Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.11 n.2 Mérida Apr./Jun. 2020 Epub Oct 23, 2020
https://doi.org/10.22319/rmcp.v11i2.5100
Articles
Ivermectin effectiveness for gastrointestinal nematode control in donkeys (Equus asinus) in the Mexican High Plateau
a Universidad Nacional Autónoma de México, Facultad de Medicina Veterinaria y Zootecnia, Circuito Exterior, Ciudad Universitaria, Av. Universidad 3000. 04510, Ciudad de México, México.
The donkey has been used as a working animal for centuries, and 96 % of the world population of this species is in developing countries. Gastrointestinal nematodes with anthelmintic resistance (AHR) are the most serious parasitic problem in equidae. This study analyzes the phenomenon of AHR to ivermectin (IVM) in donkeys, and economic thresholds, with an evaluation of the practices by the owners through surveys, are considered. Based on 53 donkeys from the Mexican High Plateau, the experiment was divided into two stages: 1) economic thresholds were determined for 53 animals, and the experimental groups were formed. 2) the IVM efficacy test was performed, and two experimental groups (n= 30) were established: the treated group and the control group, without treatment. The economic threshold of eggs per gram of feces was 600, and the threshold of body condition (BC) of 91 % of the animals was acceptable (2.5 to 3.5). At a higher BC, the egg discharge obtained was lower (P<0.05). Of the 100 larvae identified, 63 % were cyathostomidae, and the rest were large strongyles. In this nematode population, IVM efficacy was 100 %. Eighty, 80% of the surveyed owners admit that they use as the only strategy the treatment provided by volunteer Veterinarians, which consists of 1% IVM. This antiparasitic is still a valuable resource and must be used properly in order to prevent AHR. This is the first step toward targeted selective deworming in equidae in Mexico.
Key words Donkeys; Equus asinus; Cyathostomidae; Nematodes; Ivermectin; Anthelmintic resistance
El burro se ha empleado como animal de trabajo durante siglos y el 96 % de la población mundial se encuentra en países en desarrollo. Los nematodos gastrointestinales con resistencia antihelmíntica (RAH) son el problema parasitario más grave de los équidos. Este estudio analiza el fenómeno de RAH a la ivermectina (IVM) en burros, y se consideran umbrales económicos con la evaluación de las prácticas por parte de los propietarios a través de encuestas. Con 53 burros del Altiplano Mexicano, el experimento se dividió en dos etapas: 1) en 53 animales se determinaron umbrales económicos y se formaron los grupos experimentales. 2) se realizó la prueba de la eficacia de la IVM y se establecieron dos grupos experimentales (n= 30), grupo tratado y grupo testigo sin tratamiento. El umbral económico de huevos por gramo de heces fue de 600 y el umbral de la condición corporal (CC) del 91 % de los animales fue aceptable (2.5 a 3.5). A mayor CC, la descarga de huevos obtenida fue menor (P<0.05) de las 100 larvas identificadas, el 63 % fueron ciatostómidos, el resto fueron grandes estrongílidos. En esta población de nematodos, la eficacia de la IVM fue del 100 %. Con las encuestas, el 80 % de los propietarios admite utilizar como única estrategia el servicio de los médicos veterinarios zootecnistas voluntarios, el cual consiste en IVM al 1%. La IVM es un recurso todavía valioso y debe utilizarse adecuadamente para evitar RAH. Este es el primer paso para la desparasitación selectiva dirigida en équidos en México.
Palabras clave Burros; Equus asinus; Ciatostómidos; Nematodos; Ivermectina; Resistencia antihelmíntica
Introduction
The donkey (Equus asinus) has been used as a working animal for 5,000 yr1 and more than 96 % of the world population of this species is found in developing countries. In Mexico, it is estimated that there are around 3.3 million donkeys2). In the Mexican High Plateau, they are mainly used for agricultural activities2. In some cases, these donkeys suffer from poor nutrition, as they are fed with agricultural debris supplemented with low-quality grains or commercial concentrates 1-2. Donkeys are also hosts to a large number of parasites whose life cycles are similar to that of the parasites present in horses; therefore, these can act as a significant reservoir for the infection of other equidae3. The most important parasites in these animals are certain helminths such as Anoplocephala perfoliata or Parascaris equorum, but the ones with a greater impact due to the clinical implications of the larval migration and the hypobiosis that they exhibit4,5 are those of the Strongylida family, where the gastrointestinal nematodes (GIN) of the subfamily Cyathostominae, also known as small strongyles, are located, and those of the subfamily Strongylidae (Strongylus spp.), known as large strongyllids6,7. These gastrointestinal nematodoses in donkeys are perhaps one of the greatest challenges in clinical management, since donkeys with significantly high helminth loads may be apparently healthy and rarely exhibit clinical signs, unlike horses8.
It is known that, between horses and donkeys, there are great differences in behavior and physiology, and specifically in the mechanism through which they metabolize certain drugs and, in turn, respond differently to the pathologies that affect them9. This is not reflected in the pharmaceutical industry, since donkeys have a limited economic impact compared to horses9. Therefore, due to underdosing, there is the possibility of finding donkeys with chemical-resistant GIN, as has been demonstrated in other domestic species10,11. In domestic animals worldwide, GIN and their anthelmintic resistance (AHR) represent health, economic and productive problems12,13. The phenomenon of AHR is widely studied in Mexico and Latin America, especially in ruminants12,14 and other equidae5. Several studies carried out in some ecological zones in the high plateau and the central parts of the country measure the antiparasitic effect of ivermectin (IVM) in equines15; however, their efficacy in donkeys has never been measured. In the Mexican High Plateau region, veterinary doctors in the field have provided, at least twice a year for more than 10 yr, free deworming to donkeys with 1% oral IVM (personal communication Prado-Ortiz, O.), since the development of the AHR of the parasite population is imminent. Due to these practices, it is relevant to carry out studies that reveal the current panorama of the AHR in the region.
At present, in order to address AHR, it must first and foremost know relevant aspects of the epidemiology of the disease at the regional, local and particular levels11,16,17. For this purpose, it is necessary to establish certain criteria that are characteristic of economic thresholds, such as the distribution of the elimination of eggs per gram of faeces (EPG), the body condition (BC) values, and the identification of the genus of nematodes existing in the ecological study area, as well as to detect potential practices that may indicate treatment failures and cause AHR18,19. This study analyzes the phenomenon of AHR in donkeys and considers these thresholds based on the evaluation of owner practices. This contributes to the planning of different strategies to delay AHR and prolong the effectiveness of a drug, with a view to achieving Integrated Parasitic Control (IPC) in donkeys in the future.
Material and methods
The study was carried out in four communities located in the Mexican High Plateau: Aljibes and San Pedro, in the municipality of Tecozautla, Hidalgo, and Santa Rosa Xajay and Vaquerias, in the municipality of San Juan del Río, Querétaro.
Animals
Donkeys from the above communities were selected because these receive continuous support from volunteer Veterinarian (VET), who provided information on the owners of the donkeys and data on their treatment protocols. The last anthelmintic treatment applied consisted of 1% IVM applied orally, six months prior to the experiment. It is worth mentioning that the donkeys of these communities have been cared for by these doctors for more than 10 yr and they have never been administered any other family of anthelmintics or drugs.
Experimental design
Fifty-three (53) donkeys (Equus asinus) of either sex were selected within an age range of 3 mo to 25 yr. The experiment was divided into two stages. In the 1st stage, all 53 animals allowed the determination of the economic thresholds (explained below) in the ecological study area and the formation of the 2nd stage experimental groups on the pre-treatment day. In the 2nd stage, two experimental groups of 15 animals each were established: a treatment group (Tg) and a control group, without treatment (Cg). Following the methodology suggested by the World Association for the Advancement Veterinary Parasitology (WAAVP) 20-22, the animals were selected at random, provided that they had a minimum of 150 EPG. The ranges exhibited by each group were 150 to 2,850 HPG for the Cg, and 150 to 2,150 EPG for the Tg. On day zero, the Tg was administered 1% IVM orally, at the recommended dose of 0.2 mg/kg body weight21. In order to calculate the individual dose of the drug, the approximate body weight was obtained using “The Donkey Sanctuary” weight estimator23,24. Fecal samples were collected from both groups on days 7 and 14 post-treatment21.
Economic thresholds
Eggs per gram of feces (EPG)
In order to determine the EPG number, stool samples were obtained directly from the donkeys' rectum21,22. The samples were kept at 4 ºC until they were processed, using the modified McMaster technique20-22 at the Research Laboratory of the Department of Parasitology of the Faculty of Veterinary Medicine (FMVZ, Spanish acronym) of UNAM.
Body condition (BC)
The BC was obtained from the visual estimation of the animal, on a scale of 1 to 5, in increments of 0.5, using The Donkey Sanctuary's Body Condition Score Chart23.
Identification of genera of gastrointestinal nematodes
Stool cultures were performed in order to obtain the infecting larvae (L3), using the culture technique described by Figueroa et al25 and the larval migration technique with the Baermann device, with an incubation period of 10 d25. Subsequently, the L3 were collected and washed using the GIN larval cleaning technique by density gradients with 40% sucrose25,26. Finally, 100 L3 of the cultures from the pre-treatment day and from the 7th and 14th post-treatment days were identified (25,26).
Fecal egg count reduction test (FECRT)
The percentage of efficacy of the drug was determined using the FECRT, performed according to the guidelines of the WAAVP21,22,27. McMaster tests were performed on d 1 pre-treatment for the Tg (n= 15) on d 7 and 14 post-treatment for the Cg (n= 15).
Donkey owner surveys
An exploratory survey28,29 was conducted with 25 donkey owners. The questionnaire included questions about the medical care provided by the doctors, specifically deworming, as well as the type of confinement and feed given to their animals, with the aim of detecting management failures and causes that may generate AHR. In the event that the owner provided the complementary treatment to that applied by the VET, the survey considered issues such as frequency criteria, application procedures, and application of other anthelmintics 29.
Statistical analysis
The correlation data of BC and EPG were analyzed with the R TM statistical package30. The FECRT values were calculated using the RESOTM software31 according to the formula: Efficacy (%) = (Cg pretreatment EPG - Tg post-treatment EPG / Cg pretreatment EPG) x10020-22. A parasite population is considered susceptible to an AH when the efficacy percentage is higher than 95% and the lower limit of the 95% confidence interval is above 90%. AHR is considered suspicious when a population meets only one of the two criteria20-22,31.
Results
Economic thresholds
Eggs per gram of feces (EPG)
In the 1st stage, 98.1 % (n= 52) of the stool samples were positive for strongylid-type eggs. The EPG elimination range at this stage ranged from 0 to 3,000 EPG, as shown in Figure 1. Based on the distribution and on the estimated median value of 350 EPG and third quartile value of 600 EPG, it was determined that the economic threshold for donkeys in the Mexican High Plateau is 600 HPG.

Green bars= animals with <350 EPG;
Orange bars= animals with 350 to 600 EPG;
Red bars= animals with >600 EPG.
Figure 1 Distribution of egg elimination per gram of feces (EPG) in donkeys of the Mexican High Plateau
Body condition (BC)
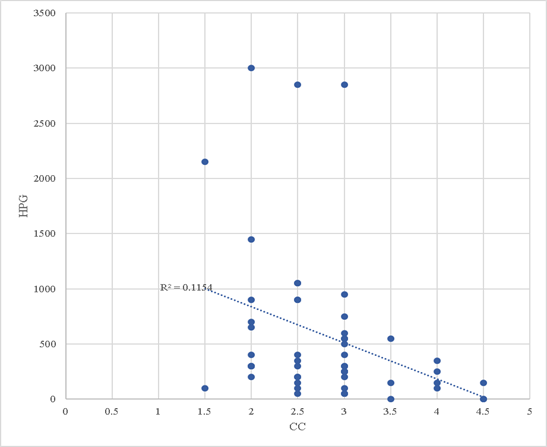
The BC of most of the animals (91 %) was acceptable (2.5 to 3.5); three animals exhibited values of 4.5 (overweight), and two, of 1.5 (poor), according to the reference estimate of condition and weight (2.3). In addition, a highly significant negative correlation (P= 0.01) was observed; thus, there is evidence to assume that at higher BC the egg discharge obtained is lower (P<0.05), with a 95% confidence interval. (0.07 to 0.56). The determination coefficient was 0.1154 (Figure 2). Therefore, for each increase of 0.5 in BC, the egg discharge is reduced by 0.33 %. However, this decrease in egg removal may vary within a range of 0.07 to 0.56 %.
Identification of gastrointestinal nematode Genera
One hundred (100) infective larvae were identified, of which 63 % were observed to be cyathostomidae (Figure 3). Small strongyles included species of the genera Poteriostomum, Gyalocephalus, and Oesophagodontus. The most frequent species among the larger strongyles was Strongylus edentatus, followed by S. equinus and S. vulgaris.
Test to determine the effectiveness of ivermectin FECRT
At the 2nd stage of the experiment, the results of the FECRT test indicated that the oral administration of 1% IVM had an efficacy of 99 % in the Tg on d 7 post-treatment, and of 100 % on d 14, with a 95% confidence interval; therefore, this parasite population is considered to be susceptible to treatment with 1% IVM. In contrast, the elimination range for the Cg was 100 to 850 EPG on d 14.
Donkey owner surveys
The average age of the animals, most of which were males (79 %) was 11.05 yr. Only 40 % of the animals coexist in pens, some of them in common accommodations in each community, during the dry season, when they do not carry out daily agricultural activities, although in times of sowing and harvesting, these animals are mostly housed in pens as a nocturnal enclosure, after the workday. In the case of grazing animals, the owner comments that the animals graze on land of his own property, used exclusively for planting such products as corn and beans. Eighty, 80 % admit to using parasite control as the only strategy that is provided by the volunteer medical service. The remaining 20 % did not know the name of the commercial product or the active ingredient, and they administer the product without knowing the weight of the animal or the appropriate dose. It should be noted that the owners indicate that the treatment is applied during the period prior to the rains, or up to twice a year (Table 1).
Table1 Responses to the survey on deworming practices carried out on donkey owners in the Mexican High Plateau
| Question | % | (n) | |
|---|---|---|---|
| What kind of management do you give to your animals? | Grazing | 36 | 9 |
| Night confinement | 24 | 6 | |
| Pen | 40 | 10 | |
| Where do they graze? | Exclusive plot | 36 | 9 |
| Shared plot | 20 | 5 | |
| Number of animals/pen | Average | NA | 1.3 |
| In addition to the services provided by VET, do you deworm your animals? |
Yes | 20 | 5 |
| No | 80 | 20 | |
| What product do you use? | Does not remember | 8 | 2 |
| “Paste” (does not know the name) | 12 | 3 | |
| Do you check the expiration date? | Yes | 4 | 1 |
| No | 16 | 4 | |
| Which is the dose of the product you regularly apply? | Does not know/ Does not remember | 12 | 3 |
| By “cm”/ The whole product | 8 | 2 | |
| Route of administration | Oral | 20 | 5 |
| Intramuscular | 0 | 0 | |
| Subcutaneous | 0 | 0 | |
| Does not know | 0 | 0 | |
| Who applies the product? | ZVD | 8 | 2 |
| Owner | 12 | 3 | |
| Other | 4 | 1 | |
| Weighing | Yes | 0 | 0 |
| No | 20 | 5 | |
| Frequency | As recommended by the VET | 0 | 0 |
| Semestral | 12 | 3 | |
| Annual | 8 | 2 | |
| When do you practice this management? | Previous to breeding time | 0 | 0 |
| Before birth | 0 | 0 | |
| Before the rainy season | 20 | 5 | |
| After the rainy season | 0 | 0 | |
| Other | 0 | 0 |
Discussion
At present, no studies of donkeys of the Mexican High Plateau exist showing the population dynamics measured in the seasonal variation of EPG elimination, like those previously performed in Argentina32, and including the identification of parasitic species in the area; nor are there studies on the economic threshold of EPG typical of the ecological study area indicating whether the animal is a candidate for deworming or not, like for other species33,34, or on the potential relationship between thresholds such as the EPG and the BC of donkeys in the ecological study area.
Parasitic diagnosis in donkeys is poor, and therefore the establishment of thresholds has not been a priority. In previous works2, a median of 600 EPG was observed, and specifically in a tropical ecological zone, a median of 1,000 EPG was determined, confirming the differences that exist between each zone. These variations speak, above all, of the epidemiology of parasites; hence the importance of defining economic thresholds for EPG in particular.
In order to determine the economic threshold criteria for EPG, this study identified the median of the distribution as 350 EPG, which means that 50 % of the animals are routinely dewormed. This involves an expensive and unnecessary deworming program, as some do not require the medication. The challenge here begins with the so-called phenomenon of superdispersion, in regard to which several authors35,36 agree that only 20 to 25 % of the animal population are infected with parasites (as they are great eliminators) and are likely candidates for treatment. Also estimated was the value of the third quartile (75 %), which was 600 EPG, considered the economic threshold of EPG in donkeys of the Mexican High Plateau. This would indicate that only 25 % of this population would be a candidate for treatment, as long as they presented other criteria of economic thresholds that reflect an apparent parasitosis. Another aspect to be assessed by this study of the dispersion of the GIN is that the donkeys represented by the green and orange bars indicate the GIN-resistant population, and those animals represented by the red bars that do not exhibit clinical signs or depression rare the GIN-resilient population. These two resistant and resilient populations are most probably the relevant and redeemable refuge population in the face of the phenomenon of AHR, which are crucial concepts for understanding the phenomenon of parasitism and its hosts37. Considering also the ecological zone, the elimination phenomenon observed in the Cg stands out, which after 14 d reduced the EPG value to a range of 100 to 850 when before the treatment it was 150 to 2,850. One hypothesis with regard to this involves the role of endemic plants with bioactive components against GIN, studied in other species38, as well as the hypobiosis phenomenon, discussed below.
Cyathostomidae have gained importance due to the encystment of larvae in the intestinal submucosa and of hypobiotic larvae39-41. At the same time, it is known that in countries where in the autumn and winter period, when this study was carried out, is marked, the larvae that may exist in the intestinal mucosa are undetectable21,39; therefore, knowledge of the annual dynamics of the parasite population may provide a better overview of the existing nematodoses and allow the development of strategies for their treatment.
It is worth mentioning that the BC obtained in 91 % of the donkeys has acceptable values of 2.5 to 3.5, which indicates that the animals, despite their environmental conditions, express the rusticity that characterizes them and withstand parasitosis3 , a phenomenon that helps reaffirm why not all donkeys require treatment under fixed schemes. On the other hand, when the correlation was made between the values of the BC and the EPG, it was observed that there is an apparent reduction of 0.33 % of the EPG when the BC increases by 0.5. This study defined that the EPG, the BC and their close relationship are valuable thresholds in the management of gastrointestinal nematodosis in donkeys. It is important to note that, although there are studies where a relationship between the BC and the parasite load in donkeys is not observed2,3,23. Yoseph et al40 suggest the measurement of this economic threshold, as long as it is met with a high-quality, forage-based diet and proper management of an anthelmintic treatment.
As parasitoses are one of the diseases that go unnoticed by the owner, several authors41 mention the importance of reestablishing the association between doctor and producer/owner for the design of parasite management and control strategies, together with the growing concern about AHR in Mexico, due to the constant and indiscriminate use of AH. This happens mainly with IVM.
In this study, the effectiveness of IVM was found to be 100 %. However, there are studies42,43 in which it is mentioned that, since the tests for the effectiveness of AH in equines have not yet been completely standardized, there may be a margin where the validation of a resistant population requires additional tests. Other authors44 also highlight the importance of developing comparative tests such as the LMIT (larval migration inhibition test), in which the sensitivity to IVM of the population of cyathostomidae present in equines can be known. As a complement to this study, the assessment of the ERP (egg reappearance period) after treatment is suggested, since, although the FECRT indicates that the parasite population is 100 % sensitive to the assessed AH, it has been observed that this threshold may expose a latent resistance process. In equines, the observed ERP was 8 to 13 wk for IVM. At The Donkey Sanctuary, in the UK, this ERP in donkeys that have had little exposure to the drug is 6 wk44 and has probably diminished due to the development of "juvenile" specimens that were not eliminated during the treatment45. The ineffectiveness of the drug against hypobiotic larvae may trigger AHR, due to their constant exposure to IVM; therefore, the selection pressure of the parasite is increased, resulting in a low ERP, and therefore resistant species in the next generation44. Further studies are required in order to verify this.
It is not possible to speak of the effectiveness of a drug or AHR if the practices that owners or veterinarians carry out with respect to deworming are not evaluated in parallel. In this study, probable causes of AHR and potential failures in deworming were detected through surveys.
The entire donkey population was treated, including seemingly healthy individuals. This increases the selection pressure of the parasites, making subsequent generations resistant and reducing the existence of refuge hosts within the population; for this reason, reserving such a valuable resource as IVM for those animals that actually require treatment prevents the generation of new species without sensitivity to the drug. This same AH was dosed without knowing the exact body weight of the animal and was utilized as a systematic or suppressive treatment (e. g. twice a year). These actions involve the risk that parasites may acquire AHR without efficient elimination or control and transmit it genetically to their offspring. A clear example is the reduced action of the IVM against hypobiotic larvae, where the selection pressure on the parasite is increased. The owner's ignorance regarding the applied products and the adequate doses, or the aim of management and its correct implementation, as mentioned above, may result in unremarked treatments and in drug underdose or overdosing.
Thus, the implementation of new parasite control tools, which focus on reducing the selection pressure of parasites for various AH, has become one of the main requirements of parasite management41. In response to this need, different tools are being integrated into a management and control strategy. IPC aims at slowing the growth of parasitic populations with a high proportion of individuals genetically resistant to one or more AH46. The scheme proposes to integrate various principles based on the parasitic population dynamics of a known herd or stable. This scheme includes the TST16, which is perceived as viable in this region.
Conclusions and implications
Although the development of resistance to IVM is not yet present, it has been observed that these communities are at a critical point; therefore, the correction of failures in anthelmintic treatment and the prevention of probable causes of AHR can help doctors, together with the owners, to take care of a unique resource such as this AH through the correct and rational use of new molecules available in the market, the evaluation and the obtainment of their own thresholds, and the development of tools for their implementation, setting the precedent of awareness of the correct use of AHs, and thus improving the quality of the welfare of donkeys.
Acknowledgements
To Veterinarian Omar Prado Ortiz for the valuable technical help received during the development of this research.
REFERENCES
1. The Donkey Sanctuary. Svendsen ED [compilador]. Manual Profesional del Burro. Reino Unido: Whittet Books; 1997. [ Links ]
2. Burden F, Du Toit N, Hernández Gil M, Prado Ortiz O, Trawford AF. Selected health and management issues facing working donkeys presented for veterinary treatment in rural Mexico: some possible risk factors and potential intervention strategies. Trop Anim Health Prod 2010;42:597-605. [ Links ]
3. Burden F, Thiemann A. Donkeys Are Different. J Eq Vet Sci 2015; 35:376-382. [ Links ]
4. Corning S. Equine cyathostomins: a review of biology, clinical significance and therapy. Parasites & Vectors 2009;2(suppl 2):S1. [ Links ]
5. Canever RJ, Braga PRC, Boeckh A, Gycajuck M, Bier D, Molento MB. Lack of Cyathostomin sp. reduction after anthelmintic treatment in horses in Brazil. Vet Parasitol 2013;194:35-39. [ Links ]
6. Taylor MA, Coop RL, Wall RL. Veterinary Parasitology. 4a ed. Oxford, Reino Unido: Wiley-Blackwell; 2015. [ Links ]
7. ITIS. Integrated Taxonomic Information System. Encyclopedia of Life (EOL). https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search _value=63957#null . Consultado 1 Mar, 2017. [ Links ]
8. Matthews J, Burden F. Common helminth infections of donkeys and their control in temperate regions. Eq Vet Ed 2013;25:461-467. [ Links ]
9. Grosenbaugh DA, Reinemeyer CR, Figueiredo MD. Pharmacology and therapeutics in Donkeys. Eq Vet Ed 2011;23:523-530. [ Links ]
10. Vercruysse J, Claerebout E. Treatment vs non-treatment of helmint infections in cattle: defining the threshold. Vet Parasitol 2001;98:195-214. [ Links ]
11. Kaplan R, Vidyashankar A. An inconvenient truth: Global worming and anthelmintic resistance. Vet Parasitol 2002;186(1-2):70-78. [ Links ]
12. Nari A, Eddi C. Control Integrado de las parasitosis. Reunión de especialistas en parasitología Veterinaria de Argentina, Brasil, Chile, Paraguay y Uruguay. Tandil, Argentina; 2002. [ Links ]
13. Torres Acosta JFJ, Hoste H. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Ruminant Res 2008;77:159-173. [ Links ]
14. González Garduño R, Torres Hernández G, López Arellano ME, Mendoza-de-Gives P. Resistencia antihelmíntica de nematodos parásitos en ovinos. Rev Geo Ag 2012; 48(49): 63-74. [ Links ]
15. Rosado-Aguilar JA, Flota-Burgos GJ, Rojas-Becerril R, Trinidad-Martínez I. Frequency and ivermectin resistance of gastrointestinal nematodes in equine farms from Mexico [summary].13th Int Cong Parasitol. Mexico. 2014. [ Links ]
16. Van Wik J, Hoste H, Kaplan RM, Besier RB. Targeted selective treatment form worm management-How do we sell rational programs to farmers. Vet Parasitol 2006;139:336-346. [ Links ]
17. Berk Z, Laurenson YCSM, Forbes AB, Kyriazakis I. Modelling the consequences of targeted selective treatment strategies on performance and emergence of anthelmintic resistance amongst grazing calves. IJPl: Drugs and Drugs Resistance 2016;6:258-271. [ Links ]
18. Martínez Ortiz-de-Montellano C, Torres Acosta JFJ. Control integrado de los nematodos gastrointestinales en rumiantes. Consejo Técnico Nacional de Sanidad Animal. Tuxtla Gutiérrez, Chiapas. 2011. [ Links ]
19. Vineer HR, Vande Velde F, Bull K, Claerebout E, Morgan ER. Attitudes towards worm egg counts and targeted selective treatment against equine cyathostomins. Prev Vet Med 2017;144: 66-74. [ Links ]
20. Duncan JL, About EM, Arundel JH, Eyseker M, Lei TR, Krecek RC et al. World association for the advancement of veterinary parasitology (WAAVP). Second ed. Guidelines for evaluating the efficacy of equine anthelmintics. Vet Parasitol 2002;103(1-2): 1-18. [ Links ]
21. Wood IB, Amaral NK, Bairden K, Duncan JL, Kassai T, Malone JB et al. World Association for the advancement of veterinary parasitology (WAAVP). Second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet Parasitol 1995;58:181-213. [ Links ]
22. Geurden T, van Doorn D, Claerebout E, Kooyman F, De Keermaecker S, Vercruysse J et al. Decreased strongyle egg re-appearance period after treatment with ivermectin and moxidectin in horses in Belgium, Italy and The Netherlands. Vet Parasitol 2014;204(3-4):291-296. [ Links ]
23. The Donkey Sanctuary. Condition scoring and weight estimation. Devon, Reino Unido: The Donkey Sanctuary. 2013. [ Links ]
24. The Donkey Sanctuary. Condition scoring and weight estimation. Devon, Reino Unido: The Donkey Sanctuary 2014; Devon, Reino Unido: The Donkey Sanctuary 2014; https://www.thedonkeysanctuary.org.uk/sites/sanctuary/files/document/142-1423234830-donkey_health_and_welfare.pdf . Consultado Mar 30, 2017. [ Links ]
25. Figueroa JA et al. Examen coproparasitoscópico. En: Rodríguez Vivas RI, editor. Técnicas para el diagnóstico de parásitos con importancia en salud pública y veterinaria. 1ª. ed. México: AMPAVE. Consejo Técnico Consultivo Nacional de Sanidad Animal. 2015:101-118. [ Links ]
26. Zajac MA, Conboy GA. Veterinary Clinical Parasitology. 8a ed. USA: Am AssocVet Parasitol . 2012. [ Links ]
27. Cabaret J, Berrag B. Faecal egg count reduction test for assessing anthelmintic efficacy: average versus individually based estimations. Vet Parasitol 2004;121:105-113 [ Links ]
28. López Romo H. La metodología de encuesta en Galindo Caceres LJ, coordinador. Técnicas de investigación en sociedad, cultura y comunicación. México: Logman; 1998:83-93. [ Links ]
29. Alegría López MA, Rodríguez Vivas RI, Torres Acosta JFJ, Ojeda Chi MM, Rosado Aguilar JA. Use of Ivermectin as endoparasitide in tropical cattle herds generates resistance in gastrointestinal nematodes and the tick Rhipicephalus microplus (Acari: Ixodidae). J Med Entomol 2015;52:214-221. [ Links ]
30. R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2018. ISBN 3-900051-07-0, URL http://www.R-project.org. [ Links ]
31. Rodríguez Vivas RI [editor]. Técnicas para el diagnóstico de parásitos con importancia en salud pública y veterinaria. 1a Ed. México: AMPAVE / Consejo Técnico Consultivo Nacional de Sanidad Animal. 2015:355-403. [ Links ]
32. Fusé L, Saumell CA, Iglesias L. Variación estacional del parasitismo interno en equinos: Fenómeno de hipobiosis de los pequeños estrongílidos (Cyathostominae) en Tandil, Buenos Aires, Argentina. Rev Med Vet 2013;94(3):62-72. [ Links ]
33. Martinez Ortiz-de-Montellano C, Cervantes Morali J, Figueroa Castillo JA, Torres Acosta JFJ. Distribution of gastrointestinal nematodes egg counts in sheep from Mexico Center south and its implications of parasitic integrated control. Int Conf World Assoc AdvancVet Parasitol . Kuala Lumpur, Malasia; 2017. [ Links ]
34. Martínez-de-Castro Dubernard A, Dominguez Hernández Y, Quiroz Rocha G, Saldaña Hernandez N, Ojeda Robertos N et al. Importance of egg per gram treshold determination in goats from Mexican plateau: To deworm or not to deworm?. Int Conf World Assoc AdvancVet Parasitol . Kuala Lumpur, Malasia; 2017. [ Links ]
35. Sréter T, Molnár V, Kassai T. The distribution of nematode egg counts and larval counts in grazing sheep and their implications for parasite control. Int J Parasitol 1994; 24(1):103-108. [ Links ]
36. Hoste H, Le Frieleux Y, Pommaret A. Distribution and repeatability of feacal egg counts and blood parameters in dairy goats naturally infected with gastrointestinal nematodes. Res in Vet Sci 2011;70(1):57-60. [ Links ]
37. Van-Houtert MFJ, Sykes AR. Implications of nutriction for the ability of ruminants to withstand gastrointestinal nematode infections. Int J Parasitol 1996;26(11):1151-1167. [ Links ]
38. Peachey LE, Pinchbeck GL, Matthews JB, Burden FA, Mulugueta G, Scantlebury CE et al. An evidence-based approach to the evaluation of ethnoveterinary medicines against strongyle nematodes of equids. Vet Parasitol 2015;210:40-52. [ Links ]
39. Zachary JF. Pathologic basis of Veterinary diseases. 6th ed. USA; 2017. [ Links ]
40. Yoseph S, Smith DG, Teklu F, Firew T, Betere Y. Seasonal variation in the parasite burden and body condition of working donkeys in East Shewa and West Shewa Regions of Ethiopia. Trop Anim Health Prod 2005;37(1):35-45. [ Links ]
41. Statford CH, McGorum BC, Pickles KJ, Matthews JB. An update on cyathostomins: Anthelmintic resistance and diagnostic tools. Eq Vet J 2011;43:133-139. [ Links ]
42. Molento MB, Nielsen K, Kaplan RM. Resistance to avermectin/milbemycin anthelmintics in equine cyathostomins - Current situation. Vet Parasitol 2012;185:16-24. [ Links ]
43. Mejía ME, Fernández Igartúa BM, Schmidt EE, Cabaret J. Multispecies and multiple anthelmintic resistance on cattle nematodes in a farm in Argentina: the beginning of high resistance?. Vet Res 2003;34:461-467. [ Links ]
44. McArthur CL, Handel IG, Robison A, Hodgkinson JE, Bronsvoort B, Burden F. et al. Development of the larval migration inhibition test for comparative analysis of ivermectin sensitivity in cyathostomin populations. Vet Parasitol 2015;212:292-298. [ Links ]
45. Lyons ET, Tolliver SC, Collins SS. Probable reason why small strongyle EPG counts returning “early” after ivermectin treatment of horses on a farm in Central Kentucky. Parasitol Res 2009;104(3):569-574. [ Links ]
46. Waller PJ, Echevarría F, Eddi C, Maciel S, Nari A, Hansen JW. The prevalence of anthelmintic resistance in nematodes parasites of sheep Southern Latin America: General overview. Vet Parasitol 1996;62:181-187. [ Links ]
Received: October 05, 2018; Accepted: April 05, 2019











 text in
text in