Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.11 n.2 Mérida Apr./Jun. 2020 Epub Oct 23, 2020
https://doi.org/10.22319/rmcp.v11i2.5010
Articles
Semen quality of hair sheep supplemented with Moringa oleifera (Moringaceae) and Trichanthera gigantea (Acanthaceae)
a Tecnológico Nacional de México, Instituto Tecnológico de Conkal, División de Estudios de Posgrado e Investigación, Ave. Tecnológico s/n Conkal, 97345, Yucatán, México.
b Tecnológico Nacional de México, Instituto Tecnológico de Chiná, Campeche, México.
c Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Campo Experimental Mocochá, Yucatán, México.
It was assessed the effect of the inclusion of Moringa oleifera Lam. and Trichanthera gigantea (Bonpl.) Nees in the diet of hair (Pelibuey) sheep on the quality of their semen. During 90 d, the diets of 15 sheep (24 kg ± 3.95) were divided into three treatments: T1: integral diet with 40 % of M. oleifera + Taiwan grass (Pennisetum purpureum Schumach.), T2: integral diet with 40 % of T. gigantea + Taiwan grass, and T3: 40 % commercial feed + Taiwan grass. The daily weight gain (DWG), carcass yield (CY), testicular development (DT) determined by the diameter (AE) and the scrotal circumference (SC) of these sheep were determined, and the volume, concentration, motility (CASA), viability (SYBR-14/IP), mitochondrial activity (J-C1) and acrosomal integrity (FITC-PSA) of their sperm samples were assessed. No differences were found (P>0.05) in the GDP and RC. Differences were found (P<0.05) in DT, T1 (AE= 48.84 ± 5.99 mm, CE=$9.29 ± 1.13 cm) and T3 (AE= 48.83 ± 4.34 mm, C = 26.62 ± 1.27 cm) exhibited higher values than T2 (44.57 ± 5.59 AE= mm, CE= 25.42 ± 1.50 cm); as for viability, T2 (62.90 ± 6.10 %) and T1 (54.00 ± 6.61 %) had higher percentages than T3 (24.45 ± 7.56 %); in motility, T1 (93.9 ± 2.1 %) and T2 (88.6 ± 1.9 %) had a higher percentage than T3 (71.9 ± 4.0 %). The inclusion of M. oleifera and T. gigantea in the diet allows to obtain a GDP, RC and DT similar to those of commercial feed and increases the number of viable sperm cells by more than 20 %; it also improves certain parameters of motility, thereby improving the reproductive potential of the stallions.
Keywords Viable sperm cells; motility; Pennisetum purpureum; channel performance
Se evalúo el efecto de la inclusión de Moringa oleifera Lam. y Trichanthera gigantea (Bonpl.) Nees en la dieta de ovinos de pelo (Pelibuey) sobre su calidad seminal. Durante 90 días, las dietas de 15 ovinos (24 kg ± 3.95) se dividieron en tres tratamientos: T1: dieta integral con 40 % de M. oleifera + pasto Taiwán (Pennisetum purpureum Schumach.), T2: dieta integral con 40 % de T. gigantea + pasto Taiwán y T3: 40 % alimento comercial + pasto Taiwán. A estos ovinos se les determinó la ganancia diaria de peso (GDP), rendimiento de la canal (RC), desarrollo testicular (DT) (determinado por el diámetro (AE) y la circunferencia escrotal (CE)); y a sus muestras de esperma se les evalúo el volumen, concentración, motilidad (CASA), viabilidad (SYBR-14/IP), actividad mitocondrial (J-C1) e integridad acrosomal (FITC-PSA). No se encontraron diferencias (P>0.05) en la GDP y RC. Se encontraron diferencias (P˂0.05) en DT, T1 (AE= 48.84 ± 5.99 mm, CE= 26.48 ± 1.13 cm) y T3 (AE= 48.83 ± 4.34 mm, C = 26.62 ± 1.27 cm) presentaron valores más elevados que T2 (AE= 44.57 ± 5.59 mm, CE= 25.42 ± 1.50 cm); en la viabilidad, T2 (62.90 ± 6.10 %) y T1 (54.00 ± 6.61 %) poseen mayores porcentajes que T3 (24.45 ± 7.56 %); en la motilidad, T1 (93.9 ± 2.1 %) y T2 (88.6 ± 1.9 %) tuvieron mayor porcentaje que T3 (71.9 ± 4.0 %). La inclusión de M. oleifera y T. gigantea en la dieta permite obtener una GDP, RC y DT similar al alimento comercial e incrementa más del 20 % de las células espermáticas viables; también mejora algunos parámetros de motilidad, mejorando con esto el potencial reproductivo de los sementales.
Palabras clave Células espermáticas viables; Motilidad; Pennisetum purpureum; Rendimiento de canal
Introduction
The high demand for sheep meat forces producers to improve all its processes aimed at reducing production costs, to increase the production and reproduction parameters in order to increase the productivity per unit area, to improve animal welfare, and to propose a sustainable approach to ensure the permanence of the system through time1.
In tropical areas, the atmosphere is a determining factor for livestock production, and in particular for sheep production, which must deal, among other factors, with a marked seasonality in the production of forage, forcing producers to implement supplementation strategies (mainly with commercial feed) in order to maintain optimal levels of production; this has generated a dependency on the feed, as well as a considerable increase in production costs2. Within this context, the use of tree species with fodder potential has become relevant in animal feed3,4,5, because its implementation allows to generate strategies of partial replacement of grains in the diet6. However, the use of tree species with fodder potential is conditioned by their high nutrient content, tolerance to pruning, steady production of biomass and content of secondary compounds that may affect the animals that consume them7,8. This is one of the main reasons that limit their use for animal feed9, since it can generate estrogenic effects that inhibit, among others, the growth and maintenance of the reproductive system. In the reproductive system of males, the affectations by presence of secondary compounds can be: an increased number of immature sperm with a significant decrease in motility, a reduction of the lumen of seminiferous tubules, an increase in the apoptotic index of germ cells, prostatic atrophy, and affectation of spermatogenesis10,11.
Within this context, various species such as Moringa oleifera Lam. (Moringaceae) and Trichanthera gigantea (Bonpl.) Nees (Acanthaceae) are alternative dietary supplements8. The presence of secondary metabolites has been reported in these; for example, García12 recorded the presence of phenols, flavonoids and tannins that precipitate the protein and condensed tannins; on the other hand, Balercia et al13, Rajimakers et al14 and Ghasi et al15 observed in M. oleifera and T. gigantea, the presence of riboflavin, niacin, folic acid, pyridoxine, protein, vitamins A, B, C, and E, as well as amino acids and high levels of β-carotene; consumption of these antioxidants helps to improve semen quality by eliminating free radicals found in the seminal plasma. For this reason, it is necessary to study the effect that these plants may generate in the animals that consume them. Therefore, the objective was to evaluate the effect of the inclusion of M. oleifera and T. gigantea in the diet of Pelibuey sheep on the seminal quality.
Material and methods
The study was carried out in the agricultural and livestock production and research area of the Technological Institute of Conkal, Yucatán, Mexico (21°05' N and 89°32' W), which is located at an altitude of 7 meters above sea level and has an AW0 climate, with an annual precipitation of 900 mm and an average temperature of 29 ºC.
Treatments, animals and management
Fifteen (15) male pubertal Pelibuey sheep (aged 6 ± 0.5 mo) with an average weight of 24 kg ± 3.95 were utilized and distributed in a fully random way in three treatments (n=5): T1= integral diet with 40 % M. oleifera + Taiwan grass (Pennisetum purpureum Schumach.). T2= integral diet with 40% Trichanthera gigantea + Taiwan grass. T3= integral diet + Taiwan grass. The amount of feed offered was 4.7 % of the live weight during the 90 days of the experiment (the consumption was adjusted every seven days). Table 1 shows the contents of the percentage composition of the experimental diets, and Table 2, the proximate analysis.
Table 1 Percentage composition of the experimental diets
| Component | Diets (%) | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| Moringa | 16 | 0 | 0 |
| Trichanthera | 0 | 16 | 0 |
| Molasses | 4.24 | 3.72 | 7 |
| Minerals | 1.2 | 1.08 | 3 |
| Canola | 3.2 | 5.88 | 21 |
| Corn | 8.28 | 8 | 5 |
| Sorghum | 7.08 | 5.32 | 4 |
| Taiwan grass | 60.0 | 60.0 | 60.0 |
T1: intergral diet with 40% M. oleifera + Taiwan grass (Pennisetum purpureum Schumach.), T2: integral diet with 40% T. gigantea + Taiwan grass, and T3: commercial feed + Taiwan grass.
Table 2 Proximal chemical analysis of the offered diets
| Diets and grass |
Dry matter |
Crude protein |
Neutral detergent fiber |
Acid Detergent Fiber |
Ashes |
|---|---|---|---|---|---|
| T1 | 90.83 | 13.81 | 20.22 | 16.18 | 1.01 |
| T2 | 91.54 | 14.01 | 26.27 | 14.21 | 0.95 |
| T3 | 98.82 | 23.16 | 22.83 | 13.47 | 0.98 |
| Taiwan grass | 90.96 | 8.00 | 67.26 | 42.21 | 0.97 |
T1: intergral diet with 40 % M. oleifera + Taiwan grass (Pennisetum purpureum Schumach.), T2: integral diet with 40% T. gigantea + Taiwan grass, and T3: commercial feed + Taiwan grass
Development of lambs
To determine the development of lambs, the animals were weighed every seven days; prior to each weighing, the animals underwent a period of 18 h of fasting. They were weighed on a T31P (OHAUS) electronic platform scale with a capacity for 300 kg, with a margin of error of 0.1 kg. The daily weight gain was determined through the analysis of these weights16.
Testicular development
The measurement of the scrotal circumference was taken with a plastic measuring tape, while the testicular diameter was registered using a TRUPER brand digital caliper 17.
Semen quality
For the assessment of the semen quality, after the 70-days experimental period (at age 8 ± 0.7 mo), six semen samples per animal (two per week) were collected in each treatment using an estrogenized female dummy and the artificial vagina method with a rubber sleeve (Liner). Warm water at 45 ⁰C and air were introduced by blowing through a valve in order to obtain the temperature and pressure required to stimulate ejaculation18. Once the ejaculates were obtained, they were transported to the laboratory in isothermal conditions (35 to 37 ⁰C) for evaluation. At the laboratory, the samples were placed in a double boiler at 37 ⁰C for immediate evaluation.
The following were determined in the collected samples:
1) The volume of the ejaculate, with a graduated pickup tube.
2) The sperm concentration was determined with the CASA system (ISAS®v1 (Proiser R&D, Valencia, Spain) by diluting 5 µl of pure semen in 995 µl of distilled water (1:200); 9 µl of the diluted sample were then placed in a Bürker chamber for analysis. Sperm motility was evaluated by depositing 5 µl of diluted sample at a concentration of 30 x 106 spermatozoa/ml on a Makler® counting chamber (Sefi Medical Instruments, Haifa, Israel), and four recordings were made of each sample; the dilution was performed with the commercial diluent (Triladyl ® + distilled water + 20 % egg yolk). The parameters evaluated for this variable were: percentage of total motility (TM), percentage of progressive motility (PM), curvilinear speed (CLS, µm/s), rectilinear speed (RLS, µm/s), average speed (AS, µm/s), linearity index (LIN), straightness index (STR), oscillation index (WOB), average amplitude of lateral displacement of the head (ALH), sperm head beat frequency (HBF).
3) The sperm viability was assessed by staining with the Fluorochrome SYBR-14/IP (Live/Dead® kit L-7011, InvitrogenTM); 1 µL of SYBR-14 (10 µM) was mixed with 1 µl IP (12 µM) in 100 µl of diluted sperm sample and kept for 10 min at 37 ⁰C; 5 µl of the stained sample were subsequently placed on a glass slide preheated at 37 ⁰C for evaluation in a (CX-31, Olympus, Tokyo, Japan) fluorescence microscope with a wavelength of 488 nm.
4) The mitochondrial activity was determined using the J-C1 fluorochrome (153 µM, Molecular Probes® T-3168, InvitrogenTM).1 µl of the fluorochrome was mixed with 100 µl of diluted sperm sample and kept for 10 min at 37 ⁰C; 5 µl of the stained sample were subsequently placed on a glass slide preheated to 37 ⁰C for evaluation under a fluorescence microscope with a wavelength of 488 nm.
5) The integrity of the acrosomes was evaluated with the FITC-PSA fluorochrome (100µg/mL, L-0770, Sigma-AldrichTM), adding 5 µl of the fluorophore in 100 µl of diluted sperm sample and was kept for 30 min at 37 ⁰C; 5 µl of the incubated sample were subsequently placed on a slide and were evaluated in a fluorescence microscope with a wavelength of 485 nm.
The data obtained were analyzed using the PROC GLM procedure of the statistical package SAS 9.4 for Windows (Inst. Inc. Cary, NC, USA); a repeated means variance analysis (ANOVA) a Tukey mean comparison test were performed in order to evaluate the effect of diet on the sperm quality.
Results and discussion
Productive behavior
The daily weight gains of the studied animals did not exhibit any differences (P˂0.05) between treatments. These gains are greater than those recorded by Madera-Solis et al19, who with different levels of inclusion of Leucaena leucocephala (Lam.) de Wit found daily weight gains of 25, 41.67, 50, and 75 g in Pelibuey sheep. For their part, Rivers et al20 reported profits of 54, 87 and 56 g/d when assessing the use of Morus sp. and Gliricidia sepium Kunth ex Walp. as substitutes for concentrated feed for growing lambs. Daily revenues of 45.5, 96.3, 124.5 and 106.4 g with different levels of inclusion of hay from Hibiscus rosa-sinensis L. are also reported in growing sheep4. The profits obtained in this study are less than those mentioned by others16, which recorded daily gains of 229 ± 10 g/d for Pelibuey-Katadin animals housed in elevated cages and fed a commercial concentrate with a 16 % crude protein content and Cynodon nlemfuensis Vanderyst hay.
When assessing the performance of the carcass, no differences were found (P˂0.05) between treatments: 50.79, 49.44 and 46.94 % for treatments T1, T2 and T3, respectively; these values are similar to those registered by Magaña-Monforte et al16, who obtained a yield of 49.1 ± 0.58 with crossbred animals of the Pelibuey-Katahdin breeds. The yields of this study were lower than those found in another research21, which cites a carcass output of 52.8 % for whole males, 56.64 % for castrated males, and 55.87 % for females. The proper nutritional balance present in the assessed diets generated an adequate, similar productive response in the study animals, compared to other researchers performed under similar conditions. These results in the evaluated rations (Table 2) can be attributed to the adequate level of protein and the improved nutritional balance22; this stimulates an increase in the level of efficiency of nutrients produced by greater microbial activity in the rumen23 and ensuring metabolic stability24, positively affecting the various productive parameters evaluated in the present study (daily weight gains and carcass output).
Testicular development
Testicular volume is an indicator of the fertilizing ability of the male and of the highest fertility; also a smaller number of abnormal spermatozoa has been observed in animals with a larger scrotal circumference. In the study animals, the testicular development determined by means of the increase in scrotal circumference and diameter exhibited differences (P<0.05) between the treatments evaluated for both variables (Table 3). For the variable scrotal circumference, treatment T3 had a higher mean than T2, while T1 did not differ (p>0.05) from the other two treatments. For the scrotal diameter variable, there was no difference between treatments T1 and T3; however, both were larger than with T2.
Table 3 Scrotal circumference, scrotal diameter, and hair sheep seminal volume and concentration supplemented with M. oleifera and T. gigantea (mean ± standard error)
| Treatments | Scrotal circumference (cm) |
Scrotal diameter (mm) |
Volume (ml) |
Concentration |
|---|---|---|---|---|
| T1 | 26.48±0.33ab | 48.84±1.05a | 0.70 ± 0.05a | 1882.63 ± 202.66a x 106 |
| T2 | 25.42±0.33b | 44.57±1.05b | 0.61 ± 0.05a | 1841.92 ± 182.34a x 106 |
| T3 | 26.62±0.33ª | 48.83±1.05a | 0.63 ± 0.06a | 1014.49 ± 482.44a x 106 |
T1: integral diet with 40% M. oleifera + grass (Pennisetum purpureum Taiwan), T2: integral diet with 40 % T. gigantea + Taiwan grass, and T3: commercial feed + Taiwan grass.
(a,b) different letters between columns indicate significant differences (P<0.05).
The means for the scrotal circumference were: 26.4 ± 0.33, 25.4 ± 0.33 and 26.6 ± 0.33 cm for treatments one two and three, respectively (Table 3); they are lower than those recorded by Luna et al.25, who found averages of 28.4 cm in sheep supplemented with animal fat and palm oil; as well as those reported in other studies26,27, of 29.4 and 36.5 cm, respectively. The averages of scrotal diameter were lower than those recorded by Ballin et al.26. Testicular development is determined by various factors, mainly age, race, and nutrition27; in this regard the study animals, with an average age of 7±0.5 months, received a diet with adequate levels of protein and nutrients required for meeting the nutritional requirements according to their physiological stage, which is reflected in the weight gain and in the obtained testicular development.
Seminal evaluation
The variables of the ejaculate volume and sperm concentration did not exhibit any differences (P>0.05) between treatments (Table 3). The volume for the three treatments lies within the range considered normal, between 0.5 and 2.0 ml, and this volume varies, depending on the age, size, body condition of the animal, frequency of collection, and dexterity of the operator28. Despite this, the values reported in this experiment were lower than those reported by Mansano et al29, who obtained volumes of 1.3 ± 0.4 ml; Carrillo and Hernández30 obtained 1.41 ± 0.11 ml; for their part Córdova-Izquierdo et al31 obtained 1.11 ml; however, these two studies were performed with mature males unlike the pubertal males (7 ± 0.5 mo of age) used in the present study.
The low ejaculate volume values obtained in this study, contain a greater concentration of spermatozoa per ml. Therefore, these values are higher than those recorded in other studies, which indicate ranges of 206.4 to 711.89 × 106(30,31, but similar to those found by Domínguez et al32, of 2.19 ± 0.04 and 108 ± 8.0 × 106 spermatozoa/ml, when assessing the semen quality of F1 Katahdin × Pelibuey lambs supplemented with alfalfa. In contrast, the concentration values obtained here are lower than those found by Quintero et al33, who obtained 2,628.2 × 106 when assessing hair sheep aged 2 to 5 yr
There were no differences (P<0.05), in total motility, curvaceous speed, and AD, where treatments T1 and T2 were better than T3; and in the variables AS and RLS, T1 exhibited the best results with respect to T3; however, no differences were observed between T1 and T2. For the rest of the analyzed variables (PM, LIN, IR, OI, and BF), no differences were found between experimental groups (Table 4).
Table 4 Seminal motility parameters of hair sheep supplemented with M. oleifera and T. gigantea (mean ± standard error)
| Variables | Treatments | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| MT, % | 93.9 ± 2.1a | 88.6 ± 1.9a | 71.9 ± 4.0b |
| AS, µm/s | 100.4 ± 3.7a | 88.6 ± 3.2ab | 73.0 ± 6.8b |
| CLS, µm/s | 157.8 ± 5.0a | 146.6 ± 4.4a | 117.3 ± 9.2b |
| RLS, µm/s | 70.3 ± 3.1a | 61.3 ± 2.7ab | 54.6 ± 5.8b |
| AD, µm | 4.8 ± 0.1a | 4.6 ± 0.1a | 3.7 ± 0.3b |
| PM, % | 30.79±2.64a | 29.23±2.32a | 30.18±4.87a |
| IR, % | 64.72±1.31a | 64.98±1.15a | 69.62±2.42a |
| WOB, % | 61.96±1.63a | 59.85±1.43a | 60.78±3.01a |
| HBF | 10.32±0.25a | 10.57±0.22a | 9.89±0.47a |
| LIN, % | 42.27±1.9a | 40.85±1.67a | 44.35±3.5a |
T1= 40% of M. oleifera + Taiwan, T2= 40% of T. gigantea + Taiwan, T3= commercial feed + Taiwan.
MT= total motility; AS= average speed; CLS= curvilinear; speed; RLS= rectilinear speed; AD= amplitude of displacement; PM= progressive motility; STR= straightness index; WOB= oscillation index; HBF= sperm head beat frequency; LIN= linearity index.
a,b Values with different letters in the same row are different (P<0.05).
The results for the motility variable are higher than those recorded by Mansano et al29, of 52 %, but similar to those found in another research32, which registered an 80 % total motility and over 20 % progressive motility; however, the results of this study are lower than those recorded by Quintero et al33, who obtained 90.69 % progressive motility.
In the analysis of the plasma membrane viability variable, differences (P<0.05) were found between the assessed treatments (T1: 54 %, T2: 63 % and T3: 21 %), T2 being the treatment that exhibited the highest percentage of viability, in contrast with T3, which obtained the lowest percentages (Figure 1).
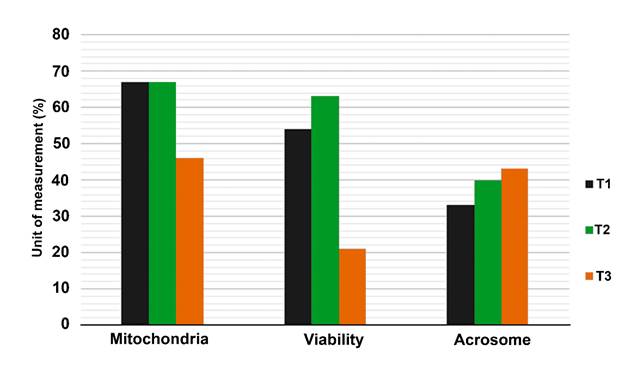
T1: integral diet with 30% M. oleifera + Taiwan grass (Pennisetum purpureum Schumach.), T2: integral diet with 30% T. gigantea + Taiwan grass, and T3: commercial feed + Taiwan grass.
Figure 1 Feasibility, mitochondrial activity and integrity of the acrosome of hair sheep supplemented with Moringa oleifera and Trichanthera gigantea
The mitochondrial activity and acrosome integrity variables did not reveal any differences (P>0.05) between treatments, while T1 exhibited the highest means for the mitochondrial activity variable, T3 showed the highest percentages for the mitochondrial activity variable (Figure 1). The integrity of the plasma and acrosome membranes and mitochondrial activity are major factors for fertilization, as an intact plasma membrane indicates that the spermatozoon is alive; an intact acrosomal membrane allows penetration of the oocyte, and the mitochondrial activity ensures the presence of energy required to move the spermatozoon (Figure 2). For this reason, it is essential to include it in the assessments on semen quality31. The results observed in this study agree with those recorded by Cordova-Izquierdo et al31 who obtained 68.5 % of the integrity of the plasma membrane by assessing sheep of the Suffolk race. They also match the results of Rubio-Guillen et al34, who recorded 42 % when evaluating West African sheep. However, the percentages obtained in this study are lower than those found in other researches30,32,33 but are consistent with the values estimated by Domínguez et al32 for acrosomal integrity.

A. Living (green) and dead (red or orange) sperm heads stained with SYBER-14/IP fluorochromes; viability of the acrosome located in the apical region using the fluorochrome FITC-PSA (colorless: viable; green: corrupted). B. Mitochondrial activity located in the middle region of the tail stained with the fluorophore JC1, with activity (yellow or orange) and without activity (green).
Figure 2 Sperm cells from sheep fed with Trichanthera gigantea or Moringa oleifera
The parameters recorded in the assessment of the semen quality as well as of the testicular development may be attributed to the adequate nutritional balance of the assessed diets, as sperm production and quality, and testicular size, are directly influenced by nutrition. An increase in the nutritional level increases the frequency of pulses of LH and increases levels of FSH35, hormones that promote spermatogenesis and androgen secretion36.
The animals assessed in the present study are pubescent, animals, and animals in this condition have a greater amount of immature, dead or defective spermatozoa, which in turn produce high amounts of free radicals that affect those spermatozoa that are in good conditions, since free radicals cause a cascade of lipid peroxidation affecting mainly the unsaturated fatty acids of the plasma membrane of the sperm, causing loss of sperm function and cell apoptosis, and therefore are one of the leading causes of death of sperm cells37.
It has been observed that the inclusion of antioxidants in the diet improves the percentage of live sperm cells because antioxidants eliminate the free radicals that are found in the seminal plasma and confer protection to the spermatozoa13,14; Priyadarshani and Varma38 observed a higher percentage of live cells when they used M. oleifera in the diet of mice. This result is similar to what has been observed in the present study and may be ascribed to the contribution of antioxidants (beta-carotene) contained in M. oleifera and T. gigantea15.
The secondary compounds present in both assessed species may be related to the values found in the present study; therefore, further studies determining the presence and concentration of these would contribute greater evidence in this regard.
Conclusions and implications
The replacement of 40 % of commercial feed by M. oleifera or T. gigantea in the diet of Pelibuey sheep promotes adequate testicular development, and improves the sperm viability by more than 20 %, as well as certain parameters of motility -such as total motility, average speed, curvilinear speed, rectilinear speed, and the average amplitude of lateral motility of the sperm head-, whereby the reproductive potential of the stallions may be improved.
Acknowledgments
This work was funded partly by the CONACYT Basic Science Project 164592 and by project 5304.14-P of the National Technological Institute of Mexico. We are grateful for the anonymous reviewers’ suggestions and comments on the manuscript.
REFERENCES
1. Altieri MC, Nicholls CI. Agroecología. Teoría y práctica para una agricultura sustentable. México: Programa de las Naciones Unidas para el Medio Ambiente Red de Formación Ambiental para América Latina y el Caribe; 2000. [ Links ]
2. Ramírez MEI. Productive response of pelibueysheep diet supplemented with Moringa oleifera Lam. (Moringaceae) and Trichanthera gigantea (Bonpl.) Nees (Acanthaceae). Int J Agric Sci 2017;8(2):172-176. [ Links ]
3. Galindo JD. Impacto de los árboles, los arbustos y otras leguminosas en la ecología ruminal de animales que consumen dietas fibrosas. Pastos y Forrajes 2005;28:59-67. [ Links ]
4. Ruiz-Sesma DL. Evaluación nutritiva y productiva de ovinos alimentados con heno de Hibiscus rosa-sinensis. Zootec Trop 2006;24:467-482. [ Links ]
5. Apráez JE, Delgado JM, Narváez J. Composición nutricional, degradación in vitro y potencial de producción de gas, de herbáceas, arbóreas y arbustivas encontradas en el trópico alto de Nariño. Livest Res Rural Dev 2012;24(3): Livest Res Rural Dev 2012;24(3): http://www.lrrd.org/lrrd24/3/apra24044.htm . Consultado 10 Feb, 2018. [ Links ]
6. Katsube TT, Tsurunaga Y, Sugiyama M, Furuno T, Yamasaki Y. Effect of air-dryng temperature on antioxidant capacity and stability of polyphenolic compunds in mulberry (Morus alba L.) leaves. Food Chem 2009;113(4):964-969. [ Links ]
7. Sosa RE. Evaluación del potencial forrajero de árboles y arbustos tropicales para la alimentación de ovios. Téc Pecu Méx 2004;42(2):129-144. [ Links ]
8. Carmona J. Efecto de la utilización de árboreas y arbustivas forrajeras sobre la dinámica digestiva en bovinos. Rev Lasallista Investig 2007;4(1):40-50. [ Links ]
9. Rivera LJ, Ramon UJ. Factores antinutricionales del recursos forrajero disponible en Yucatán como una posibilidad para mitigar el efecto de los gases invernadero causados por los rumiantes. In: Dumonteil E editor. Contribución de la biotecnología al desarrollo de la Peninsula de Yucatán. Mérida: Fondo Mixto CONACYT-Gobierno del Estado de Yucatán. 2012;99-115. [ Links ]
10. Lenis YG. Efectos de los fitoestrógenos en la reproducción animal. Rev Fac Nac Agron 2010;63(2):5555-5565. [ Links ]
11. Retana-Márquez S, Hernández H, Flores JA, Muñoz-Gutiérrez M, Duarte G, Vierlma J, Fitz-Rodríguez G, Fernández IG, Keller M, Delgadillo JA. Effects of phytoestrogens on mammalian reproductive physiology. Trop Subtrop Agroecosyt 2012;15:129-145. [ Links ]
12. García D. Evaluación química de especies no leguminosas con potencial forrajero en el estado de Trujillo Venezuela. Zootec Trop 2006;24:401-415. [ Links ]
13. Balercia G, Armeni T, Mantero F, Principato G, Regoli F. Total oxyradical scavenging capacity toward different reactive oxygen species in seminal plasma and sperm cells. Clin Chem Lab Med 2003;41(1):13-19. [ Links ]
14. Raijmakers MT, Roelofs HM, Steegers EA, Steegers-Theunissen RR, Mulder TP, Knapen MF, Wong WY, Peters WH. Glutathione and glutathione S-transferases A1-1 and P1-1 in seminal plasma may play a role in protecting against oxidative damage to spermatozoa. Fertil Steril 2003;79(1):169-172. [ Links ]
15. Ghasi S, Nwobobo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam. in high-fat diet fed wistar rats. J Ethnopharmacol 2000;69(1):21-25. [ Links ]
16. Magaña-Monforte JG, Moo-Catzin CJ, Chay-Canul JR, Aké-López JR, Segura-Correa JC, Montés-Pérez RC. Crecimiento y componentes de la canal de ovinos de pelo en jaulas elevadas. Livest Res Rural Dev 2015;27(6). [ Links ]
17. Jiménez-Severiano HRP. Evaluation of mathematical models to desribe testicular growth in Blackbelly ram lambs. Theriogenology 2010;74(7):117-1114. [ Links ]
18. Ruiz LS. Evaluación de la calidad espermática del semen ovino posdescongelación al emplear dos fuentes energéticas y dos crioprotectores. Rev Inv Perú 2015;26(1):49-56. [ Links ]
19. Madera-Solís NB, Bacab-Pérez HM, Ortiz De La Rosa B. Ganancia diaria de peso en ovinos por inclusión de una planta leguminosa (Leucaena leucocephala) en dietas basadas en pasto clon Cuba CT115 (Pennisetum purpureum). Bioagrociencias 2013;6(1):24-31. [ Links ]
20. Ríos L, Rondón Z, Combellas J, Alvarez R. Uso de morera (Morus sp.) y mata ratón (Gliricidia sepium) como sustituto del alimento concentrado para corderos en crecimiento. Zootec Trop 2005;23:49-60. [ Links ]
21. Torrescano GR, Sánchez A, Peñúñuri FJ, Velázquez J, Sierra T. Características de la canal y calidad de la carne de ovinos pelibuey, engordados en Hermosillo, Sonora. BIOtecnia 2009;11(1):41-50. [ Links ]
22. González-González NN, Gutiérrez-González D, García-López R, Fernández-Mayer A. Consumo voluntario y valores metabólicos sanguíneos en caprinos alimentados con mezclas integrales frescas de Moringa oleifera: Penisetum purpureum Clon-OM22. Avances en Investigación Agropecuaria 2015;19:25-36. [ Links ]
23. Garza-F JD, Owens FN, Welty S. Effect of post-ruminal protein infusion on feed intake and utilization of low quality hay by beef steers. Anim Sci Pap Rep 1991;134:106-113. [ Links ]
24. Borroto-Pérez A, Negrín-Brito A, Peña-López P, Vega-Báez D. Uso de moringa (Moringa oleifera) para ovinos en crecimiento, como alternativa alimentaria ambientalmente amigable. Universidad ( Ciencia 2018;7:78-90. [ Links ]
25. Luna C, Aguilar JA, Peralta JA, Velázquez JR. Efecto del aceite de palma sobre el crecimiento y capacidad reproductiva de carneros de pelo púberes. Arch Zootec 2013;62(237):45-52. [ Links ]
26. Ballín FJ, Ochoa MA, Torres G, Morón FJ, Gonzáles JM, Díaz MO. Relación de la edad, peso corporal y medidas morfométricas sobre el inicio de la pubertad en corderos polypay del altiplano potosino. Revista Cientifica LUZ 2013;23:434-439. [ Links ]
27. Moreno-Cañez E, Ortega-García C, Cáñez-Carrasco MG, Peñúñuri-Molina F. Evaluación del comportamiento posdestete en corral de futuros sementales ovinos de raza Katahdin y Pelibuey en Sonora. TECNOCIENCIA Chihuhua 2013;7(1):7-16. [ Links ]
28. Aisen EG. Reproducción ovina y caprina. Buenos Aires: Intermedica; 2004. [ Links ]
29. Mansano MM, Scott C, Souza MD, Torre LT, Vallejo AV, Ferreira SF. Viabilidad de espermatozoides ovinos mantenidos a 5⁰ y 15⁰C en diferentes sistemas de refrigeración. Revista Brasileira de Ciência Veterinária 2014;21(2):122-126. [ Links ]
30. Carrillo GD, Hernández HD. Caracterización seminal de individuos ovinos criollos colombianos de pelo en el departamento de Sucre. Rev Colombiana Cienc Anim 2016;8(2):197-203. [ Links ]
31. Córdova-Izquierdo A, Saltijeral-Oaxaca J, Muñoz-Mendoza R, Córdova-Jiménez MS, Cordova -Jimenez CA, Guerra-Liera JE. Efecto del método de obtención de semen ovino sobre la calidad espermática. Rev Electron Vet 2006;7(8):1-5. [ Links ]
32. Domínguez-Rebolledo AE, Alcaraz-Romero A, Cantón-Castillo JG, Loeza-Concha H, Ramón-Ugalde J. Efecto de la alfalfa (Medicago sativa L.) en la dieta sobre la calidad de los espermatozoides epididimarios de ovinos Katahdin con pelibuey. Reunión científica tecnológica forestal y agropecuaria Tabasco 2014 y III Simposio internacional en producción agroalimentaria tropical. Tabasco: Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias 2014:186-189. [ Links ]
33. Quintero EJA, Clemente SF, Olguín AHA. Contribución en el desarrollo de un índice de calidad del semen para la valoración de sementales ovinos. CULCYT 2016;58(13):105-109. [ Links ]
34. Rubio-Guillen J, Quintero-Montero AA, González-Villalobos DM. Efecto de la criopreservación sobre la integridad de la membrana plasmática y acrosomal de espermatozoides de toros. Rev Cient (Maracaibo) 2009;19(4):382-389. [ Links ]
35. Blanche DZ. Fertility in males: modulators of the acute effects of nutrition on the reproductive axis. Reprod Suppl 2003;59:219-233. [ Links ]
36. Prieto-Gómez B, Velázquez-Paniagua M. Fisiología de la reproducción: hormona liberadora de gonadotrofinas. Rev Fac Med UNAM 2002;45(6):252-257. [ Links ]
37. Lozano H. Factores que afectan la calidad seminal en toros. Rev Med Vet Zoot 2009;56:258-272. [ Links ]
38. Priyadarshani N, Varma MC. Effect of Moringa oleifera leaf poder on sperm count, histology of testis and epididymis of hyperglycaemic mice Mus musculus. Am Int J Res Form Appl Nat Sci 2007;14:7-13. [ Links ]
Received: August 03, 2018; Accepted: May 06, 2019











 text in
text in 


