest | 20/08/13 | 0.694 | Undisturbed | 2860 | 20 | 1.30 |
| PN | Scrubland | 25/10/13 | 1.371 | Disturbed | 2 715 | 11 | 1.47 |
| EP | Pine forest | 20/02/14 | 1.314 | Disturbed | 3 275 | 22 | 1.08 |
| EP | Pine forest | 22/02/14 | 1.228 | Disturbed | 2 864 | 9 | 1.13 |
| EP | Oak forest | 12/04/14 | 1.338 | Disturbed | 2 562 | 27 | 1.46 |
| EP | Pine-oak forest | 12/04/14 | 1.710 | Disturbed | 3 012 | 6 | 0.90 |
| PN | Coniferous forest | 01/08/14 | 1.195 | Undisturbed | 3 140 | 15 | 1.14 |
| PN | Pine forest | 31/07/14 | 1.424 | Disturbed | 3 135 | 6 | 0.67 |
| PN | Desert Rosetophyllous scrub | 31/07/14 | 1.379 | Disturbed | 2 254 | 14 | 1.07 |
| EP | Coniferous forest | 09/08/14 | 0.882 | Undisturbed | 3 015 | 15 | 0.33 |
| EP | Coniferous forest | 10/08/14 | 1.023 | Undisturbed | 3 000 | 16 | 0.54 |
| EP | Oak forest | 10/08/14 | 1.035 | Undisturbed | 2 710 | 14 | 0.78 |
| PN | Pine forest | 29/11/14 | 1.204 | Disturbed | 3 012 | 11 | 0.82 |
| PN | Pine forest | 29/11/14 | 1.069 | Undisturbed | 2 842 | 12 | 0.37 |
| PN | Pine forest | 30/11/14 | 1.396 | Disturbed | 2 100 | 13 | 1.18 |
| PN | Pine forest | 30/11/14 | 1.455 | Disturbed | 2 720 | 14 | 0.69 |
| EP | Pine-oak forest | 24/02/15 | 1.482 | Disturbed | 2 125 | 10 | 0.90 |
| EP | Pine forest | 25/02/15 | 1.544 | Disturbed | 3 425 | 4 | 0.52 |
| EP | Pine forest | 25/02/15 | 1.412 | Disturbed | 3 305 | 7 | 0.65 |
| EP | Oak forest | 13/05/15 | 1.187 | Undisturbed | 2 405 | 10 | 0.73 |
| EP | Oak forest | 13/05/15 | 0.882 | Undisturbed | 2 256 | 10 | 0.74 |
| EP | Scrubland | 13/05/15 | 0.943 | Undisturbed | 2 109 | 8 | 0.60 |
| PN | Pine forest | 16/05/15 | 1.397 | Disturbed | 3 290 | 13 | 0.60 |
| PN | Pine forest | 17/05/15 | 0.661 | Undisturbed | 3 277 | 9 | 0.48 |
| EP | Pine forest | 14/06/15 | 0.945 | Undisturbed | 3 434 | 12 | 0.63 |
| EP | Pine forest | 14/06/15 | 1.169 | Undisturbed | 3 426 | 7 | 0.62 |
| PN | Pine forest | 30/08/15 | 1.430 | Disturbed | 2 978 | 11 | 0.51 |
| PN | Pine-oak forest | 30/08/15 | 1.064 | Undisturbed | 2 550 | 19 | 0.86 |
| EP | Scrubland | 20/10/15 | 1.483 | Disturbed | 2 266 | 14 | 0.59 |
| EP | Pine forest | 21/10/15 | 1.009 | Undisturbed | 3 324 | 7 | 0.38 |
| EP | Pine forest | 21/10/15 | 1.321 | Undisturbed | 3 138 | 9 | 0.60 |
| EP | Coniferous forest | 22/10/15 | 1.009 | Undisturbed | 3 174 | 9 | 0.53 |
| EP | Pine forest | 29/10/15 | 0.699 | Undisturbed | 2 875 | 12 | 0.62 |
| EP | Scrubland | 29/10/15 | 1.584 | Disturbed | 2 716 | 12 | 0.34 |
| PN | Pine forest | 01/11/15 | 1.577 | Disturbed | 2 232 | 18 | 0.57 |
| PN | Coniferous forest | 01/11/15 | 0.874 | Undisturbed | 3 425 | 12 | 0.70 |
| PN | Pine forest | 02/11/15 | 1.538 | Disturbed | 2 085 | 9 | 0.55 |
| PN | Pine forest | 08/11/15 | 1.234 | Disturbed | 2 385 | 11 | 0.60 |
| PN | Desert Rosetophyllous scrub | 08/11/15 | 1.531 | Disturbed | 3 330 | 9 | 0.52 |
| PN | Pine forest | 08/11/15 | 1.480 | Disturbed | 2 425 | 11 | 0.50 |
| EP | Pine forest | 11/11/15 | 1.299 | Disturbed | 2 393 | 9 | 0.56 |
| EP | Pine forest | 11/11/15 | 0.804 | Undisturbed | 2 542 | 15 | 0.35 |
In axis 1 of the canonical analysis of correspondence it was observed that the altitude explains almost by itself the graph, its magnitude reflects the high correlation that exists between altitude and vegetation. For example, Abies vejarii Martínez, Lupinus sp. and Geranium crenatifolium H. E. Moore tend to be in the higher areas along with Chrysometa sp., a spider that was only recorded in coniferous forests. Dyssodia pinnata (Cav.) B. L. Rob. is a herb found in more desertic and in lower areas; Agelenidae is a family of spiders that was mainly recorded in pine forests with scrubland elements (Figure 2).
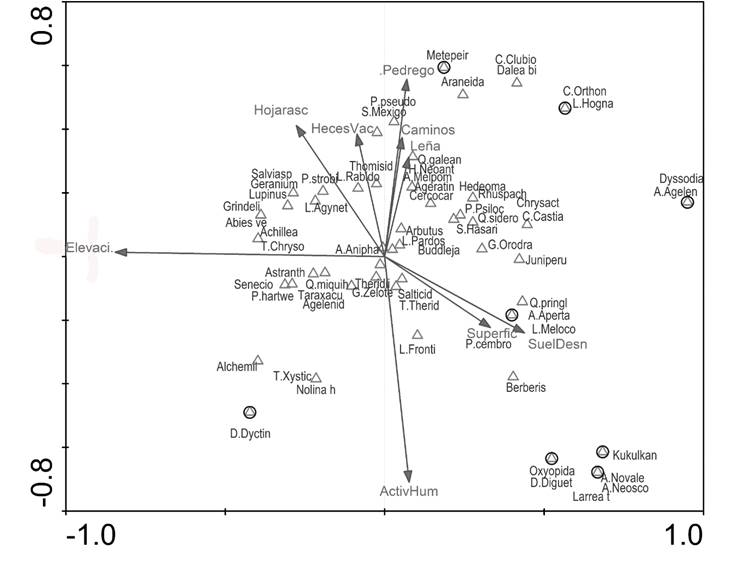
Axis 1 (50.6 %); Axis 2 (41.8 %); Hecesvac = Frequency of stool found at the site; Firewood = Frequency of plants used for fuel extraction (wood, firewood); Hojarasc = Level of litter per sampling site; Elevation = Altitude elevation; SuelDesn = Percentage of bare soil found in the area; Pedrego = Percentage of stoniness in the area).
Figure 2 Graph of canonical analysis of correspondence that indicates the dynamics of spider groups and vegetation with respect to environmental variables.
Kukulcania hibernalis, Diguetia sp., Novalena sp. and Neoscona sp. are associated with Larrea tridentata (Sessé & Moc. ex DC.) Covill from the desert rosetophyllous scrub. Hasarius sp. and Castianeira sp. were found under logs, trunks, stones and small herbs which coincide with the following plants: Hedeoma palmeri Hemsl., Rhus pachyrachis Hemsl., Chrysactinia sp. and Cercocarpus sp., from scrubland and oak forest. Pardosa sp. was located at the center of vectors, mainly in the pine forests.
The number of families identified in the study areas corresponds to 30 % of the known families in the area according to Ruíz and Coronado (2002), who document 72 families and 135 species for the states of Nuevo León and Tamaulipas. In the present investigation, a high morphospecies richness was obtained (tables 1 and 2), considering that the size of the sampled area is less than 1 % of the total area of both states. However, it is possible that the lack of identification at the species level implies an overestimation. Unidentified genera were rare and with low diversity, possibly taxa not registered for the region, although they could be juvenile organisms.
The variable that best explained the presence of spiders was leaf litter, which coincides with Podgaiski and Rodrigues (2017), who indicate that their existence and complexity are of great importance for the diversity of said invetebrate group. The disturbance to a lesser degree did not influence the presence of spiders. The above is consistent with that recorded for other environments (Bonte et al., 2004; Langlands et al., 2011). Noteworthy in particular is that of Michoacán, where the species composition differs with the disturbance, but not the diversity index (Maldonado-Carrizales and Ponce-Saavedra, 2017).
The diversity of spiders and leaf litter are linked to plant cover, since this relationship reflects a greater number and diversity of niches (Ávalos et al., 2007). The distribution of the spiders on the litter shows the dependence to a permanent cover in the ground, which provides refuge, availability of prey, aeration and the regulation of the ambient temperature, reason why said areas are more propitious for the development of the spiders (Ávalos et al., 2007).
Disturbed ecosystems, due to their characteristics, can lead to higher productivity and favor a high abundance of spiders, although their diversity does not necessarily increase (Nyffeler y Benz, 1988; Maelfait et al., 2004; Ávalos et al., 2007; Arana et al., 2014). This coincides with what was observed in the present work, in which some groups of spiders were more frequent in the disturbed areas. Pardosa sp. was located in all the altitudinal gradient and of disturbance, perhaps as a result of its dynamics to colonize practically all the territories (Nyffeler y Benz, 1988; Major et al. 2006; Arana et al., 2014). The family Lycosidae and the Pardosa genus, in particular, are the most common predators in modified ecosystems or with some degree of disturbance, which responds to their generalist condition in the use of resources. This allows them to have a greater tolerance to unfavorable conditions (Major et al., 2006; Cabrera, 2012).
Ávalos et al. (2007) indicate that Lycosidae and Linyphiidae do not together exceed 45 % of individuals in undisturbed areas, but reach up to 85 % in deteriorated areas. For this reason, Pardosa sp. as an indicator of disturbed areas; a statement shared by Urones and Majadas (2002), who point to this group as a pioneer in burned forests and during the first stages of succession. However, for the Cerro El Potosí they were also observed in areas with a low disturbance index, where they inhabit independently and dominantly, perhaps displacing other species.
Opposite to what was expected, no relationship was found between altitude and diversity of spiders, which coincides with Gobbi et al. (2006) who neither registered it with richness, nor with density in an altitudinal gradient.
Contrary to the hypothesis, no greater diversity of spiders was detected in the sites with less disturbance. Leaf litter was a factor that was associated with the distribution of the invertebrate group under study. Likewise, a high number of families of spiders was obtained, if it is considered that the sampled area is less than 1 % of the territory of Nuevo León and Tamaulipas.
Conclusions
The influence of the altitudinal gradient is not decisive in the distribution of the spiders, since there is no relationship among altitude, diversity and species richness.
Leaf litter influenced the distribution and assembly of spiders.
Pardosa sp. was recorded along the altitudinal gradient and was abundant in disturbed areas.
Acknowledgements
H. Cortés-Cabrera helped in field work; C. Salazar-Olivo helped in the identification of arachnide species; M. Salinas-Rodríguez helped with some statistical packges and literature.
REFERENCES
Arana G., R. N., M. A. Pinkus R. and E. A. Rebollar T. 2014. Spatial and temporal diversity and structure of cursorial spiders (Arachnida: Araneae) in a fragmented landscape in Yucatan, Mexico. Southwestern entomologist 39(3): 555-580. https://doi.org/10.3958/059.039.0316. [ Links ]
Arriaga C., L., J. M. Espinoza-Rodríguez, C. Aguilar-Zúñiga, E. Martínez-Romero, L. Gómez-Mendoza y E. Loa Loza. (coords.). 2000. Regiones terrestres prioritarias de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. México, D.F., México. 609 pp. [ Links ]
Ávalos G., G. D. Rubio, M. E. Bar y A. González. 2007. Arañas (Arachnida, Araneae) asociadas a dos bosques degradados del Chaco húmedo en Corrientes, Argentina. Revista de Biología Tropical 55(3-4): 899-909. [ Links ]
Bonte, D., L. Lens and J.-P. Maelfait. 2004. Lack of homeward orientation and increased mobility result in high emigration rates from low‐quality fragments in a dune wolf spider. Journal of Animal Ecology 73(4): 643-650. [ Links ]
Cabrera, G. 2012. La macrofauna edáfica como indicador biológico del estado de conservación/perturbación del suelo. Resultados obtenidos en Cuba. Pastos y Forrajes 35(4): 346-363. [ Links ]
Campuzano, E. F., G. Ibarra-Núñez, E. Chamé-Vázquez and H. Montaño-Moreno. 2016. Understory spider assemblages from a cloud forest in Chiapas, Mexico, and their relationships to environmental variables. Arthropod-Plant Interactions 10(3): 237-248. [ Links ]
Cantú, C., J. Estrada A., M. Salinas R., J. Marmolejo M. y E. Estrada C. 2013. Vacíos y omisiones en conservación de las ecorregiones de montaña de México. Revista Mexicana de Ciencias Forestales 4(17): 10-27. [ Links ]
Corcuera, P., P. L. Valverde, M. L. Jiménez, A. Ponce-Mendoza, G. De la Rosa and G. Nieto. 2016. Ground spider guilds and functional diversity in native pine woodlands and eucalyptus plantations. Environmental Entomology 45 (2): 292-300. [ Links ]
Desales-Lara, M. A., O. Francke y P. Sánchez. 2013. Diversidad de Arañas (Arachnida: Araneae) en Hábitats Antropogénicos. Revista Mexicana de Biodiversidad 84 (1): 291-305. [ Links ]
Deza, M. y J. Andia. 2009. Diversidad y riqueza de especies de la familia Araneidae (Arachnida, Araneae) en Cicra (Madre de Dios - Perú). Ecología Aplicada 8(1-2): 81-90. [ Links ]
Eggs, B. and D. Sanders. 2013. Herbivory in spiders: The Importance of pollen for orb-weavers. PLoS ONE 8(11): 1-5 e82637. doi: 10.1371/journal.pone.0082637. [ Links ]
Gobbi, M., F. Diego and F. De Bernardi. 2006. Influence of climate changes on animal communities in space and time: the case of spider assemblages along an alpine glacier foreland. Global Change Biology 12(10): 1985-1992. doi:10.1111/j.1365-2486.2006.01236.x. [ Links ]
Gómez-Rodríguez, J. F., H. Montaño, G. Ibarra-Núñez and C. A. Salazar-Olivo. 2014. Arácnidos (excepto ácaros) de Tamaulipas: listado actualizado y algunos registros nuevos. Pp. 51-74. In: Correa-Sandoval, A., J. V. Horta, J. García-Jiménez y L. Barrientos (eds.). Biodiversidad Tamaulipeca. Vol. 2. Núm. 2. Tecnológico Nacional de México - Instituto Tecnológico de Ciudad Victoria. Ciudad Victoria, Tamps., México. pp. 51-74. [ Links ]
Gómez-Rodríguez, J. F. y C. Salazar O. 2015. Arañas de la región montañosa de Miquihuana, Tamaulipas: listado faunístico y registros nuevos. Dugesiana 19(1): 1-7. [ Links ]
Langlands, P. R., K. E. Brennan, V. W. Framenau and B. Y. Main. 2011. Predicting the post‐fire responses of animal assemblages: testing a trait‐based approach using spiders. Journal of Animal Ecology 80(3): 558-568. doi:10.1111/j.1365-2656.2010.01795.x. [ Links ]
Levi, H. W. 1991. The Neotropical and Mexican species of the orbweaver genera Araneus, Dubiepeira, and Aculepeira (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology 152(4): 167-315. [ Links ]
Maelfait, J.-P., L. Baert, D. Bonte, D. De Bakker, S. Gurdebeke, S. and F. Hendrickx. 2004. The use of spiders as indicators of habitat quality and anthropogenic disturbance in Flanders, Belgium. In: Samu, F. and C. Szinetár (eds.). European Arachnology 2002. Plant Protection Institute (Budapest) and Berzsenyi College (Szombathely). Akaprint Kft, Budapest, Hungría. pp. 129-141. [ Links ]
Major, R. E., G. Gowing, F. J. Christie, M. Gray and D. Colgan. 2006. Variation in wolf spider (Araneae: Lycosidae) distribution and abundance in response to the size and shape of woodland fragments. Biological Conservation 132(1): 98-108. [ Links ]
Maldonado-Carrizales, J. y J. Ponce-Saavedra. 2017. Arañas Saltarinas (Araneae: Salticidae) en dos sitios contrastantes en grado de antropización en Morelia Michoacán, México. Entomología Mexicana 4(1):597−603. [ Links ]
Martorell, C. and E. M. Peters. 2005. The measurement of chronic disturbance and its effects on the threatened cactus Mammillaria pectinifera. Biological Conservation 124(2): 199-207. https://doi.org/10.1016/j.biocon.2005.01.025. [ Links ]
Nyffeler, M. and G. Benz. 1988. Feeding ecology and predatory importance of wolf spiders (Pardosa spp.) (Araneae, Lycosidae) in winter wheat fields. Journal of Applied Entomology 106(1-5): 123-134. doi: 10.1111/j.1439-0418.1988.tb00575.x. [ Links ]
Podgaiski, L. R. and G. G. Rodrigues. 2017. Spider community responds to litter complexity: insights from a small-scale experiment in an exotic pine stand. Iheringia. Série Zoologia 107: 1-8. https://dx.doi.org/10.1590/1678-4766e2017007. [ Links ]
Rivera-Quiroz, F. A., U. Garcilazo-Cruz and F. Álvarez-Padilla. 2016. Ciberdiversidad de arañas (Araneae: Araneomorphae) en un fragmento ecoturístico de selva tropical en Xilitla, México. Revista Mexicana de Biodiversidad 87 (3): 1023-1032. http://dx.doi.org/10.1016/j.rmb.2016.07.011. [ Links ]
Rodríguez-Rodríguez, S. E., K. P. Solís-Catalán and A. Valdez-Mondragón. 2015. Diversity and seasonal abundance of anthropogenic spiders (Arachnida: Araneae) in different urban zones of the city of Chilpancingo, Guerrero, Mexico. Revista Mexicana de Biodiversidad 86(4): 962-971. https://doi.org/10.1016/j.rmb.2015.09.002. [ Links ]
Ruíz C., E. y J. M. Coronado B. 2002. Artrópodos terrestres de los estados de Tamaulipas y Nuevo León, México. Centro de Investigación y Desarrollo Agropecuario, Forestal y de la Fauna - Universidad Autónoma de Tamaulipas, Serie Publicaciones Científicas Núm. 4. Cd Victoria, Tamps., México. 377 p. [ Links ]
Ubick, D., P. Paquin and P. E. Cushing. 2005. Spiders of North America: an identification manual. Oxford/American Arachnological Society. New York, NY, USA. 377 p. [ Links ]
Uma, D. B. and M. R. Weiss. 2012. Flee or fight: Ontogenetic changes in the behavior of Cobweb spiders in encounters with spider-hunting. Environmental Entomology 41(6): 1474-1480. [ Links ]
Urones, C. y A. Majadas. 2002. Cambios en la comunidad de Araneae durante la sucesión postfuego en matorrales mediterráneos de montaña. Revista Ibérica de Aracnología 5: 19-28. [ Links ]
Willett T., R. 2001. Spiders and other arthropods as indicators in old-Growth versus logged redwood stands. Restoration Ecology 9(4): 410-420. [ Links ]
World Spider Catalog. 2018. World Spider Catalog. Version 19.5. Natural History Museum Bern, online at Natural History Museum Bern, online at http://wsc.nmbe.ch , accessed on Oct 04, 2018. doi: 10.24436/2). [ Links ]
Received: March 08, 2018; Accepted: October 09, 2018











 text in
text in 


