Every breeding program depends on the genetic variability in the population, but it is often neglected. One of the methods used to evaluate the impact of selection on the genetic variability is studying the population genetic structure, which can be done through pedigree analysis1. The effective population size (Ne) is a parameter widely used to indicate risk of inbreeding depression or even extinction risk. In the last 50 yr, the effective population size of several sheep breeds has drastically decreased, leading in some cases to inbreeding depression2,3,4. Moreover, several sheep breeds were extinct in the 20th century due to reduction of Ne. Of the 1,495 sheep breeds recorded up to december 1999, only 656 were not at risk of extinction until that time5.
Another important genetic population parameter is the number of ancestors explaining the genetic variability of a breed, because many commercial breeds usually have a reduced number of sires in mating. There are previous studies reporting large differences between the total number of ancestors and the number of ancestors that explain 50 % of the genetic variability in different sheep breeds6-9. Additionally, the ratio between the effective number of founders (fe) and the effective number of ancestors (fa) indicates whether a population is under bottleneck effect. Examples of strong bottleneck effect have been observed in some sheep breeds1,9.
The coefficient of inbreeding expresses the probability that two alleles at one locus will be identical by descent10. Inbreeding causes a reduction in individual genetic merit for some productive traits, possibly due to the increase in homozygous genotypes for deleterious recessive alleles or a reduction of heterozygous genotypes10. However, the depressive effect is relatively minor at low levels of inbreeding. Therefore, monitoring of inbreeding is indicated for maintenance or reduction of inbreeding level of a population11. An increase in the average inbreeding coefficient along generations has been observed in some sheep breeds6,8, and the most efficient way to control long-term inbreeding is to use breeding flocks with low average relatedness (AR). Thus, AR is another important parameter in population genetics.
Estimates of population parameters have been rarely reported for Santa Inês sheep12,13 and the only one study reported estimates of inbreeding effect on phenotypic traits12. These authors estimated inbreeding effects only for body weight traits, but did not estimated such effects on breeding values. Thus, the inbreeding effect on many traits and their breeding values remains unknown for Santa Inês sheep. The present study aimed to evaluate the genetic population structure of Santa Inês flocks, through pedigree information, and to estimate the effect of inbreeding on growth traits as well as for estimates of breeding values.
Dataset
An initial dataset was preliminary edited based on a file containing 11,781 animals with productive information and 12,322 animals in the pedigree, belonging to 16 different flocks. After consistency analysis, animals with missing productive information or without genetic connection between at least two different flocks were discarded. Records with errors or incomplete information or contemporary groups (CGs) with fewer than five animals with valid measurements were eliminated. As well as CGs in which the animals were the offspring of only one sire and the information was outside the acceptable range, i.e., 3 standard deviation above or below the mean of the trait, were also removed. Additionally, records were checked to ensure that: there were no duplicate records; no progeny was born before neither of their two parents; progeny only appeared as progeny, but not as sire and/or dam in the same record; sires only appeared as sires, but not as dams; dams only appeared as dams, but not as sires.
The final dataset included 11,564 animals in the pedigree, born from 2003 to 2011, which is maintained by the Sergipe State Association of Goat and Sheep Breeders (Associação Sergipana de Criadores de Caprinos e Ovinos - ASCCO). Traits recorded were weights at birth (BW1), 60 (BW60), 180 (BW180), and 270 (BW270) days of age. Daily weight gains were calculated from birth to 60 (DWG1), 60 to 180 (DWG2), and 60 to 270 (DWG3) days of age.
Population parameters
The software ENDOG14 was used to estimate inbreeding coefficient (F)15, effective population size (Ne)14 and average relatedness coefficient (AR). POPREP16 was used to estimate effective number of founders (fe), ancestors (ƒa), effective number of founders genomes (fge) and number of generations traced (g). Additionally, it was estimated the genetic diversity loss average due to bottlenecks and genetic drift. A complete description of these parameters can be found in Gutiérrez and Goyache14.
Breeding value prediction and Inbreeding effect analysis
All traits were tested for data normality applying Shapiro-Wilk test, at 5% significance level, using Statistical Analysis System17, before estimating genetic parameters. Estimates of variance components and breeding values were obtained by restricted maximum likelihood (REML), with a multitrait animal model, using the software VCE618 (for variance components) and PEST19 (for breeding values). In this analysis, the matrix model can be described as follows:
where:
y is the vector of phenotypic values;
b is the vector of fixed effects of contemporary group, and the covariates dam’s age and animal’s age; X is the incidence matrix that relates the observations in y to fixed effects in b;
a is the vector of direct additive random effect; Z is the incidence matrix that relates the observations in y to direct additive random effects in a;
m is the vector of maternal additive random effect;
M is the matrix that relates the observations in y to maternal additive effect in m;
e is the vector of random residual term.
The maternal component Mm was adjusted only for the traits BW1, BW60 and DWG1. The dataset used in this study had a low number of calves per ewe. Thus, the permanent maternal effect and the litter environmental effect were tested but presented problems such as non-convergence or inconsistent estimates of parameters. Therefore, it was chose not to include those effects in the final model. In addition, the dataset showed a low number of inbred Dams and, therefore, it was not include this effect in the model.
The assumptions of the models for analyzes could be simply represented as follows:
The (co)variance matrix for additive genetic effects is G = G  A, where A is the relationship matrix and G is the additive genetic (co)variance matrix. The (co)variance matrix for maternal genetic effects is M = M
A, where A is the relationship matrix and G is the additive genetic (co)variance matrix. The (co)variance matrix for maternal genetic effects is M = M A, where M is the genetic maternal (co)variance matrix. R = I
A, where M is the genetic maternal (co)variance matrix. R = I R0 is the residual (co)variance matrix between the seven traits. GxM is the covariance between genetic additive and maternal effects.
R0 is the residual (co)variance matrix between the seven traits. GxM is the covariance between genetic additive and maternal effects.
The contemporary group (CG) consisted of animals from the same farm (45 levels), sex (male or female), birth type (single or twins), year (2003 to 2011), and season of birth (dry or rainy). Contemporary groups with less than three animals were removed from the analysis. Table 1 shows the number of CG per trait and descriptive statistics for all traits.
Table 1 Sample size (N), number of contemporary groups (CG), mean and standard deviation (SD) of the traits
| Traits1 | N | CG | Mean | SD |
|---|---|---|---|---|
| Birth weight | 10232 | 291 | 3.63 | 0.80 |
| Weight at 60 d (weaning) | 6277 | 319 | 15.94 | 5.77 |
| Weight at 180 d | 4541 | 403 | 31.9 | 11.16 |
| Weight at 270 d | 3328 | 374 | 39.7 | 14.5 |
| Daily weight gain from birth to 60 d | 5786 | 319 | 171.73 | 64.87 |
| Daily weight gain from 60 to 180 d | 3229 | 403 | 149.43 | 67.84 |
| Daily weight gain from 60 to 270 d | 1863 | 374 | 69.17 | 26.83 |
| Dam | 4742 | ---- | ---- | ---- |
| Sire | 391 | ---- | ---- | ---- |
1Weight and weight gain were measured in kilograms.
For testing inbreeding effect on the phenotypic values it was used the mixed model:
where:
In addition, inbreeding effect on breeding values were also tested and the model was as follows:
Pedigree completeness
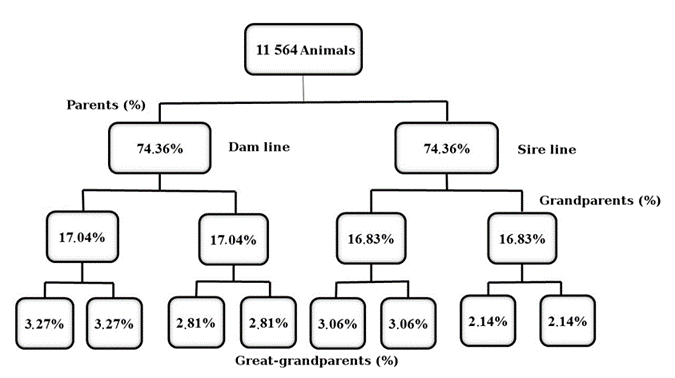
Percentages of animals with known pedigrees decreased with the passing of the generations, from over 70% in the first generation to less than 5% in the third (Figure 1). This result may be a consequence of ASCCO had been started the phenotypic and pedigree records recently (about 3-4 generations), which may explain the little-known ancestry of the animals studied here. Loss of information from one generation to another in the present study was higher than those reported in previous studies with Santa Inês flocks12,13. Pedrosa et al12 found known ancestry from parents to great-grandparents of 77, 59.5, and 38.75 %, while Teixeira Neto et al13 found 80.84, 73.78, and 67.75 %. Previous studies about other sheep breeds reported varied levels of pedigree completeness. High levels were reported for Bharat Merino sheep, with values of 91.01, 82.63, 74.91, 67.10, and 57.78 % for the first, second, third, fourth, and fifth generation, respectively8. However, for other sheep breeds were reported pedigree completeness less than those observed in the present study, especially in the first generation back (11.88 % for sire and 69.38 % for dam) in Guilan sheep20, and (57 % for sire and 15 % for dam) in Mehraban sheep21.
The number of equivalent generations is the parameter that best describes the quality of a pedigree and higher value for this parameter indicates a more completeness pedigree. In the present study, this value was low (Table 2) indicating that, even with a reasonable amount of information (11,564 individuals), the average relatedness was less in this Santa Inês dataset. In previous studies with Santa Inês, higher values were found, 2.2612 and 4.6713, due to higher pedigree completeness. A low number of equivalent generations is common in sheep breeds with early conservation and breeding programs6, resulting in pedigrees with a low degree of depth and incomplete information. For this reason, a reduced number of equivalent generations was also reported in several sheep breeds2,6,9,21.
Table 2 Genetic parameters of the gene origin for Santa Inês Flocks in Northeast of Brazil
| Genetic parameters | Value |
|---|---|
| Reference population | 11,564 |
| Number of ancestors | 3,984 |
| Effective number of founders (f e ) | 285 |
| Number of founding animals | 486.84 |
| Effective number of ancestors (f a ) | 273 |
| Number of ancestors explaining 50% | 146 |
| Effective number of founders genomes (f ge ) | 35.71 |
| Average of genetic diversity loss | 0.0094 |
| Inbreeding (F) | 1.40% |
| Average relatedness coefficient (AR) | 0.47% |
| Average number of equivalent generations | 0.94 |
| Average number of complete generations | 0.83 |
| Average number of maximum generations | 1.09 |
| Inbreeding increment (ΔF) in equivalent generations | 0.95 |
| Inbreeding increment (ΔF) in complete generations | 0.97 |
| Inbreeding increment (ΔF) in maximum generations | 0.73 |
| Effective population size (Ne) in equivalent generations | 52.62 |
| Effective population size (Ne) in complete generations | 51.28 |
| Effective population size (Ne) in maximum generations | 68.83 |
| Generation Interval Father-Son | 3.46 |
| Generation Interval Father-Daughter | 3.33 |
| Generation Interval Mother-Son | 3.40 |
| Generation Interval Mother-Daughter | 3.28 |
Structure and genetic diversity
The effective population size (Ne) changed with time (Figure 2), being highest in 2004 (665) and lowest in 2010 (45). The largest effective size was observed for the maximum generation (Table 2). The variation in effective population size (Ne) over time has also been observed in other sheep breeds. Values of Ne ranging from 41.8 to 31.3 in Morada Nova sheep22, while values of Ne from 280.2 to 12.4 for Segureña sheep has been reported6. Therefore, the average effective population size (
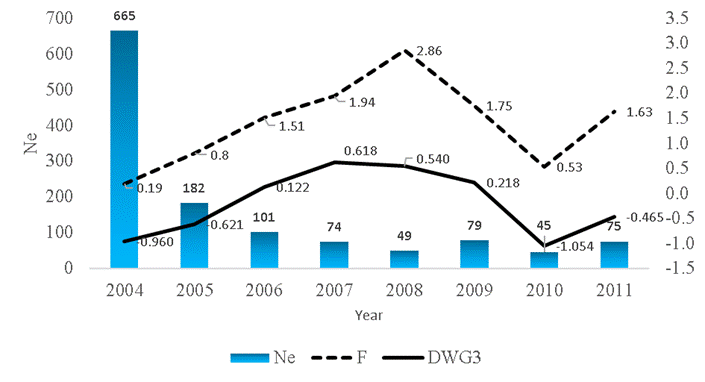
Figure 2: Average inbreeding (F), effective population size (Ne) and average breeding values for DWG3 per year of birth
A reference population of 11,564 Santa Inês sheep was evaluated, with 3,984 ancestors. In this population, the effective number of founders (ƒe) was 285, while the number of founding animals was 486.84. The number of ancestors explaining 50% of the genetic variation was 146 and the effective number of ancestors was 273. The effective number of founders genomes (f ge ) was 35.71. Therefore, some Santa Inês sires were used more intensely, in detriment to others, which may have contributed to the loss of genetic variability. All ancestors would contribute in the same way throughout the generations, but for many sheep breeds the total number of ancestral is much larger that number of ancestral explaining 50 % of genetic variability8,9,22.
Largest inbreeding increment was observed in equivalent generation (Table 2). The highest number of inbred animals was concentrated between 0 and 10 % of the inbreeding coefficient and just 263 animals showed F>10 %. Generation interval for each of the four parent-offspring pathways demonstrated an average of 3.37 (Table 2). Estimates of average inbreeding coefficient ranged from zero to 6.25% along the generation (Table 3) and average inbreeding was 1.40 % when only inbred animals were considered. The estimates of average relatedness coefficient ranged from 0.22 to 0.52 % along the generation (Table 3) and general average relatedness coefficient was 0.47 %. The inbreeding coefficient had its lowest value in 2004 (0.19 %) and a higher value in 2008 (2.86 %) (Figure 2).
Table 3 Number of animals (N) per generation with their respective average inbreeding coefficient (F) and average relationship coefficient (AR)
| Generations | N | F (%) | AR (%) |
|---|---|---|---|
| 1 | 7,562 | 0.00 | 0.22 |
| 2 | 2,901 | 0.88 | 0.45 |
| 3 | 844 | 2.42 | 0.51 |
| 4 | 210 | 3.81 | 0.51 |
| 5 | 57 | 3.82 | 0.52 |
| 6 | 1 | 6.25 | 0.43 |
Ideally, f e equals f a , or the difference is always as low as possible. Ratios much higher than 1.0 indicate a strong bottleneck effect, which may be due to small number of sires used in mating. This ratio in the present study (1.04 %) suggests that the majority of ancestors were founders and an insignificant genetic bottleneck. Despite the good fe/fa ratio in the present study, f e and f a had values much lower than the reference population and ancestors (Table 2), indicating that the animals evaluated here have a narrow genetic origin. Another study with Santa Inês reported a higher (1.35) fe/fa ratio12, demonstrating genetic variability reduction caused by the imbalance between ancestral and founders and the higher bottleneck effect. For other sheep breeds, a fe/fa close to one was also reported, such as 1.0 in Morada Nova sheep22, and 1.18 in Iran-black sheep7, and 1.12 in Segureña sheep6. However, large values were also reported in Xalda sheep (2.02)1 and in Kermani sheep (2.07)9. Finally, an average of genetic diversity loss of 0.0094 was detected over the studied period, demonstrating that genetic drift was significant to result in loss of genetic diversity in this population.
Inbreeding values above 10% are associated with decreased performance in sheep24. It was observed few animals (2.27 %) with consanguinity higher than 10%, and a maximum F value of 37.5 %, while several animals (97.73 %) were not inbreed or showed an inbreeding coefficient less than 10 %. These values are similar to those reported for other sheep breeds8,25. In a study with Bharat Merino sheep, 97.62 % of animals were non-inbreed or showed F< 10 %, and the highest individual inbreeding was 32.81 %8. In Iranian Shal sheep, 93.72 % of animals to be non-inbred or F<10 %, with a maximum individual inbreeding of 31.25 %25. It is noteworthy that low pedigree completeness may have underestimated individual inbreeding coefficients in the present study. Previous studies about Santa Inês sheep, with better pedigree completeness, found maximum inbreeding of 41.02 %12 and 54.83 %13, respectively; both studies have shown that the number of inbred animals increases significantly after the first years of pedigree control.
The average inbreeding coefficient of the population (inbred and non-inbred animals) was 0.36 %, but average inbreeding coefficient for inbred animals was 1.41 %. Higher values of population average inbreeding 2.33 %12 and 6.92 %13 were reported for Santa Inês sheep. In these studies, average inbreeding coefficients were 10.74 %12 and 12.57 %13 when only inbred animals were used. The lowest value found in the present study may be due to low pedigree completeness, especially in the first years, which makes computing inbreeding coefficient difficult. Small average inbreeding coefficients were reported in other sheep breeds with low pedigree completeness. Previous studies reported average inbreeding coefficients of 0.15, 1.6 and 0.60 % for whole analyzed pedigree of the Guilan20, Baluchi2, and Segureña6 sheep breeds, respectively.
The increase in inbreeding throughout the generations (Table 3) may be reflecting a better flock pedigree control and consequently higher database quality, because the inbreeding coefficient depends on the number of known generations. The inbreeding increment (ΔF) in the present study was low for all of the generations traced (Table 2), suggesting that the Santa Inês flocks under investigation were in good condition. An increase in the average inbreeding coefficient along generations was also observed in other sheep breeds6,8. An increase from zero to 7.09 (from the initial to the fourth generation) in Segureña sheep was reported6, while an increase from zero to 1.54 (from the initial to the sixth generation) was reported to Bharat Merino sheep8. The low AR values obtained in the present study (Table 3), show that the flocks are in a good situation, increasing the probability of mating among unrelated individuals. Another Santa Inês dataset12 also showed a low estimate for AR (0.73 %), evidencing the great variability of this breed.
Inbreeding effect on phenotype and breeding values
For phenotypic values, the individual inbreeding had no effect (P>0.05) on BW60, BW180, BW270, and DWG1 (Table 4), but significant effects (P<0.05) were observed for BW1 (0.0054 ± 0.0015), DWG2 (-0.9837 ± 0.3025), and DWG3 (-0.5628 ± 0.2377). For breeding values, depression inbreeding effect were significant (P<0.05) for all traits, except DWG1. For the Santa Inês sheep, only one previous study12 tested inbreeding effect on phenotypic traits, but they evaluated only BW1, BW60 and BW180. They observed a reduction of 34, 52, and 204 grams per %∆F (equivalent to a traditional inbreeding coefficient of 2.2 % when 2.26 generations in the pedigree are known) in the weight of Santa Inês for BW1, BW60, and BW180, respectively. The present analysis for record traits did not confirm these findings, because it was found a positive inbreeding effect on BW1, where each 1 % of inbreeding increased 5.4 g in this weight, and no significant effects were observed for BW60, BW180 and BW270 (Table 4). Several previous studies with other sheep breeds reported depressive inbreeding effect on birth weight21,26,27 where each 1% of inbreeding resulted in decreases ranging from -0.7 g per 1% F in Polish Olkuska sheep26 to -51 g per 1% F in Thalli sheep27. For other pre and post-weaning body weights, there are many results indicating depressive effects in different sheep breeds as well. Depression-inbreeding effect for BW60, with values range from -33 to -48 g per 1% F were reported27,28. However, studies with Iranian Shal sheep25 and Segureña sheep6 did not any significant inbreeding effect on body weight.
Table 4 Regression coefficients of the effects of inbreeding on the performance traits
| Phenotype value | Breeding value | |||||
|---|---|---|---|---|---|---|
| Trait | Estimate | Standard Error |
P-value | Estimate | Standard Error |
P-value |
| BW at birth | 0.0054 | 0.0015 | 0.0004 | -0.0049 | 0.0006 | <0.0001 |
| BW at 60 d | 0.0252 | 0.0132 | 0.0555 | -0.0162 | 0.0030 | <0.0001 |
| BW at 180 d | -0.0568 | 0.0299 | 0.0575 | -0.0347 | 0.0104 | 0.0009 |
| BW at 270 d | -0.0623 | 0.0430 | 0.1469 | -0.0448 | 0.0131 | 0.0006 |
| DWG from birth to 60 d | -0.2318 | 0.2200 | 0.2921 | -0.0461 | 0.0573 | 0.4213 |
| DWG from 60 to 180 d | -0.9837 | 0.3025 | 0.0012 | -0.2846 | 0.0868 | 0.0011 |
| DWG from 60 to 270 d | -0.5628 | 0.2377 | 0.0180 | -0.0524 | 0.0212 | 0.0137 |
BW= body weight; DWG= daily weight gain.
In some previous studies, no-significant effects are many times attributed to the low level of inbreeding of the animals as consequence of low pedigree completeness. In the present study, the dataset had also low pedigree completeness (Figure 1); consequently, large number of animals (7,562) showed F close to zero. Another hypothesis for no-significant effect is the reduced number of animals showed both F>0 and phenotypic record. To avoid this second problem, we decided to evaluate the effect of inbreeding on the breeding values. The results (Table 4) indicated a significant inbreeding effect on breeding values of all body weights (BW1, BW60, BW180 and BW270). In addition, the regression coefficients were negative, which is more consistent with previous studies reported in sheep26,27,28.
Regression coefficients for DWG2 and DWG3 were higher than those (−0.263 ± 0.116) reported for daily weight gain from 90 to 365 in Sandyno sheep4, and lower than those found for daily weight gain from 90 to 180 (−1.810 ± 0.017) and from 90 to 365 (−1.345 ± 0.083) in Baluchi sheep2. Finding effect for daily weight gain and no effect for body weight seems incoherent, but it is easy to explain. The number of animals with records for BW60, BW180 and BW270 was different from the number of animals with records for DWG2 and DWG3, because to calculate the daily weight gain we need the same animal to have four information (the initial and final BW, and the initial and final ages). It is not a reality for all animals in our dataset. When used breeding values this problem was resolved, because all animal has the estimates of breeding values for all traits. It is possible observed in Table 4 that this incoherence practically not existed when we estimated inbreeding effect on breeding values (Table 4), suggesting a more consistent result.
It could be observed that the evaluated population had a low pedigree completeness and small average inbreeding. This population presented a decrease in the effective population size over the generations and an increase of the endogamy, which can compromise its genetic variability. The inbreeding had significant effect on BW1, DWG2 and DWG3 when evaluated phenotypic records. When was evaluated breeding values, the inbreeding effect was significant for all traits, except for DWG1. Regression coefficients obtained for breeding values suggested a more consistent analysis, because they were negative and significant for both BW (all ages) and post-weaning DWG. On the other hand, positive and significant regression inbreeding coefficients was found only for BW1 (in phenotypic analysis), but it was not found similar results for this trait in breeding value analysis. In both analysis, the inbreeding effect on growth traits were mainly negative, which implies the need to avoid related mating on the studied flocks of Santa Inês sheep.











 text in
text in