Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.14 n.2 Mérida Apr./Jun. 2023 Epub June 26, 2023
https://doi.org/10.22319/rmcp.v14i2.6093
Articles
Effect of Xoconostle (Opuntia matudae Scheinvar) on methane concentration and ruminal variables during in vitro fermentation of corn stover
a Universidad Autónoma del Estado de Hidalgo (UAEH). Instituto de Ciencias Agropecuarias. Avenida Universidad Km. 1 s/n Exhacienda Aquetzalpa, 43600. Tulancingo de Bravo, Hidalgo, México.
b Universidad Autónoma de San Luis Potosí (UASLP). Facultad de Estudios Profesionales Zona Huasteca. San Luis Potosí, México.
c Universidad de la Cañada (UNCA). Oaxaca, México.
The effect of the addition of xoconostle on in vitro ruminal fermentation of corn stover was determined in order to reduce methane emission. Previous studies have shown that xoconostle contains bioactive compounds with potential antimicrobial activity that enhance ruminal fermentation. Zero point zero percent, 2.0 %, 4.0 % and 6.0 % of xoconostle were added. The following were determined: chemical composition of the substrates, phenolic compounds, antioxidant capacity, in vitro disappearance of dry matter (IVDDM), the production of volatile fatty acids (VFAs) and the kinetic variables of gas production. The volume of methane was measured using the technique of capturing carbon dioxide in sodium hydroxide solution. The content of protein, ether extract, total phenols and antioxidant activity significantly increased (P<0.05) with the addition of xoconostle. The IVDDM also increased with the addition of xoconostle. Regarding the production of propionic acid, it increased significantly (P<0.05) with 6.0 % of xoconostle. The kinetic parameters obtained by the best fit of the experimental data showed a higher digestion rate and lower methane production with the addition of 4.0 and 6.0 % of xoconostle. The use of xoconostle as an additive in ruminant diets decreases methane production in vitro so it can be an alternative to mitigate the increase in the greenhouse effect and benefit the cultivation of a commercially not very appreciated fruit.
Keywords Xoconostle; Corn stover; Ruminal fermentation; Climate change; Enteric methane
Se determinó el efecto de la adición de xoconostle en la fermentación ruminal in vitro de rastrojo de maíz con objeto de reducir la emisión de metano. Estudios previos han demostrado que el xoconostle contiene compuestos bioactivos con actividad antimicrobiana potencial que mejoran la fermentación ruminal. Se adicionaron el 0.0%, 2.0%, 4.0% y 6.0% de xoconostle. Se determinó la composición química de los sustratos, compuestos fenólicos, capacidad antioxidante, desaparición in vitro de la materia seca (DIVMS), la producción de ácidos grasos volátiles (AGV) y las variables de cinética de producción de gas. El volumen de metano se midió utilizando la técnica de captura de bióxido de carbono en solución de hidróxido de sodio. Con la adición del xoconostle se incrementó significativamente (P<0.05) el contenido de proteína, extracto etéreo, fenoles totales y actividad antioxidante. La DIVMS también se incrementó con la adición del xoconostle. Respecto a la producción de ácido propiónico, ésta se incrementó significativamente (P<0.05) con el 6.0 % de xoconostle. Los parámetros cinéticos obtenidos mediante el mejor ajuste de los datos experimentales mostraron una mayor tasa de digestión y menor producción de metano con la adición del 4.0 y 6.0 % de xoconostle. El uso de xoconostle como aditivo en dietas para rumiantes disminuye la producción de metano in vitro por lo que puede ser una alternativa para mitigar el incremento del efecto invernadero y beneficiar el cultivo de un fruto comercialmente no muy apreciado.
Palabras clave Xoconostle; Rastrojo de maíz; Fermentación ruminal; Cambio climático; Metano entérico
Introduction
According to the UN, in studies carried out between 2009-2019 by the Economic Commission for Latin America and the Caribbean (ECLAC), Latin American countries are very vulnerable to the effects of climate change, so it is urgent to adopt short-term measures to reduce their impact on ecosystems1. The natural balance of greenhouse gases (GHGs), which are found in greater proportion, carbon dioxide (CO2) and methane (CH4), has experienced an imbalance in recent decades due to various anthropogenic activities resulting in an accumulation in the atmosphere, CO2 increased from 315 ppm in 1960 to 410 ppm in 20192; CH4 from 1,770 ppb in 2000 to 1,860 ppb in 20193. The proportion of CH4 accumulated per year is lower than that of CO2, but its global warming potential is 25 times higher4. Of the anthropogenic sources of CH4, the agricultural sector is one of the largest contributors, the emission of enteric CH4 as a result of the digestive process of ruminants is approximately 115 million tons per year and corresponds to 20 % of global emissions5. Of the strategies used to mitigate enteric CH4 emissions, the incorporation of additives in animal feed appears to be the most promising for its practicality and economy.
Several phenolic compounds contained in some vegetables have potential antimicrobial activity, which can improve ruminal fermentation and decrease the emission of CH46. Among the endemic plants of Mexico that have this characteristic, some species of the genus Opuntia that produce acid fruits, known as xoconostles, are an important source of phenolic compounds, with potential antimicrobial activity7,8,9. Despite the extensive number of studies regarding the reduction of GHGs caused by methanogenesis in ruminants, studies using cacti as forage are few, and it can be considered that there are no studies to date that relate the decrease of ruminal CH4 with secondary metabolites of xoconostle. It is important that livestock producers have access to technologies that allow them to reduce CH4 emissions in a way that ensures animal safety and welfare, and that, on the other hand, is economically viable. Therefore, the objective of this research was to evaluate the effect of xoconostle on the in vitro fermentation of corn stover (Zea mays).
Material and methods
Area of study
The study was conducted in the Multidisciplinary, Animal Nutrition and Special Analysis laboratories of the Institute of Agricultural Sciences of the Universidad Estatal Autónoma de Hidalgo (UAEH, for its acronym in Spanish), in Tulancingo de Bravo, Hidalgo, Mexico.
Collection and preparation of samples
Fruits of xoconostle (Opuntia matudae Scheinvar cv. Rosa) in a state of commercial maturity, harvested in the state of Hidalgo, Mexico (Figure 1), were cut into slices and dehydrated for 72 h in an air flow oven (Felisa 242 A, Mexico) at 60 °C. It was subsequently ground (Weg Crusher, Mexico) and passed through a 2 mm diameter mesh. The corn stover was obtained from the University Ranch of the Autonomous University of the State of Hidalgo and was dehydrated and pulverized in the same way as the xoconostle.
The ruminal fluid was obtained from two sheep (Hampshire, 54 kg live weight ± 2.4) via cannula in the rumen. All surgical procedures were carried out based on the protocol of the Institutional Ethics Committee for the Care and Use of Laboratory Animals of the UAEH and in accordance with the guidelines of the Law of Protection and Decent Treatment for Animals of the government of the state of Hidalgo, Mexico10. The sheep received commercial antiparasitic (Ivermectin, Bayer. 200 mcg/kg live weight), vitamins ADE (Vigantol ADE fuerte 2 mL) and were fed ad libitum with corn stover and mineral premix (Multi-Brick Triple, Malta-Cleyton) for 15 d prior to taking the ruminal fluid.
Physicochemical characterization
The physicochemical characterization of the substrates (corn stover and xoconostle) and of the established treatments: 100 % corn-0 % xoconostle (0%Xoco); 98 % corn-2 % xoconostle (2%Xoco); 96 % corn-4 % xoconostle (4%Xoco) and 94 % corn-6 % xoconostle (6%Xoco) was carried out by proximate analysis, determining: moisture content; mineral content (Mi); ether extract (EE); crude protein (CP); crude fiber (CF)11. The proportion of neutral detergent fiber (NDF) and acid detergent fiber (ADF) was determined as described by Van Soest12, digestions were carried out using filter bags (Ankon F57 USA). In both cases, a bag without substrate was used as a blank to perform the calculations.
Determination of total phenols
The total phenol content was determined using the Folin-Ciocalteu13 method, the calibration curve was constructed from a standard solution of gallic acid (1 g/L H2O), and 0-20 ppm dilutions were obtained. To 250 μL of each dilution, 5 mL of Folin-Ciocalteu 1/10 was added in a light-tight container and left to stand for 8 min. Subsequently, 4 mL of Na2CO3 7.5 % was added and it was kept 2 h in the dark. The absorbance was read at 765 nm (A765) (Spectronic-Genesys 5, USA) using distilled water as a blank. Approximately 150 mg of sample, corn stover or xoconostle, was placed in 2 mL vials adding 1.5 mL of methanol and 100 μL of NaF 2 mM in order to inhibit polyphenol oxidase (PPO). They were placed in a light-tight container and stirred at room temperature for 30 min. They were centrifuged at 10,510 xg (Hermle Z36 HK, Germany) for 20 min at 4 °C. Aliquots of 250 μL of supernatant were treated in the same way as dilutions to construct the calibration curve, absorbance was read at 765 nm (A765) and the corresponding calculations were made.
Determination of antioxidant capacity
ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) method
The ABTS•+ radical was obtained after the reaction of 10 ml of ABTS solution (7 mM) with 10 ml of potassium persulfate solution (2.45 mM). The reaction was carried out at room temperature and in the dark with constant stirring for 16 h14. Once the ABTS•+ radical was formed, it was diluted with ethanol (20 %) until the absorbance 0.7 (±0.1) was adjusted to 754 nm (A754) using ethanol (20 %) as a blank. As a reference antioxidant, dilutions of ascorbic acid 0-100 ppm and gallic acid 0-10 ppm were used to obtain the calibration curves. Approximately 1 g of sample was suspended in 9 mL ethanol (50 %) in light-tight centrifuge tubes, and they were stirred at room temperature for 30 min. It was centrifuged at 10,510 xg at 4 °C for 20 min; 200 μL of supernatant, dilution of ascorbic or gallic acid was added with 2 mL of standardized ABTS•+, allowed to stand 6 min and A754 was determined using ethanol (20 %) as a blank. The calibration curves were used to convert the absorbances obtained in the samples to ascorbic acid equivalents (AAE) or gallic acid equivalents (GAE) respectively.
DPPH (2,2-diphenyl-1-picrylhydrazyl) method
The method developed by Brand-Williams15 with some modifications to that described by Kim16 was used, where the absorbance of the DPPH• radical 200 μM in methanol (80 %) stirred for 2 h in a light-tight container and adjusted its absorbance to 515 nm (A515) to 0.7±0.1 with methanol (80 %) was measured after the reaction with a reference antioxidant. As a reference antioxidant, dilutions of ascorbic acid 0-50 ppm and gallic acid 0-5 ppm were used to obtain the calibration curves. Approximately 1 g of sample was suspended in 9 mL ethanol (50 %) in light-tight centrifuge tubes and stirred at room temperature for 30 min. It was centrifuged at 10,510 xg at 4 °C for 20 min; 0.5 mL of supernatant or ascorbic acid dilution was added with 2.5 mL of standardized DPPH•, allowed to stand for 1 h and A515 was determined using methanol (80) as a blank.
pH and in vitro degradation of dry matter (IVDDM)
The pH was measured (HANNA, HI2211, Romania) at the end of the incubation time and the contents of each bottle were transferred to 50 mL polysulfone tubes, which were centrifuged (HERMLE, Z326K, Germany) at 15,130 xg for 15 min. The supernatant was removed by decantation and the solid material was dried at 65 °C for 48 h. The IVDDM was calculated as the difference between the weight of the initial dry matter and the weight of the residual dry matter and was expressed as g/100 g DM.
Determination of volatile fatty acids (VFAs)
At the end of fermentation, 1.6 mL of the liquid fraction of the fermentation bottles was placed in 2.5 mL vials containing 0.4 mL of HPO3 25 % w/v, they were stored at 5 °C. Subsequently, they were centrifuged at 15,130 xg 15 min. The concentration, millimoles/liter (mM L-1) of VFAs was determined in a gas chromatograph Claurus 500, Perkin Elmer, USA, provided with autosampler, 15 m capillary column (ELITE-FFAP, Perkin Elmer, USA) and flame ionization detector (FID). The carrier gas was N2 at 60 psi, H2 and extra dry air were used to generate the flame. The temperatures of the oven, injector and column were 120, 250 and 250 °C, respectively. The retention time was 1.22, 1.55 and 2.02 min for acetic, propionic and butyric acids, respectively. Previously, a calibration curve was constructed with standard solutions of acetic, propionic and butyric acids17.
Fermentation and gas production
In glass bottles with a capacity of 125 mL, 0.5 g of substrate corresponding to each corn stover-xoconostle treatment was deposited. The ruminal fluid was filtered through eight layers of gauze and stored at 39 °C under anaerobic conditions until use. To each bottle and under continuous flow of CO2, 40 mL of culture medium and 4 mL of ruminal fluid were added. Per liter of solution the culture medium contains: 1 g NH4HCO3; 8.74 g NaHCO3; 1.43 g Na2HPO4; 1.55 g KH2PO4; 0.15 g MgSO4.7H2O; 0.017 g CaCl2.2H2O; 0.013 g MnCl.4H2O; 0.0013 g CoCl.6H2O; 0.01 g FeCl3; 1.29 mL of 0.1 % resazurin solution as an indicator and 37 mL of reducing solution containing 0.21 g Na2SO4 and 1.5 mL of 0.1N NaOH solution. The bottles were hermetically closed (Manual crimper, Wheaton, USA) by means of a silicone stopper and a vial capsule with a removable center. Similar containers with only ruminal inoculum were included as blanks. The bottles were incubated in a water bath at 39 °C. The volume of gas produced (ml) inside each bottle at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 18, 22, 26, 30, 42, 54, 66, 78, 92 h of incubation was recorded by displacement of water volume by puncturing through the silicone stopper using a hypodermic needle coupled to a graduated glass column containing water. After each measurement, the gas was released by equalizing the internal and external pressure of the bottles18.
The rate at which gas production takes place depends on the characteristics of the rumen microbiota present19; as well as on the type of substrate, pH, Redox potential20, resulting in different kinetic profiles. The mathematical description of these profiles allows comparing characteristics of the substrates or the fermentation environment. The fit of the experimental data to the Logistic model using the Sigma Plot 12© software allowed obtaining Equation 1; where y (mL g-1 DM) denotes the amount of accumulated gas produced per gram of dry matter (DM) at time t (h) during incubation. A (mL g-1 DM) represents the maximum gas production at infinite time. to (h) is the incubation time in which half of A has been produced and b is a dimensionless constant that determines the characteristic profile and therefore the inflection point of the curve21.
The indicator inflection point of the lag phase (L) or Equation 2 resulting from dy/dt is:
Considering the disappearance of substrate (P) as a kinetics of the first order, the digestion rate of the substrate (S) (Equation 3) for values of b>1 increases until reaching a maximum (Smax) when the size of the microbial population no longer limits the fermentation of the feed. The time in which Smax is reached is given by the resolution of dS/dt=0 (Equation 4)21.
Methane determination
The volume of CH4 was measured using the technique described by Torres-Salado22 with the following modifications: the biodigester bottle was coupled by means of a Taygon® hose (2.38 mm internal Ø and 30 cm long) with hypodermic needles (20 G x 32 mm) at the ends to an inverted vial and fully filled with NaOH 2N. The gas originated by the fermentation of the substrate flows through the NaOH 2N, where CO2 reacts and forms sodium carbonate. The residual gas is insoluble in the solution and corresponds to CH4, where the amount is quantified according to the ml of NAOH 2N displaced through another hypodermic needle placed in the silicone stopper as an outlet valve and measured with a graduated cylinder.
Statistical analysis
A completely randomized design was used, the statistical model used was:
Where
Yij Response variable of the ij-th experimental unit;
μ Effect of the overall mean;
t i Effect of the i-th treatment;
εij Effect of the experimental error associated with the i-th experimental unit.
Each treatment had five independent repetitions, with the experimental unit being a bottle with 500 mg of substrate. Data analysis was performed using ANOVA and comparison of means with the Tukey test adjusted to a significance level α=0.05.
Results and discussion
Physicochemical characterization and antioxidant capacity of the substrates used
The concentration of moisture, CP, EE and ash in Xoconostle (Table 1) is similar to that reported by Sánchez-González23, where the variation in these nutrients depends on maturity and culture conditions. Information about the proportion of NDF, ADF is scarce to null because this fruit is mainly used in human food, where the relevance is due to its beneficial and antioxidant properties, as reported by Morales and Espinoza-Muñoz24,25. However, being a cactus fruit, the concentration of NDF is similar to the prickly pear (52 % vs 40.74 %) based on what is reported in the NRC26. The content of phenolic compounds and antioxidant activity in the corn stover used in this study (Table 1) is close to that reported by Vázquez-Olivo27 for corn stover with values of 219 GAE/100 g sample. Regarding xoconostle, the content of total phenols is consistent with what has been reported by other studies28,29.
Table 1 Physicochemical characterization and antioxidant capacity of the substrates used
| Corn stover (%DB) | Xoconostle (%DB) | ||
|---|---|---|---|
| Dry matter | 92.97 ± 0.003 | 87.27 ± 0.002 | |
| Minerals | 7.45 ± 0.001 | 13.13 ± 0.002 | |
| Crude protein | 3.46 ± 0.004 | 4.82 ± 0.004 | |
| Ether extract | 0.78 ± 0.020 | 4.07 ± 0.008 | |
| Nitrogen-free extract | 88.31 ± 0.002 | 77.98 ± 0.001 | |
| Neutral detergent fiber | 68.05 ± 0.008 | 40.74 ± 0.024 | |
| Acid detergent fiber | 36.99 ± 0.016 | 30.34 ± 0.067 | |
| Total phenols | 246.93 ± 0.206 mg GAE 100 g-1 | 740.59 ± 0.461 mg GAE 100 g-1 | |
| Antioxidant activity |
DPPH | 4.94 ± 0.013 mg GAE 100 g-1 | 21.42 ± 0.028 mg GAE 100 g-1 |
| 22.77 ± 0.037 mg AAE 100 g-1 | 66.80 ± 0.076 mg AAE 100 g-1 | ||
| ABTS | 10.87 ± 0.050 mg GAE 100 g-1 | 19.73 ± 0.260 mg GAE 100 g-1 | |
| 99.74 ± 0.048 mg AAE 100 g-1 | 191.43 ± 0.273 mg AAE 100 g-1 | ||
Average values ±SD of three repetitions. GAE (gallic acid equivalents) AAE (ascorbic acid equivalents).
The physicochemical characterization of the treatments is shown in Table 2, where it is observed that the content of DM decreases when the content of xoconostle increases, this is because the moisture contained in the fruit is greater than that of corn stover, on the contrary, the concentration of Mi and CP increases proportionally in the treatments since, when replacing stover with xoconostle, the latter contains a higher percentage of these components, as shown in Table 1. Regarding the EE, when the amount of stover substituted for xoconostle increases, the proportion of this fraction in the treatments increases substantially, since the magnitude of the extract determined in the xoconostle is 4.21 times greater than in that of corn stover. Some varieties of xoconostle such as Opuntia matudae Scheinvar cv. Rosa contain saturated fatty acids such as palmitic and myristic; and polyunsaturated as oleic and linoleic24. Despite the increase in EE (Table 2), the concentration of lipids in the treatments of this study is considered not to influence methanogenesis since its effect has been observed from 50 g kg-1 of dry matter in the diet30. The NFE includes assimilable carbohydrates and crude fiber, this parameter decreases when the amount of corn stover replaced with xoconostle increases since the latter, despite containing more simple carbohydrates than corn stover, has a lower proportion of structural carbohydrates, 18 % less ADF. This decrease in ADF results in better digestibility of the feed, since the supply of non-structural carbohydrates, up to a certain limit, reduces the lag phase and improves the energy content of the diet being decisive in the production of ruminal bacterial protein. The increase in the percentage of xoconostle as a substitute also increased TF content and antioxidant activity. The presence of phenolic compounds could have an effect on some rumen microorganisms; it has been shown that these secondary metabolites of plants have an inhibitory effect, Diaz-Solares and others evaluated the content of phenols and flavonoids as well as the antimicrobial capacity of extracts of Morus alba leaves, finding abundant presence with activity against S. aureus, E. coli, P. aeruginosa, K. pneumoniae and β hemolytic S., suggesting its use in animal feed31. Hayek and Ibrahim7 report inhibitory effect of aqueous extracts of xoconostle on E. coli. It has been shown that phenolic compounds contained in some vegetables improve ruminal fermentation and decrease methane production32,33,34.
Table 2 Physicochemical characterization of treatments
| 0% Xoco | 2% Xoco | 4% Xoco | 6% Xoco | |||
|---|---|---|---|---|---|---|
| (%DB) | ||||||
| Dry matter | 92.97 ± 0.003a | 92.85 ± 0.000b | 92.74 ± 0.005c | 92.62 ± 0.003d | ||
| Minerals | 7.45 ± 0.001a | 7.57 ± 0.000b | 7.68 ± 0.005c | 7.80 ± 0.003d | ||
| Crude protein | 3.46 ± 0.004a | 3.49 ± 0.000b | 3.52 ± 0.001c | 3.54 ± 0.000d | ||
| Ether extract | 0.78 ± 0.020a | 0.85 ± 0.000b | 0.91 ± 0.003c | 0.98 ± 0.002d | ||
| Nitrogen-free extract | 88.31 ± 0.002a | 88.09 ± 0.001b | 87.89 ± 0.010c | 87.67 ± 0.006d | ||
| Neutral detergent fiber | 68.05 ± 0.008a | 67.48 ± 0.003b | 66.94 ± 0.028c | 66.37 ± 0.016d | ||
| Acid detergent fiber | 36.99 ± 0.016a | 36.85 ± 0.000b | 36.72 ± 0.006c | 36.58 ± 0.004d | ||
| Total phenols, mg GAE 100 g-1 | 246.93±0.206a | 257.30±0.051b | 266.97±0.505c | 277.34±0.287d | ||
| Antioxidant activity |
DPPH | GAE | 4.94 ± 0.013a | 5.29 ± 0.002b | 5.61 ± 0.017c | 5.96 ± 0.010d |
| AAE | 22.77 ± 0.037a | 23.73 ± 0.005b | 24.56 ± 0.045c | 25.48 ± 0.026d | ||
| ABTS | GAE | 10.87 ± 0.05a | 11.06 ± 0.001b | 11.23 ± 0.009c | 11.42 ± 0.005d | |
| AAE | 99.74 ± 0.048a | 101.67±0.010b | 103.460.094c | 105.39 0.053d | ||
Average values ± SD of five repetitions. GAE (gallic acid equivalents); AAE (ascorbic acid equivalents).
abcd Values on the same row with different superscript are different (P<0.05).
pH and IVDDM
The pH values of the ruminal fluid of the treatments at the end of fermentation showed no significant difference (P>0.5) (Table 3). The use of a buffer solution based on bicarbonate and phosphates in the culture medium probably maintained the pH with values above 6.0 during the fermentation time favoring the digestibility of the DM. pH values below 6.0 inhibit the development of cellulolytic bacteria (i.e., diets with high concentrate content) and result in longer times in the lag phase (L) and a decrease in the IVDDM35,36. One of the quality parameters of forages is the IVDDM, as it indicates the efficiency with which ruminants can metabolize them. In this study, the treatment of 6% Xoco had a higher IVDDM than the control (P<0.05). The treatment of 4% Xoco did not present a statistic difference with respect to the control, but it did show a tendency to increase, there was no significant difference (P>0.05) between both treatments, this increase in the IVDDM due possibly to the addition of non-structural carbohydrates of the xoconostle caused a better fermentation, resulting in a lower lag phase and a higher digestion rate (Table 3 ). In vitro digestibility of NDF (IVDNDF) showed no difference between treatments (P>0.05), which could indicate that the bioactive compounds of xoconostle have no activity on cellulolytic bacteria and that the degradation of structural carbohydrates is not affected. Regarding total nitrogen, there was no significant difference between the treatments (P>0.05) but, the concentration at the end of the experiment probably increased as a result of the bacterial action on the proteins solubilizing nitrogen.
Table 3 Response variables after 92 h of fermentation
| 0% Xoco | 2% Xoco | 4% Xoco | 6% Xoco | ||
|---|---|---|---|---|---|
| pH | 6.43 ± 0.059 | 6.43 ± 0.019 | 6.44 ± 0.019 | 6.43 ± 0.019 | |
| IVDDM, % | 73.97 ± 4.73ab | 72.76 ± 3.07a | 78.99 ± 4.70bc | 80.44 ± 4.71c | |
| IVDNDF, % | 89.31 ± 1.94 | 88.67 ± 1.27 | 88.61 ± 2.55 | 89.69 ± 2.48 | |
| Nitrogen | Initial | 0.0338 ± 0.003 | 0.0338±0.002 | 0.0338±0.001 | 0.0339±0.002 |
| Total, mg d l-1 | Final | 0.036 ± 0.002 | 0.04 ± 0.003 | 0.04 ± 0.003 | 0.04 ± 0.002 |
Average values ±SD of five repetitions.
abc Values in the same row with different superscript are significantly different (P<0.05).
Production of volatile fatty acids (VFAs)
The concentration of VFAs (mM L-1) in the ruminal fluid as a result of fermentation is shown in Table 4, where it is observed that the treatment of 6% Xoco has a difference in the amount of propionic acid generated (P<0.05), which suggests that xoconostle has an effect on the rumen microbiota directing ruminal fermentation towards a decrease in available H+, necessary for the production of CH433, another possibility is that the metabolism of phenolic compounds contained in xoconostle increases the synthesis of propionic acid, which also decreases the production of methane but without having a direct effect on rumen microorganisms (Ku Vera, et al)37.
Table 4 Concentration and proportion of VFAs originated by in vitro fermentation of corn stover and xoconostle
| 0% Xoco | 2% Xoco | 4% Xoco | 6% Xoco | |
|---|---|---|---|---|
| Acetic, mM L-1 | 35.6 ± 2.43 | 35.8 ± 0.862 | 33.1 ± 0.100 | 33.8 ± 02.34 |
| Propionic, mM L-1 | 14.8 ± 0.898a | 14.5 ± 0.412a | 13.8 ± 0.350a | 16.85 ± 0.845b |
| Butyric, mM L-1 | 5.2 ± 0.340 | 5.1 ± 0.186 | 4.9 ±0.165 | 5.4 ± 0.793 |
| Total, mM L-1 | 55.6 | 55.4 | 51.8 | 56.05 |
| Acetic, % | 64.0 | 64.6 | 63.9 | 60.3 |
| Propionic, % | 26.6 | 26.2 | 26.6 | 30.1 |
| Butyric, % | 9.4 | 9.2 | 9.5 | 9.6 |
| Acetic/Propionic | 2.40 | 2.46 | 2.40 | 2.00 |
Average values ±SD of three repetitions.
ab Values in the same row with different superscript are significantly different (P<0.05).
Gas production
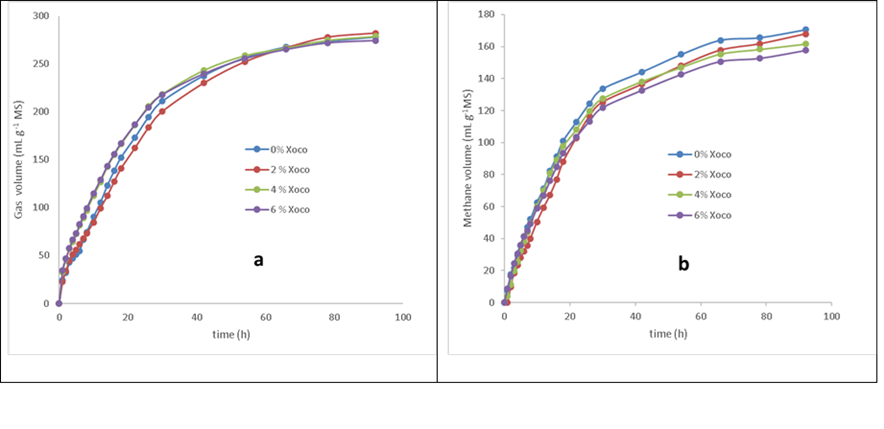
The production profiles of total accumulated gas and methane gas (Figure 2a and 2b) show that the treatments have a sigmoid behavior during the 92 h of incubation following the logistic behavior38, the mathematical model used21 allowed a good fit of the data (R2 >0.99). Regarding the accumulated volume of total gas, the treatment of 2% Xoco was different (P<0.05), observing a greater production of gas compared to treatments of 4 and 6% Xoco (Table 5), probably increasing the amount of phenolic compounds contained in the xoconostle has a consequence on the activity of the rumen microbiota, similar results were determined in previous studies39. The incorporation of phenolic compounds such as tannins in ruminal fermentation has an effect on the microbiota since they can bind to the cell wall of microorganisms, causing morphological changes or secretion of extracellular enzymes or they can bind to enzymes causing changes in their metabolism40. The production profiles of CH4 (Figure 2b) show that the volume produced tends to decrease in response to the incorporation of xoconostle, with the treatment of 6% Xoco being where the effect is most evident. There are several studies that address the reduction of enteric methane from the incorporation of phenolic compounds, such as that by Tiemann41, which evaluated the effect of two legumes rich in condensed tannins on methane emissions in lambs, achieving a reduction of up to 24 % but reducing the digestibility of dry matter. In this study, an 8.5 % reduction was achieved in the treatment of 6% Xoco without having this negative effect.
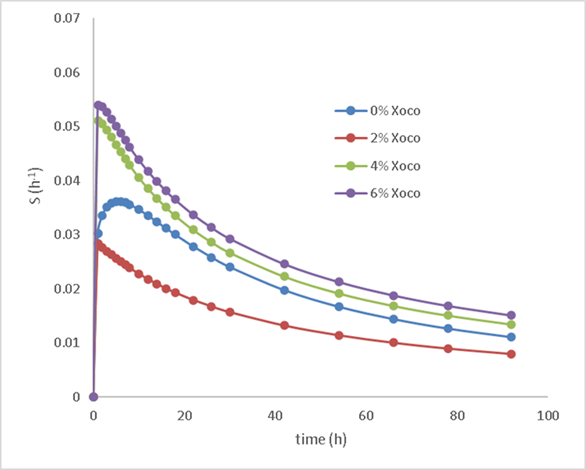
The kinetic parameters resulting from the fit to the Logistic model are shown in Table 3 and through Equation 4, they determine tsmax for the study treatments, being 5.3, 2.8, 0.9 and 1.1 h for 0% Xoco, 2% Xoco, 4% Xoco and 6% Xoco respectively (Figure 3). A reduction in the time to reach the maximum digestion rate of the substrate represents better digestibility and therefore lower methane production.
Table 5 Kinetic parameters obtained from the fit to the Logistic model
| 0% Xoco | 2% Xoco | 4% Xoco | 6% Xoco | |
|---|---|---|---|---|
| Atotal gas, ml g DM-1 | 334.88a | 395.48b | 356.84a | 351.26a |
| ACH4, ml g DM-1 | 193.60a | 188.70a | 177.2a | 184.3a |
| b (-) | 1.1978a | 1.0031b | 0.9474b | 0.9325b |
| to, h | 21.142a | 33.902b | 20.598a | 19.97a |
| Smax, h-1 | 0.026a | 0.037b | 0.049c | 0.052d |
| tSmax, h | 5.32a | 2.84b | 0.90c | 1.10bc |
| L, h | 2.76a | 1.41b | 0.44b | 0.54b |
| R2 | 0.9938 | 0.9942 | 0.9945 | 0.9947 |
Atotal gas (total gas CO2 + CH4); ACH4 (methane produced); S (digestion rate); tSmax (time to reach Smax); L (lag phase).
abcd Values on the same row with different superscript are significantly different (P<0.05).
Conclusions and implications
The addition of xoconostle in an in vitro fermentation of corn stover increased the digestion rate and reduced the lag phase, which translates into an improvement in the digestibility of the substrate. The bioactive compounds of xoconostle increase propionic acid production by 13.1 % when 6 % of xoconostle is added and reduce methane production by 8.5 % with the addition of 4 % of xoconostle.
Literatura citada
1. Bárcena A, Samaniego JL, Peres W, Alatorre JE. La emergencia del cambio climático en America Latina y el Caribe:¿seguimos esperando la catástrofe o pasamos a la acción? Santiago, ONU:CEPAL; 2020. [ Links ]
2. Friedlingstein P. Global Carbon Projet: https://www.globalcarbonproject.org/. Consultado 21 May, 2021. [ Links ]
3. Saunois M, Stavert AR, Poulter B, Bousquet P, Canadell JG, Jackson RB, et al. The Global Methane Budget 2000-2017. Earth Syst Sci 2020;12:1561-1623. [ Links ]
4. Solomon S, Quin D, Manning M, et al. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. New York, USA: Cambridge University Press; 2007. [ Links ]
5. Duin EC, Wagnerb T, Shimab S, Prakasha D, Cronina B, Yáñez-Ruiz DR, et al. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. PNAS 2016;133(22):6172-6177. [ Links ]
6. Khang DN, Ngoc AD, Preston R. Effect of cassava leaf meal and coconut cake on methane production in an in vitro incubation using cassava root pulp and urea as substrate. LRRD 2019;31:(128). [ Links ]
7. Hayek SA, Ibrahim SA. Antimicrobial activity of xoconostle pears (Opuntia matudae) against Escherichia coli O157:H7 in Laboratory Medium. Int J Microbiol 2012;2012 (368472). [ Links ]
8. Roldán-Cruz CA, Ángeles-Santos A, Hernández-Fuentes AD, Santos-Fernández SA, Campos-Montiel RG. Efecto inhibitorio de bacterias patógenas con extractos de xoconostle asistidos por ultrasonido. Inv Des Cs Tec de Alim 2016;1(1):214-219. [ Links ]
9. Espinosa-Muñoz V, Roldán-Cruz C, Hernández-Fuentes AD, Quintero-Lira A, Almaraz-Buendía I, Campos-Montiel RG. Ultrasonic-assisted extraction of phenols, flavonoids, and biocompounds with inhibitory effect against Salmonella typhimuriun and Staphylococcus aureus from cactus pear. J Food Proc Eng 2017;40(2). [ Links ]
10. Instituto de Estudios Legislativos del Estado de Hidalgo. Ley de Protección y Trato Digno para los Animales en el Estado de Hidalgo. Ley publicada en el Periódico Oficial 9 Bis, el 28 de febrero de 2005. http://www.congreso- hidalgo.gob.mx/biblioteca_legislativa/leyes_cintillo/Ley%20de%20Proteccion%20y%20Trato%20Digno%20para%20los%20Animales.pdf. [ Links ]
11 . AOAC. Official Methods of Analysis. 17th ed. USA: Association of Official Analytical Chemist. 2000. [ Links ]
12 . Van-Soest PJ, Robertson JB. Analysis of forages and fibrous foods a Laboratory Manual for Animal Science. Ithaca, NY: Cornell University; 1985. [ Links ]
13. García-Martínez E, Fernández-Segovia I, Fuentes-López A. Determinación de polifenoles totales por el método de Folin-Ciocalteu. Valencia: Universitat Politécnica de Valencia; 2015. [ Links ]
14 . Kuskoski E, Asuero AG, Troncoso AM, Mancini-Filho J, Fett R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciênc Tecnol Aliment Campinas 2005;25(4):726-732. [ Links ]
15 . Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 1995;(28):25-30. [ Links ]
16 . Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem 2002;(50):3713-3717. [ Links ]
17 . Erwin E, Marco G, Emery E. Volatile fatty acid analysis of blood and rumen fluid by gas chromatography. J Dairy Sci 1961;(44):1768-1771. [ Links ]
18 . Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Tech 1994;(48):185-197. [ Links ]
19 . Demeyer D. Rumen microbes and digestion of plant cell walls. Agric Envir 1981;(6):295-337. [ Links ]
20 . Hidayat H, Newbold CJ, Stewart C. The contributions of bacteria and protozoa to ruminal forage fermentation in vitro, as determined by microbial gas production. Anim Feed Sci Tech 1993;(64):77-89. [ Links ]
21 . Groot J, Cone J, Williams BA, Debersaques F, Lantinga E. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim Feed Sci Tech 1996;(64):77-89. [ Links ]
22 . Torres-Salado N, Sánchez-Santillán P, Rojas-García A, Herrera-Pérez, J, Hernández-Morales J. Producción de gases efecto invernadero in vitro de leguminosas arbóreas del trópico seco mexicano. Archiv Zootec 2018;67(257):55-59. [ Links ]
23 . Sanchez-González N, Jaime-Fonseca MR, San Martín-Martínez E, Zepeda LG. Extraction, stability, and separation of betalains from Opuntia joconostle cv. using response surface methodology. J Agric Food Chem 2013;(61):11995-12004. [ Links ]
24 . Morales P, Barros L, Ramírez-Moreno E, Santos-Buelga C, Ferreira I. Xoconostle fruit (Opuntia matudae Scheinvar cv. Rosa) by-products as potential functional ingredients. Food Chem 2015;(185):289-297. [ Links ]
25. Espinosa-Muñoz V, Roldán-Cruz C, Hernández-Fuentes AD, Quintero-Lira A, Almaraz-Buendía I, Campos-Montiel RG. Ultrasonic-assisted extraction of phenols, flavonoids, and biocompounds with inhibitory effect against Salmonella typhimuriun and Staphylococcus aureus from cactus pear. J Food Proc Eng 2017;40(2):e12358. [ Links ]
26 . NRC. National Research Council. Nutrient Requirements of Small Ruminants: Sheep, goats, cervids, and new world camelids. Washington, DC, USA: National Academy Press; 2007. [ Links ]
27 . Vazquez-Olivo G, López-Martínez LX, Contreras-Angulo L, Basilio-Heredia J. Antioxidant capacity of lignin and phenolic compounds from corn stover. Waste Biomass Valor 2017;(10):95-102. [ Links ]
28 . Medina-Pérez G, Peralta-Adauto L, Afanador-Barajas L, Fernández-Luqueño F, Pérez-Soto E, Campos-Montiel R, Peláez-Acero A. Inhibición de la actividad enzimática de ureasa, elastasa y β-glucuronidasa mediante la aplicación de extractos acuosos de Opuntia oligacantha CF Primeras frutas ácidas: ensayo in vitro en condiciones digestivas simuladas. Apl Sci 2021;11(16):7705. [ Links ]
29 . Pérez-Soto E, Cenobio-Galindo A, Espino-Manzano S, Franco-Fernández M, Ludeña-Urquizo F, Jiménez-Alvarado R, et al. The addition of microencapsulated or nanoemulsified bioactive compounds influences the antioxidant and antimicrobial activities of a fresh cheese. Molecules 2021;(26):2170. [ Links ]
30 . Dohme F, Machmüller A, Wasserfallen A, Kreuzer M. Comparative efficiency of various fats rich in medium-chain fatty acids to suppress ruminal methanogenesis as measured with RUSITEC. Canadian J Anim Sci 2000;80:473-482. [ Links ]
31 . Diaz-Solares M, Lugo-Morales Y, Fonte-Carballo L, Castro Cabrera I, López-Vigoa O, Montejo-Sierra IL. Evaluación de la actividad antimicrobiana de extractos frescos de hojas de Morus alba L. Pastos Forrajes 2017;40(1):43-48. [ Links ]
32 . Mcallister TA, Martínez T, Bae HD, Muir AD, Yanke LJ, Graham AJ. Characterization of condensed tannins puriefed from legume forages: chromophore production, protein precipitation and inhibitory effects on celullose digestion. J Chem Ecol 2005; 31(9):2049-2068. [ Links ]
33 . McAllister T, Newbold C. Redirecting rumen fermentation to reduce methanogenesis. Australia J Exp Agric 2008;(48):7-13. [ Links ]
34 . Patra AK, Saxena J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J Sci Food Agric 2011;(91):24-37. [ Links ]
35 . Grant R, Mertens D. Influence of buffer pH and raw corn starch addition on in vitro fiber digestion kinetics. J Dairy Sci 1992;7(10):2762-2768. [ Links ]
36 . Mouriño F, Akkarawongsa R, Weimer P. Initial pH as a determinant of cellulose digestion rate by mixed ruminal microorganisms in vitro. J Dairy Sci 2001;(84):848-859. [ Links ]
37. Ku-Vera J, Castelán-Ortega O, Galindo-Maldonado F, Arango J, Chirinda N, Jiménez-Ocampo R. et al. Strategies for enteric methane mitigation in cattle fed tropical forages. Animal 2020;453-463. doi:10.1017/S1751731120001780. [ Links ]
38. Schofield P, Pitt R, Pell A. Kinetics of fiber digestion from in vitro gas production. J Anim Sci 1994;(72):2980-2991. [ Links ]
39. Espino-García JJ, Campos-Montiel RG, González-Lemus U, Torres-Cardona MG, Sánchez-Santillán P, Peralta-Ortiz JJ, Almaraz-Buendía I. Fermentación in vitro de rastrojo de maíz combinado con Xoconostle (Opuntia matudae Sheinvar) y su efecto en la producción de gas. LRRD 2020;32(2):31. [ Links ]
40 . Smith HA, Zoetendal E, Mackie RI. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Micro Ecol 2005;(50):197-205. [ Links ]
41 .Tiemann TT, Lascano CE, Wettstein HR, Mayer AC, Kreuzer M, Hess HD. Effect of the tropical tannin-rich shrub legumes Calliandra calothyrsus and Flemingia macrophylla on methane emission and nitrogen and energy balance in growing lambs. Animal 2008; 2(5):790-799. [ Links ]
Received: November 21, 2021; Accepted: December 21, 2022











 text in
text in