Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.12 n.1 Mérida Jan./Mar. 2021 Epub Sep 20, 2021
https://doi.org/10.22319/rmcp.v12i1.5999
Technical notes
Histopathology and PCR detection of bovine fibropapillomatosis in cattle in San Luis Potosí, Mexico
a Universidad Autónoma de San Luís Potosí. Facultad de Agronomía y Veterinaria. Carretera San Luis-Matehuala Km 14.5, Ejido Palma de la Cruz, 78321 Soledad de Graciano Sánchez, SLP, México.
Bovine papillomavirus (BPV) occurs worldwide and has myriad signs, including cutaneous papillomas, fibromas and fibropapillomas. Histology and PCR were used to identify the presence of BPV in tissue samples collected from cattle manifesting skin lesions suggestive of papillomas, fibromas and fibropapillomas in production units in the state of San Luis Potosí, Mexico. Eleven skin biopsies were taken from animals between 5 and 18 months’ age in stabled, semi-stabled and pastured beef and dairy production systems. Lesions were suggestive of papillomas, fibropapillomas and squamous cell carcinomas. Samples were evaluated by histopathology. Detection of BPV was also done using DNA extracted from the samples and analyzed by PCR with the FAP59/FAP64 and MY09/MY11 oligonucleotide pairs. The lesions were classified into fibromas (45.45 %) and fibropapillomas (54.54 %). Lesion type distribution exhibited no patterns by anatomical location, animal age, production system or end purpose. Most (72.72 %, n= 8) of the samples were positive for BPV by PCR; 45.45 % (n= 5) with the FAP pair and 54.54 % (n= 6) with the MY pair. This is the first study identifying the presence of BPV in San Luis Potosí. The results will be useful in establishing detection and control measures to improve production system health measures and end product quality.
Key words Bovine papillomavirus; Bovine fibropapillomatosis; Histopathology; PCR; San Luis Potosí
El objetivo del trabajo fue determinar la presencia del papilomavirus bovino (PVB) en muestras de tejidos de bovinos con lesiones cutáneas sugerentes de papilomas, fibromas y fibropapilomas en unidades de producción de ganado bovino en el estado de San Luis Potosí. Se obtuvieron 11 biopsias de piel de bovinos de entre 5 y 18 meses provenientes de sistemas de producción estabulado, semiestabulado y de agostadero con fines productivos de carne y leche, con lesiones sugerentes de papilomas, fibropapilomas y carcinomas de células escamosas. Estas muestras se procesaron mediante histopatología y se realizó extracción de ADN para la detección del PVB mediante PCR con los oligonucleótidos FAP59/FAP64 y MY09/MY11. Las lesiones se clasificaron en fibromas (45.45 %) y fibropapilomas (54.54 %) sin que se observara distribución de un tipo de lesión específica de acuerdo a la localización anatómica, edad, sistema de producción o fin zootécnico. El 72.72 % (n= 8) de las muestras mostraron resultados positivos para PVB mediante PCR; 45.45 % (n= 5) con los oligos FAP y 54.54 % (n= 6) con los oligos MY. Hasta donde se sabe, este es el primer estudio que describe la presencia de PVB en el estado de San Luis Potosí, por lo que estos resultados aportan datos útiles para establecer medidas de detección y control necesarias para mejorar las condiciones zoosanitarias de los animales.
Palabras clave Papilomavirus bovino; Fibropapilomatosis bovina; Histopatología; PCR; San Luis Potosí
Bovine papillomavirus (BPV) causes bovine fibropapillomatosis, an infectious, species-specific disease found worldwide. It mainly affects young cattle and is associated with various predisposing factors such as immunosuppression conditions, animal age, nutritional status, parasitosis, improper management, stress and immunosuppressive drugs, among others1,2. Papillomaviruses are a family of small, non-enveloped oncogenic viruses that infect birds, mammals and fish. The Papillomaviridae family comprises 29 genera that encompass 189 viral types of which 120 have been isolated from humans, 64 from mammals, 3 from birds, and 2 from reptiles2,3.
Ten BPV viral types capable of causing infection at different anatomical sites in bovines have been characterized to date. Lesion characteristics respond to viral type. For example, BPV-1 is known to produce papillomas and fibropapillomas in the penile region, while BPV-2 manifests as papillomas and fibropapillomas on the skin and in the digestive tract. Bovine papillomavirus types 3 and 8 cause skin tags, and BVP-4 has been associated with the appearance of papillomas in the gastrointestinal tract. Fibropapillomas on the udder can be caused by BVP-5 while papillomas on the udder are associated with BVP-6, -9 and -103-7.
Fibromas, papillomas or fibropapillomas are benign proliferative neoplasms. They can be exophytic or endophytic, solitary or multiple, partially delimited, plaque-like and papillary. Their appearance can vary from that of a grain of rice to the texture of cauliflower, and their texture can be dry or firm. They can become necrotic and detach, and may exhibit secondary bacterial contamination8,9.
Lesions caused by BVP can regress spontaneously or remain for six to eighteen months. Depending on their location, multiple lesions can lead to loss of body condition. Clinical signs of BVP vary by location on the body; for example, if located in the interdigital space, they can cause pain, leading to lameness or prostration. Rarely do BVP lead to clinical manifestations in the gastrointestinal tract although they can cause anorexia or bloat. When infecting the mammary gland BVP can make milking difficult or complicate with secondary infections and generate mastitis. Lesions in the vagina or on the penis can interfere with intercourse, may bleed or become infected and can interfere with reproduction10-12.
On a microscopic level papillomas consist of papillary projections of squamous epithelium, supported by fibrovascular stroma. These epithelial projections exhibit marked hyperplasia and hyperkeratosis, as well as ortho- and parakeratosis. In some papillomas, keratinocytes, mainly those of the stratum spinosum, have abundant clear cytoplasm or a perinuclear halo and pyknotic nuclei, which are called koilocytes (cells with cytopathic changes). Conditions present in certain regressing papillomas include reduction of epidermal hyperplasia, increased fibroblast proliferation, collagen deposits, and lymphocyte infiltration. Fibropapillomas have two components: a lining epithelium which alternates with fibrous tissue arranged in short interlocking bundles, and reactive fibroblasts. The lining epithelium does not exhibit cytopathological changes, but does have marked hyperplasia and plexiform acanthosis1,13,14. In large lesions, the epithelium may erode and come to resemble fibroids, in which proliferation of fibroblasts with dense collagen deposits has been observed15.
Different strategies have been developed for using PCR to detect BPV in fibromas and fibropapillomas. The FAP59/FAP64 oligonucleotide pair, designed based on analysis of conserved regions of the human papillomavirus (HPV) L1 gene, has proven effective to this end. In addition to being useful in detecting a wide spectrum of HPV types in skin tumors and healthy skin, it has also been applied in detection of cutaneous papillomaviruses in various species, including BPV types 1-12. Similarly, the MY09/MY11 pair, originally designed to detect mucosa- and genital-associated HPV types, have been shown capable of amplifying regions of the L1 gene in BPV types 1, 3, 5 and 616,17.
In Mexico, data on molecular detection and identification of BPV infection have only been reported for cattle in the state of Tamaulipas18. No epidemiological data on the incidence, prevalence, or the BPV viral types most frequently involved in the development of papillomas or fibropapillomas in cattle have been reported from other regions of the country. The present study objective was to identify the presence of different BPV types in tissue samples from skin lesions with a histopathological diagnosis of papillomas and/or fibropapillomas collected from cattle from two regions in the state of San Luis Potosí, Mexico.
Incisional and excisional biopsies of skin exhibiting lesions suggestive of fibromas, papillomas, and/or fibropapillomas were collected from eleven animals in two regions of San Luis Potosí (Table 1, Figure 1).
Table 1 Descriptive information on sampled cattle
| ID | Breed | Age
(months) |
Sex | Production system |
Location |
|---|---|---|---|---|---|
| 1 | Swiss-Zebu | 18 | Female | Stabled | Tamuín |
| 2 | Swiss-Zebu | 5 | Male | Pastured | Éban |
| 3 | Lidia | 6 | Male | Stabled | Villa de Reyes |
| 4 | Swiss Cross | 6 | Male | Semi-stabled | Villa de Zaragoza |
| 5 | Holstein | 15 | Female | Stabled | Soledad de Graciano Sánchez |
| 6 | Holstein | 10 | Female | Stabled | Soledad de Graciano Sánchez |
| 7 | Holstein | 10 | Female | Stabled | Soledad de Graciano Sánchez |
| 8 | Holstein | 12 | Female | Stabled | Soledad de Graciano Sánchez |
| 9 | Holstein | 13 | Female | Stabled | Soledad de Graciano Sánchez |
| 10 | Zebu Cross | 7 | Male | Pastured | Tamuín |
| 11 | Swiss Cross | 8 | Male | Semi-stabled | Tamasopo |

Figure 1 Sample site locations (red dots) in San Luis Potosí; at sites where more than one sample was collected the number is indicated
Each tissue sample was divided into two portions. One was placed in 15 ml Falcon tubes containing 10% formalin and evaluated by histopathology. The other was placed in a phosphate buffer solution (PBS) at pH 7.2 and stored in a cryogenic container until later PCR analysis. All samples were transported to the Immunology and Virology Laboratory of the Autonomous University of San Luis Potosí (Universidad Autónoma de San Luis Potosí - UASLP) for processing.
The sample portions intended for histopathological evaluation were fixed in 10% formalin, embedded in paraffin and processed by routine histological techniques. Thin sections (3 to 5 µm) were cut and stained with hematoxylin and eosin (H&E).
Extraction of DNA from the collected samples was done by processing 25 mg tissue with the DNeasy Blood & Tissue reagent set (Qiagen, Valencia, California, USA) following manufacturer instructions. The extracted DNA was stored at -80 °C until use. Its purity and quantify were verified with a Nano-200 drop spectrophotometer (Allsheng, Beijing, China). Integrity of the DNA was verified with 2% TAE-agarose gel electrophoresis.
The PCR analysis was run using two oligonucleotide pairs: FAP-59/FAP-64 (Macrogen, Seoul, South Korea) (FAP-59: 5'-TAACWGTIGGICAYCCWTATT-3'; FAP-64: 5'- CCWATATCWVHCATITCICCATC-3'), and MY-09/MY-11 (IDT, San Diego, California, USA) (MY-09: 5'-CGTCCAAAAGGAAACTGAGC-3'; MY-11: 5'-GCACAGGGACATAACAATGG-3'). Both oligonucleotide pairs detect the open reading frame of the gene for the majority protein of the L1 capsid, which is highly conserved in all types of bovine and human PV.
The PCR mixtures (50 µl) were prepared with the Invitrogen PCR Reagent Set (Invitrogen, Massachusetts, USA). These mixtures contained 1 × buffer, 200 µM DNTp, 2mM MgCl2, 20 pM oligonucleotides, 5 UI Taq polymerase and 10 ng DNA. Analyses were run in a Multigene Optimax Thermal Cycler (LabNET, California, USA). For the MY09/MY11 pair, the PCR program was 10 min initial denaturation at 94 °C; 35 cycles of 90 sec at 94 °C, 60 sec at 50 °C and 90 sec at 72 °C; and a final extension of 5 min at 72 °C. For the FAP59/FAP64 pair, the program was 10 min initial denaturation at 94 °C; 45 cycles of 90 sec at 94 °C, 90 sec at 50 °C and 90 sec at 72 °C; and a final extension of 5 min at 72 °C.
The PCR products were analyzed by electrophoresis of 5 µl of product on 2% TAE-agarose gel at 80 V for 80 min. The gel was then impregnated with ethidium bromide (Sigma-Aldrich, Missouri, USA) and an image taken with a Gel Doc EZ System photodocumenter (Bio-Rad, Hercules, California, USA).
All eleven skin biopsies exhibited common histological characteristics such as irregular hyperplasia and marked hyperkeratosis of the epidermis, erosions, ulcers and serocellular crusts. Some also had papillary projections supported by fibrovascular stroma alternating with dense collagen and ballooning degeneration of the epithelium, with inclusion bodies. Proliferation of mature fibrous connective tissue was observed in the dermis, interspersed with reactive fibroblasts, dense collagen, newly formed lymphatic vessels, and multiple aggregates of lymphocytes, plasma cells and macrophages. In one tissue section, scattered atypical epithelial cells were observed which exhibited loss of the nucleus cytoplasm relationship, abundant intensely eosinophilic cytoplasm, a large nucleus with notches, chromatin displaced to the periphery and one to three nucleoli evident (Figure 2).

) Fibropapilloma: Epidermis exhibits irregular hyperplasia and marked diffuse hyperkeratosis, as well as formation of papillary projections supported by fibrovascular stroma. H&E, 4X; B) Fibroma: Epidermis exhibits irregular hyperplasia and marked diffuse hyperkeratosis, dermis exhibits proliferation of fibrous connective tissue interspersed with congested blood vessels and aggregates of lymphocytes and plasma cells. H&E, 4X; C) Fibroma: Epidermis exhibits irregular hyperplasia and marked hyperkeratosis, and ballooning degeneration of keratinocytes present in different strata, dermis exhibits proliferation of reactive fibroblasts and aggregates of lymphocytes and plasma cells. H&E, 10X; D and E) Fibroma: keratinocytes exhibit ballooning degeneration and presence of amorphous amphophilic structures compatible with inclusion bodies (Arrow). H&E, 100X; F) Fibroma: some epithelial cells show marked anisokaryosis with loss of the nucleus cytoplasm relationship, abundant intensely eosinophilic cytoplasm, a large nucleus with notches, chromatin displaced to the periphery, and 1 to 3 evident nucleoli. H&E, 40X.
Figure 2 Histological lesions representative of the fibromas, papillomas and fibropapillomas identified in the bovine skin samples
The lesions observed histologically were suggestive of a benign viral-type neoplastic process compatible with fibromas and fibropapillomas. This conclusion is based on characteristics such as irregular hyperplasia and epidermal hyperkeratosis; presence of papillary projections of the epidermis; proliferation of mature fibrous connective tissue interspersed with reactive fibroblasts, dense collagen, and aggregates of lymphocytes, plasma cells and macrophages. Koilocytes and intranuclear amphophilic inclusion bodies were also observed (Table 2).
Table 2 Production type, histology and PCR results
| ID | Production type | Histological Diagnosis | PCR FAP | PCR MY |
|---|---|---|---|---|
| 1 | Beef | Fibroma | - | + |
| 2 | Beef | Fibropapilloma | - | - |
| 3 | Exhibition | Fibropapilloma | - | + |
| 4 | Beef | Fibroma | + | + |
| 5 | Dairy | Fibropapilloma | + | + |
| 6 | Dairy | Fibropapilloma | + | + |
| 7 | Dairy | Fibroma | - | - |
| 8 | Dairy | Fibroma | - | - |
| 9 | Dairy | Fibropapilloma | - | - |
| 10 | Beef | Fibroma | + | - |
| 11 | Beef | Fibropapilloma | + | - |
| Total | 5 | 6 | ||
In the FAP59/FAP64 PCR analysis an amplicon was observed between 480 and 538 bp. In 45.45 % (n= 5) of the samples, this corresponds to the PCR amplification product of the BPV L1 gene. No PCR product was observed in 27.27 % (n= 3) of the samples. Overall, 72 % of the samples analyzed with this oligonucleotide pair were positive for DNA sequences corresponding to the BPV L1 gene (Figure 3, Table 2).
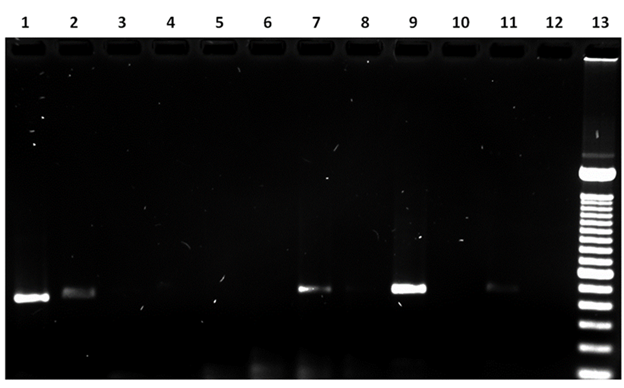
Lane 1: sample 5, 485 bp amplicon; Lane 2: sample 6, 511 bp amplicon; Lane 7: sample 10, 521 bp amplicon; Lane 9: sample 11, 515 bp amplicon; Lane 11: sample 4, 513 bp amplicon; Lane 12: negative control (no DNA); Lane 13: 100 bp ladder. Lanes 3, 4, 5, 6, 8 and 10: no PCR product observed.
Figure 3 FAP59/FAP64PCR results
In the MY09/MY11 analysis, a band was present between 470 and 490 bp in samples 1, 2, 5, 6, 8 and 9 (54.5% of all samples). Again, this corresponds to the PCR amplification product of the BPV L1 gene (Figure 4).

Lane 1: sample 5, 472 bp amplicon; Lane 2: sample 6, 479 bp amplicon; Lane 5: sample 9, 492 bp amplicon; Lane 6: sample 1, 488 bp amplicon; Lane 8: sample 3, 483 bp amplicon; Lane 9: sample 4, 486 bp amplicon; Lane 12: negative control (no DNA); Lane 13: 100 bp ladder. Lanes 3, 4, 7, 10 and 11: no PCR product observed.
Figure 4 MY09/11PCR results
Most of the examined lesions were indicative of fibroids. However, some papillomas and fibropapillomas can exhibit regression or morphological changes during their evolution which make them resemble fibroids; their presence depend on animal chronicity and immune status13,15. Given the timing of the sampling, it is therefore highly probable that the incidence of fibroids reported here constitutes an overestimate since lesion etiology and evolution can follow a common pattern.
The analyzed samples were collected from skin from different anatomical regions and no pattern of lesion restriction to a specific body region was observed. These results coincide with a previous study reporting that skin lesions associated with BPV infection can be generalized and that appearance site may correlate to BPV virus type4.
The lesions sampled here were only cutaneous, suggesting that the viral types involved were most probably BPV-2, BPV-3, BPV-6, BPV-8, BPV-9 or BPV-10. This possibility requires confirmation through PCR product sequencing.
It seems this is the first study addressing molecular level detection of BPV in cattle in the state of San Luis Potosí. The present results will help to identify areas of opportunity in which greater detection and control is needed to improve animal health and the quality of livestock products.
The most frequent lesions observed in the epidermis during the histological analysis were irregular hyperplasia, marked diffuse hyperkeratosis and ballooning degeneration of keratinocytes, while in the dermis they were proliferation of reactive fibroblasts, fibrous connective tissue and aggregates of lymphocytes and plasma cells. These characteristics are widely reported as lesions suggesting papillomavirus infection19,20.
Previous studies in Japan using the FAP-59/FAP-64 pair reported a 100 % BPV prevalence in skin lesions21, while a study in Iran found a 12.5 % prevalence in Holstein cattle22. Overall infection frequency for any BPV type was 72 % in the present study. This level is only slightly lower than the 86 % BPV positive sample frequency reported in Brazil23, and just above the 2 to 70 % positive sample frequency reported in the state of Tamaulipas, Mexico18. In 27.27 % of the present samples the histological lesions were highly suggestive of viral infection, but PCR results were negative for BPV. In these cases a possible association of BPV with these lesions cannot be ruled out due to the genomic integration process which occurs in the natural history of BPV infection4,24.
Papillomavirus infections have been described worldwide but genotype regional prevalence varies1,25,26. In the present study, samples were collected from cattle in the central and Huasteca regions of San Luis Potosí. Climate in the former is dry arid while in the latter it is subtropical. The present sample is too small to make conclusions about prevalence in these two regions, but ranchers in the Huasteca region report a higher incidence of lesions suggestive of BPV infection. This would agree with a previous report of a probable association between cutaneous papillomatosis frequency and tropical rainy climates27.
This is the first description of BPV in cattle from the state of San Luis Potosí, Mexico. Frequency in the evaluated samples was high, but similar to that found in a region bordering the state. In the present study there were no apparent patterns based on animal age or breed, or production system type. The BPV present in San Luis Potosí has not yet been characterized to the viral type level. This is a vital next step since it will allow characterization of specific distribution patterns (clusters) and consequent development of biological strategies aimed at viral types.
Acknowledgements
Isaura Méndez Rodríguez received a Ph.D. grant from the CONACYT (Mexico).
REFERENCES
1. Díaz RV, Duch CE, Gómez AD, Duato EGL, Rico LB. Papilomatosis bovina: epidemiología y diversidad de papilomavirus bovinos (BPV). Rev Complutense Cienc Vet 2012;6(2):38-58. [ Links ]
2. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. Virus taxonomy: VIIIth report of the International Committee on Taxonomy of Viruses. California, USA: Academic Press; 2005. [ Links ]
3. De Villiers EM, Fauquet C, Broker TR, Bernard HU, Zur Hausen H. Classification of papillomaviruses. Virology 2004;324(1):17-27. [ Links ]
4. Borzacchiello G, Roperto F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet Res 2008;39(5):1. [ Links ]
5. Ogawa T, Tomita Y, Okada M, Shirasawa H. Complete genome and phylogenetic position of bovine papillomavirus type 7. J General Virol 2007;88(7):1934-1938. [ Links ]
6. Tomita Y, Literak I, Ogawa T, Jin Z, Shirasawa, H. Complete genomes and phylogenetic positions of bovine papillomavirus type 8 and a variant type from a European bison. Virus Genes 2007;35(2):243-249. [ Links ]
7. Hatama S, Nobumoto K, Kanno T. Genomic and phylogenetic analysis of two novel bovine papillomaviruses, BPV-9 and BPV-10. J General Virol 2008;89(1):158-163. [ Links ]
8. Pattar J. Autogenous vaccination and immunomodulation for management of cutaneous papillomatosis in a crossbred cow. Intas Polivet 2013;14(2):423-425. [ Links ]
9. Munday J. Bovine and human papillomaviruses: a comparative review. Vet Pathol 2014;51(6):1063-1075. [ Links ]
10. Corteggio A, Altamura G, Roperto F, Borzacchiello G. Bovine papillomavirus E5 and E7 oncoproteins in naturally occurring tumors: are two better than one? Infectious Agents and Cancer 2013;8(1):1. [ Links ]
11. Knight CG, Munday JS, Rosa BV, Kiupel M. Persistent, widespread papilloma formation on the penis of a horse: a novel presentation of equine papillomavirus type 2 infection. Vet Dermatol 2011;22(6):570-574. [ Links ]
12. Salib, FA,Farghali HA. Clinical, epidemiological and therapeutic studies on Bovine Papillomatosis in Northern Oases, Egypt in 2008. Vet World 2011;4(2)53-59. [ Links ]
13. Guzmán LSO, Barboza Q, González RRA. Biología del virus del papiloma humano y técnicas de diagnóstico. Medicina Universitaria 2010;12(49):231-238. [ Links ]
14. Henry M, Ioffe O. Squamous premalignancy of the cervix: advantages of a 2-tiered versus 3-tiered terminology. AJSP: Reviews & Reports 2013;18(4):177-182. [ Links ]
15. Jang JS, Kim JH, Shin TK, Cho GJ, Kwon OD. A case of cutaneous fibroma in a Korean indigenous cattle. J Vet Clin 2008;25(3):200-201. [ Links ]
16. Forslund O, Antonsson A, Nordin P, Stenquist BO, Hansson BG. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J General Virol 1999;80(9):2437-2443. [ Links ]
17. Antonsson A, Hansson BG. Healthy skin of many animal species harbors papillomaviruses which are closely related to their human counterparts. J Virol 2002;76(24):12537-12542. [ Links ]
18. Rojas-Anaya E, Cantú-Covarrubias A, Álvarez JFM, Loza-Rubio E. Detection and phylogenetic analysis of bovine papillomavirus in cutaneous warts in cattle in Tamaulipas, Mexico. Canad J Vet Res 2016;80(4):262-268. [ Links ]
19. Jarrett WF, Campo MS, Blaxter ML, O'neil BW, Laird HM, Moar MH, et al. Alimentary fibropapilloma in cattle: a spontaneous tumor, nonpermissive for papillomavirus replication. J Natl Cancer Inst 1984;73(2):499-504. [ Links ]
20. Campo MS. Bovine papillomavirus and cancer. The Vet Journal 1997;154(3):175-188. [ Links ]
21. Ogawa T, Tomita Y, Okada M, Shinozaki K, Kubonoya H, Kaiho I, et al. Broad-spectrum detection of papillomaviruses in bovine teat papillomas and healthy teat skin. J General Virol 2004;85(8):2191-2197. [ Links ]
22. Babaahmady E, Taherpour K. Verrugas en los pezones de vacas lecheras. REDVET. Revista electrónica de Veterinaria 2011;12(6):1-6. [ Links ]
23. Santos EUD, Silva MAR, Pontes NE, Coutinho LCA, Paiva SSL, Castro RS, et al. Detection of different bovine papillomavirus types and co‐infection in bloodstream of cattle. Transboundary Emerging Diseases 2016;63(1):e103-e10863. [ Links ]
24. Agrawal R, Pelkonen J, Rytkönen M, Mäntyjärvi RA. Integration of bovine papillomavirus type 1 DNA and analysis of the amplified virus-cell junctions in transformed primary mouse fibroblasts. J General Virol 1992;73(1):201-206. [ Links ]
25. Orozco ANM, Padilla MHJ. Manual alternativas de tratamiento contra la papilomatosis bovina [tesis doctoral]. Managua, Nicaragua: Universidad Nacional Agraria; 2016. [ Links ]
26. Charry DJV, Hinojosa LMB. Estudio de papilomatosis bovina en cinco propiedades de ganadería de leche, en cantón Pedro Vicente Maldonado en la provincia de Pichincha [tesis de licenciatura]. Quito, Ecuador: Universidad de las Américas; 2011. [ Links ]
27. Violet L, Montes D, Cardona J. Frecuencia de papilomatosis en bovinos (Bos taurus) del departamento de Córdoba, Colombia. Revista Colombiana de Ciencia Animal-RECIA 2017;9(2):294-300. [ Links ]
Received: August 21, 2018; Accepted: March 11, 2020











 text in
text in 


