Introduction
This review has been divided into two parts. Firstly, a brief review is made of some anatomical and physiological aspects of the reticular groove in ruminants, as well as the techniques used to study the functioning of the reticular groove closure reflex. In the second part, those aspects that may stimulate or inhibit the reticular groove reflex, and the manipulation techniques of this reflex, as well as their applications in veterinary medicine.
Gastric groove is an anatomical structure, located in the stomach of ruminants. It extends from the orifice of cardia until near the pylorus, through the lesser curvature of reticulum, omasum and abomasum. It is divided into three segments: reticular groove (Sulcus reticuli), omasal groove (Sulcus omasi) and abomasal groove (Sulcus abomasi)1-3. While some authors only consider two structures: the reticular groove and the omasal groove, ranging from the cardia to the omasoabomasal orifice.
Various names have been used for this structure, such as reticular leak, gastric groove, gastric channel, oesophageal channel or oesophageal fluting. The scientific nomenclature represented by the Nomina Anatomica Veterinaria4 includes the Latin term "Sulcus reticulari".
The mechanism of the oesophageal groove is a primary, almost exclusive of lactating animals; which provides ruminant animals the possibility of a physiological gradual adaptation from monogastric to ruminant stomach. When stimulated, muscular tissue contracts, adopting a hollow structure forming a duct along the wall of the reticulum, which connects the esophagus (cardia) to the reticulo-omasal orifice5. The reticulo-omasal orifice remains open allowing the flow of milk5-7, which is of great interest in new born animals, as it allows the colostrum and milk to pass directly to the abomasum, without falling into the rumen and reticulum, thereby preventing abnormal fermentation. In the early hours of the beast, this powerful reflex allows immunoglobulins from colostrum to pass into the duodenum, where thanks to the high permeability of its mucosa it will be quickly absorbed in order to develop passive immunity that protects the animal from pathogens coming to its digestive tract. Besides, the high energy value of colostrum will provide sufficient energy to the animal to combat possible hypothermia and, since it has a high content of magnesium, with laxative action, it will help to expel meconium and facilitate the start of intestinal transit8,9. The activity of the reticular groove decreases after weaning and as the age of the animal advances, but can be triggered under certain conditions in the adult animal9.
Anatomical review
Embryology
The ruminant stomach represents the highest evolutionary development of all mammal species10. It originates from a fusiform dilatation of the primitive gut of the embryo, called primitive stomach. From the lesser curvature derives the reticular, the omasum and the abomasum grooves. Conversely, from the greater curvature derive the rumen, reticulum and greater curvature of abomasum1.
Differentiation of the reticular groove is early in sheep and goat and latter in cattle, showing in this last case at eight weeks of embryonic development. Molinari and Jorquera11 report that the onset of the leak in the foetuses of ruminants is simultaneous with the differentiation of the rumen and reticulum rudiments. Accordingly, the rotations experienced by these rudiments will affect the reticular groove, passing from a foetal position parallel to the axis, in the right reticular wall, to take a vertical orientation. Thus its forms an angle of 50⁰ with the main axis, developing finally a spiral structure of 180⁰.
Postnatal development
After birth, proventriculus development depends on the animal feed. At the beginning of the life of the ruminant abomasum size is slightly larger than all the proventriculus. Subsequently when diet begins to be solid, these rapidly increase their size. This development can be divided into three stages8,9.
─ From birth to 3rd week of life, the animal is considered a "non-ruminant" because its diet is exclusively dairy. High blood glucose is due to absorption of nutrients (glucose) intestine, and hence the carbohydrate metabolism is typical of a "non-ruminant".
─ Between 3 and 8 wk of life it is considered a transition period. The animal eats small amounts of solid food. Blood glucose decreases and the plasma concentration of volatile fatty acids increases, similarly to the levels of the adult animal.
─ An 8-wk-old animal will be considered as a "true ruminant". This does not occur in those cases when an animal continues exclusively breastfeeding, in which case the proventriculus remain rudimentary till 14 or 15 wk of age (Figure 1).
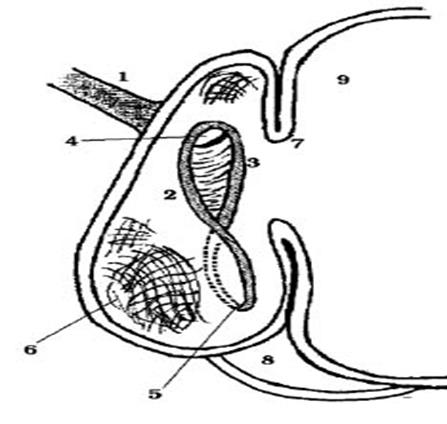
Figure 1 Reticular groove. 1: oesophagus, 2: left lip, 3: right lip, 4: cardia, 5: reticulo-omasal orifice, 6: reticular fundus, 7: ruminoreticular fold, 8: abomasum 9: rumen (Figure taken from Pochón, 2002).
Topographic locatio
The reticular groove is located in the area formed by the intersection of two imaginary lines. The first, vertical, extends, from the eighth thoracic vertebra to the costochondral junction of the left seventh rib. The other, horizontal, connects the middle third of the left seventh rib and right seventh rib. It is located between the seventh and ninth left rib and the rumen is internally in contact with the rumino-reticular content10.
Structure
The gastric groove consists of three different parts. The first is the "reticular groove" which is formed by two longitudinal muscular folds or relieves, called right and left lips, and the sulcus of gastric groove. It starts in the cardia, descends in spirals across the lesser curvature of the reticulum (right wall) in caudal direction to the left, continues dextrocaudally to the reticulo-omasal reversing in turn the position of both lips. The right lip describes a rotation around the left one, in clockwise direction; to return back to its initial position to the right, taking the muscle fibers a corkscrew disposition1,2.
From the reticulo-omasal to the omaso-abomasal orifice the gastric groove is called "sulcus of omasum". It runs through the lesser curvature of the omasum. This sulcus is interrupted by a transverse fold (omasum pillar) formed by the convergence of circular muscle fibers that reinforce the omaso-abomasal orifice. The "abomasal veils" mucosal folds of transition, extend from the abomasum to the fore mentioned pillar. In bovine they are lined by tegument from the omasum, while in sheep, both abomasal and omasal face veils are fully glandular(1, 2). The last portion of gastric groove is the "abomasal groove" which runs along the lesser curvature of the abomasum, without ridges and ending at the pyloric part1.
Innervation of the gastric groove
The control mechanism of the reticular groove is not entirely clear. It is believed to be due to the interaction between a central control and a local control6,12,13. Central control of motility occurs via the vagus nerve13. Local control can be attributed to the myenteric plexus, but the function of this plexus is little known14.
In general, the innervation is provided by the parasympathetic and sympathetic divisions of the autonomous nervous system. The parasympathetic division, efferent innervation of the stomach, consists of the ventral and dorsal vagal trunks, which accompany the oesophagus through the hiatus10. This efferent branch has excitomotor effects on the reticular groove and inhibitors of the motility of the rumino-reticulum10.
The afferent trigeminal nerve, whose stimuli are albumin, glucose and minerals in milk, copper in ovine and sodium in bovine, reinforced by cortical afferents and the effect produced by suction15. The trunks of the vagus nerve include viscerosensitive and motor afferent fibers, which will intervene in gastric reflexes through medullary centers, which in turn are influenced by the cerebral cortex and hypothalamus1,3. Schenk-Saber et al16 have shown that in adult goats and sheep the afferent nerve endings are highly developed in the lining of the reticulum, near the left lip of the reticular groove. These endings, rod, button or arrowhead-shaped, receivers are very important in regulating food passage and intake.
Various biologically active peptides, including the vasoactive intestinal peptide (VIP), have been found in neurons of the digestive tract of ruminant’s newborns and adults. It is believed that VIP plays a role in mediating non-adrenergic and non-cholinergic relaxation of reticulo-omasal orifice and the abomasum during the act of sucking7,12. This is accompanied by an increase in the concentration of VIP in the gastric and intestinal venous blood7. Reid et al7 found that an arterial infusion of VIP produces a relaxation of the reticulo-omasal and omaso-abomasal orifice, similar to that produced during the act of suckling10.
Blood supply
The blood supply of the stomach of ruminants, comes from the celiac artery, which is divided into various main branches. Gastric furrow irrigation is provided by the left gastroepiploic artery, reticular accessory artery and reticular branches of the reticular artery (coming from the left ruminal artery). Dorsal and ventral arteries of the reticular groove originate from the reticular artery; supplying the two lips of reticular groove. The veins run parallel to the arteries and drain into the portal vein. The splenic vein, important branch of the portal vein, assures the drainage of the reticular groove. Blood vessels are accompanied by right, left and cranial ruminal lymph node chains, which sometimes may be missing. Auxiliary lymphatic organs are nodes located above the bottom; there are reticulo-omasal and the atrium nodules as well10.
Histology
According Pochon10 the structural constitution of reticular groove walls comprises four layers:
─ Serous tunic, composed of connective tissue (collagen and elastic fibers) covered by mesothelium.
─ Muscular layer of mixed origin (smooth and striated). On the lips of the groove the muscular tissue is smooth with its fibers arranged longitudinally17. On the ground there are two layers of muscle tissue: the outer layer consists of smooth and striated fibers in longitudinal arrangement and the inner layer comprises only smooth muscle and its fibers are arranged perpendicularly to the longitudinal axis of the groove. The myenteric plexus is located between the two layers9,17.
─ Submucosal tunic, consisting of connective tissue (collagen and elastic fibers), where the submucosal plexus is located9.
─ Mucosal layer, composed by a stratified squamous epithelium, the muscularis mucosa and lamina propria. It has longitudinal folds on the lips, and its epithelium is darker and folded. Unguiculiform papillae, thick conical buds, with cornified epithelium, slightly curved and even twisted from the base, are found near the reticulum-omasal orifice10.
The existence of mucosal glands, especially in adult animals, and serous and mixed type in pre-ruminants (newborns) has been demonstrated.
Reticular groove physiology
Ruminants begin life similarly to monogastric animals in all matters referred to digestion, absorption and metabolism of the main nutrients. Thus, the drink consumed to pass directly to the abomasum, preventing its penetration in rumino-retículum18, where the processes of coagulation of casein by rennet action are produced. The transition from the abomasal contents, in a first moment liquid moment, and subsequently solidificated, is slowed so as to allow the action of pepsin and lipase enzymes to reduce protein and lipid to a more suitable form for intestinal digestion10,19. Alterations in the function of the reticular groove make lots of milk falling into a still immature rumen cavity which will cause significant digestive disturbances short and/or long-term19.
The motility of the reticular groove is initiated by the contraction of smooth and striated fibers of the muscular layer by two movements. The first one, shortening, occurs by joining the right and left lip, allowing direct passage of 30 to 40 % of the liquid volume to the abomasum. The closure is completed with a wave of investment in rotation around the axis that runs along the length of the right lip, allowing the passage of 75 to 90 % of liquid ingested to the abomasum. The reflex of the reticular groove also acts on other organs, being accompanied by transient inhibition of the reticulum and rumen contractions during breastfeeding action15,17, expansion of the reticulo-omasal orifice, opening omasal groove and abomasal distention5,10.
Denac et al14 studied the effect of vasoactive intestinal peptide (VIP) in muscle fibers incubated in an organic solution derived from the reticular groove, reticulo-omasal orifice and omasal cannel in calves and adult cows. Mechanical muscular activity was quantified by isometric and isotonic transducers. They noted that a relaxation of the longitudinal and circular muscle fibers of the reticular groove occurred, with lowest effect on adult cows, possibly by an involution of specific VIP receptors in the muscles of the reticular groove. Muscle relaxation of the reticulo-omasal and the longitudinal and circular fibers of omasal channel orifice in calves are also produced, the latter being less sensitive to the VIP than those of the reticular groove. Finally they concluded that VIP plays a role in the function of the reticular groove especially in the lactating calf, as an inhibitory, non adrenergic and non cholinergic transmitter.
Triggering the closure reflex of the reticular groove can occur by central or peripheral stimuli9. The reflex is initiated by the action of sucking and drinking, by stimuli produced by the sight of a bottle or food preparations. They do not seem to be affected by neither the type of liquid (water, whole milk, skim milk or whey), nor by the temperature of the milk, nor by the position taken during suction9. The reflex comes exclusively from the abomasum9. Contrary to this conclusion, Pochón10 highlights that the temperature of ingested fluid plays an important role in the closing reflex of the reticular groove, being the response more effective when the fluid is offered at body temperature.
The afferent fiber comes from the posterior region of the oral cavity after stimulation of oral and pharyngeal receptors activated by mechanical stimuli and certain substances, including some milk components20. These stimuli are transmitted to the bulbar nucleus via trigeminus nerve. The efferent pathway is formed by cholinergic parasympathetic fibers of the abdominal dorsal vagus nerve that acts stimulating reticular groove lips and inhibiting the motility of proventriculi8,9. When the pharyngeal receptors are not activated properly food is transferred to rumen and reticulum8. In the adult animal this reflex, which is vestigial, can be triggered under certain conditions as after severe water deprivation, dehydration or increased plasma osmolality21. In response antidiuretic hormone is secreted from the neurohypophysis, causing the reticular groove closure. So when the animal drinks water, it goes directly to the abomasum and small intestine to promote its rapid absorption, bypassing the reticulum and the rumen9.
The central nervous influence is demonstrated by the ability of conditioning this reflex by viewing, for example, a bottle in an adult animal22. Thus the persistence of this reflex in the adult animal depends on its handling.
Techniques for studying how it works
The attitude of the animal when it extends the neck, shakes its tail and shows an evident satisfaction when suckling vigorously, can give us an idea of progress in the reflex23. There are multiple experimental methods to study the functioning of the reticular groove in ruminants. A simple classification can be made into direct and indirect methods.
Direct methods
They are those that enable the study of the reticular groove by direct observation from outside the animal. Wester24 performed a surgical window on the left abdominal wall and the rumen calling it "ruminal fistula". Through this and by direct palpation of the lips of the groove their functioning can be perceived. The aid of a speculum and a light bulb enable a direct observation of its movements25,26.
Laparoscopic techniques. Endoscopy equipment has been used for displaying behaviour of reticular groove in ruminants. This entails making a ruminal fistula, introducing the endoscope fiber optic, and performing the extraction of residue content in the rumen and reticulum (by vacuum pumps or suction) to facilitate visibility of the reticular groove. Cinotti and Gentile27 and González-Montaña et al28 have visualized the stimulation of reticular groove though this method.
Indirect methods
They are based on tracking a known substance after being administered to the animal, which will make it possible to estimate the behaviour of the reticular groove.
( Animal sacrifice. It is the oldest known method, which consists in the administration of milk and subsequent slaughter of the animals to inspect the contents of the organs10.
( Use of coloured substances. Substances like methylene blue and eosin have been used, to stain the food and in turn the mucosa of the affected organs29. Ross30 describes the distribution of coloured solutions in the proventriculi and abomasum after necropsy. Also, it is possible to consider how long the dye takes since its intake from food to appearance in stool. It has been found that the dye passed through the reticular groove could be found in stool within 12 hours after being ingested, however this period was lengthened when it fell in the rumen and reticulum.
( Ruminal fistula and sampling of its contents. It consists in providing a liquid with a marker substance in order to observe its presence or absence in the rumen depending on the state of the groove. It also enables the removal of fluid entered through the rumen fistula31. Mikhail et al26 and González-Montaña et al28 used methylene blue as marker dissolved in water orally administered to animals.
( Abomasal fistula. This surgical technique also allows inspection of the path followed by food. If the reticular groove closure occurs, liquids administered to animals are immediately obtained through the abomasal fistula26.
( X-Ray and contrast agents. It is an old method but today it is still used. X-rays, mainly laterolateral, are taken after ingestion of liquid food with a radio-opaque (barium sulphate) dye to evaluate the performance of the reticular groove13,32,33.
At present solid radiopaque markers are used (polyethylene spheres of 1.5 mm diameter impregnated in barium "BIPS") as a contrast medium in the fluoroscopic proventriculi and abomasum of sheep34. These spheres are often used in companion animals in the diagnosis of gut motility disorders and intestinal obstructions. Poppi et al35 established a critical particle size of 1.2 mm in diameter, above which they cannot pass to the omasum and abomasum.
( Level of blood glucose and xylose. This method is based on the administration of food with glucose and determination of glucose or xilosemia in a blood plasma sample36. The amount of glucose administered varies among different authors, Encinas et al37 used a glucose concentration of 0.625 g/kg BW, unlike others26 using approximately twice the dose of glucose solution (1 g/ml/kg BW). If the reticular groove closure occurs food is directly absorbed from the abomasum in no more than 30 to 60 min, the glycaemia peak appearing 60 to 90 min after ingestion. On the contrary if the glucose enters the rumen, it will be degraded by the microflora to volatile fatty acids, increasing slowly its concentration in plasma8,9,33,38,39. Some works estimate that the reticular groove closes when the glucose peak appears in blood between 5 to 15 min postadministration26,37.
Sargison et al34 suggest that the use of glucose and xylose as markers may be incorrect due to the effects of stress management on the concentration of carbohydrates. Test of xylose absorption was performed to assess the degree of closure of the reticular groove after administration of copper sulphate in sheep. It is based on stimulating the groove and then the application by catheter of D-xylose (0.5 g/kg BW). If the closure appears it will cause an elevation of concentration of xylose in blood8,9. When xylose is directly applied into the rumen through a rumen fistula, it is fermented and is not detected in blood, but in cases when it was administered orally in the absence of reflex in the groove, the xylose solution reached the reticulum and rumen and near the reticulum-omasal orifice, went to the omasum, abomasum and intestine from which it was absorbed39. Stimulation of the reticular groove causes that when oral administration by catheter 500 ml of a 10% glucose solution takes place, a significant increase in blood sugar occurs, which is found in blood samples taken at 15 min post-application28. However, this increase is very small when stimulation of the reticular groove has not been successful.
( Electromyography. It is based on the implantation of electrodes into the muscular wall of the proventriculi, and the detection, amplification and recording of the action potential generated by the smooth muscle fibers. After stimulation of reticular groove, motility of the rumen and omasum ceases, by contrast the reticulum has small shocks38.
( Determination of strontium and chromium. Strontium chloride (SrCl2) and chromic oxide (Cr2O3) are two markers that, when taken with liquid food, are soluble and its concentration can be determined both in the rumen contents (strontium) and organ mucosae (chrome). Hedde and Ward31 used strontium (5 mg/kg BW) to evaluate the effectiveness of "ruminal bypass" in calves using various routes of administration.They noted that the reflex of groove was complete in bottle-fed animals and strontium was not detected in rumen samples. However, in those calves receiving strontium in drinking water recovery from the rumen of this substance was complete.
( Implantation of thermocouples. It is based on the use of thermocouples catheters housed by laparotomy in the rumen and in the abomasum to measure the temperature with a temperature sensitive device. When the reticular groove closure occurs, a variation in temperature is detected before in the sensor housed at abomasal level than at the one implanted in the rumen23.
( Test based on the detection of carbon 13 of octanoic acid excreted in breath. It is based on identifying a substance with a known reaction, through an enzyme that generates the reaction, wherein as a final part is released 13CO2, which diffuses to the blood, is transported to the lungs, and are exhaled through the expired air. Samples are taken from this air to be later measured in an isotope ratio mass spectrometer23.
Manipulation of the reticular groove and its use in veterinary medicine
Introduction
The closure reflex of the reticular groove in young ruminant prevents dairy food from passing through the rumen and reticulum, and ensures it goes directly through the reticulo-omasal orifice to the abomasum (Figures 2 to 5). Knowledge of physiological factors, as well as the possible pharmacological manipulations of reticular groove reflex, both to stimulate it or to inhibit it, are of great interest in oral administration of certain drugs, in their use for treatment of certain internal diseases, and even in the better use of some foods in adult ruminants as well as in lactating ones26,40.
Handling techniques
For many years, the effects of diet and management on the reflex of the reticular groove, especially in calves, have been studied. According to most researchers, suction is the most important stimulus as it is considered to cause the closing reflex of the reticular groove10. "The passage of liquid drunk from a bucket to the abomasum by calves is determined neither by temperature nor composition of the liquid nor by the position of the animal while breastfeeding, not by the act of suckling itself; but it is a result of the action of a kind of behaviour that accompanies the act of suckling"12.
Conditioning reflex
There are many experiences that suggest that the reflex can be conditioned to a variety of circumstances. The final proof that the reticular groove can be functionally conditioned was obtained when a liquid substance deposited directly into the back of the mouth and pharynx, where there are a number of nerve receptors are found, entered into abomasum, on the condition that the animal should receive visual and auditory stimuli of utensils and equipment used during the regular feed of young ruminants, perhaps olfactory stimuli also influenced15. However, when these stimuli did not exist the liquid reached rumen and reticulum41.
The strong excitation following the view of the bottle in a conditioned subject, such as an adult sheep, is enough to double or triple the volume of fluid collected from an abomasal fistula after administration in the lower oesophagus. The electromyogram indicates that an effective closure of the reticular groove is produced22. These results show that the sucking behaviour can be triggered by other stimuli than these of oropharyngeal origin, and its autonomous components do not correspond to more or less complete closure of the reticular groove. In the same line other researchers argue that induction of reticular groove closure, in milk-fed calves, requires a series of determinants, including the drunk fluid must be in contact with the local receptors of the pharynx, which should be consumed voluntarily by the animal, which must not have offensive odour or taste, and the general condition of the animal must not be altered42,43. When any of these factors is not met, the reticular groove closes incompletely, resulting in passage of the milk into the rumen and reticulum, where it is fermented by local microorganisms43.
Therefore, in some diseases this reflex may be altered, so in lactating animals there may be a failure of the afferent pathway of reflex due to a pharyngitis, the presence of oropharyngeal abscesses8,9 or infections in the larynx19. In calves under 14 d old affected by acute catarrhal enteritis non-closure of the reticular groove was observed in 11.2 % of the animals, dying 11 of the 249 patients. It was possibly due to a ruminal acidosis, due to the increased butyric acid and lactic acid fermentation or vice versa, which can cause neonatal diarrhoea by dysfunction of the reticular groove in milk feeding. These calves had marked dyskeratosis lesions of the ruminal mucosa43.
It seems that a calf or lamb needs to be in a situation of comfort, without stressful situations, for the proper functioning of the stimulus in reticular groove. So it is not surprising that a forced feeding (by catheter or bottle applied to the animal's mouth) uncomfortable for the animal, which can halt stimulus, and cause liquids to pass to reticule and rumen. However, in those animals that feed directly from the mother’s nipple, or from a bottle nipple the act of suckling may be considered as the most important stimulus in the closing reflex of the reticular groove10,22,41, and even a mechanical stimulation by the nipple or pacifier can also contribute32.
Place of administration of liquids
There are many investigations in which it has been shown that when some substances are administered using an oesophageal tube the reflex of the reticular groove closure can be inhibited19,20,29,33. Both Ørskov and Benzie44 and Van Weeren-Keverling Buisman et al20 cite that as early as in 1951 Comline and Titchen15 pointed to the nervous receptors located in the mouth and pharynx as responsible for the closure of the reticular groove.
Chapman et al33 found, a more effective treatment when large amounts of fluids are administered to dehydrated calves. In calves to whom various solutions were administered by an oesophageal catheter, it was found that the reticular groove did not lock, even after administering solutions of sodium bicarbonate, copper sulphate and guanidine hydrochloride. However, when commercial glucose solutions, amino acids and electrolytes in significant amounts, at least 2 liters, were applied there was a significant increase in blood glucose. Therefore, the results of this study suggest that the fluids intended to be absorbed in the intestine can be advantageously administered using a oesophageal tube, even if the reticular groove closure does not occur, provided that it implies a considerable amount33.
Characteristics of the fluids administered
The stimulation on oropharyngeal and lingual receptors has been attributed to protein and milk salts, which trigger the reflex of reticular groove closure22. There are many examples of investigations that have worked with fluids of different nature to cause effective closure of the reticular groove15,41,45-48. The milk supplied at different temperatures and under the same management routine, can effectively stimulate the reticular groove, but the response is more intense when milk is offered at body temperature. Also water orally administered at body temperature is able to cause a partial closure of this structure, though cold water fails to stimulate reflex10. Some research has shown that water can be recovered from the abomasum, after passing through the reticular groove, when experiences are made in conditioned animals who have been by previously accustomed to suckle from a bucket. So it appears that the conditioned reflex is probably more important than the nature of the administered liquid10,15,41,44,45,47.
Pochón10, cites that Hegland et al in 195749, concerned about the effects of diet and management, studied the destination of various fluids, such as whole milk, reconstituted skimmed milk, reconstituted whey and water, and food capsules in fistulised calves fed by regular bucket or bucket with nipple. In those cases, where capsules of varying sizes together with liquid feed was administered, the closing reticular groove leading to these capsules were located in the omasum, however if the calf was not receiving liquid feeding capsules they were placed in the reticulum. It was accepted that a capsule had passed through the reticular groove when after one minute it was neither in rumen nor in reticulum. Therefore, according Hegland et al49 any of the tested liquids were able to elicit the stimulation of the reticular groove, leading to complete closure in all tested calves during the first 6 weeks of life. They also found that the effectiveness is similar using both methods of feeding (open bucket and bucket with nipple), while feeding bucket-nipple lengthened the reflex in all calves up to 13 wk after birth, while drinking directly from bucket only it was effective in the first 6 weeks of life of the calf.
To test the possible effect of whey on the reticular groove 14 dairy calves were fed with a liquid diet46. Experimental observations were made every two weeks, beginning at 20 wk of age up to 30 wk, and the ingestion of whey made from a bucket, proceeding to palpation of lips of the groove through a ruminal fistula and the whey collection which had passed to the abomasum. The difference between what had been consumed and what had been recovered would be the amount of whey which had reached the rumino-reticular compartment. In most animals 80% of the food was collected, except at 20 wk, which was 53 %. The reflex of groove occurred 15 to 20 sec after ingestion. It was concluded that the use of whey led to the closure of the reticular groove and also the continuous ingestion of increasing amounts of whey, keeping consumption habits (schedules, dosage form and temperature), allowed the maintenance of reflex over time, with consequent digestion of milk derivative to intestine. Weight gain was greater than in those animals to whom whey was diverted into the rumen46.
Temperature of fluids given
It has already been indicated that both water and milk at body temperature can cause to greater or lesser extent, closure of the reticular groove. Cold water does not stimulate this reflex10. However for Ørskov45 stimuli as temperature and feed composition, dosage form and position of the animal are much less important on the reflex of the reticular groove than the environment surrounding the animal or its mental state at the time of ingestion of liquid food.
Method of administration of fluids
The mere sight of a bottle is enough to trigger the closure of the reticular groove in adult animals, provided they have been accustomed to eating milk or liquids this way10,22. This reflex is also present if young animals are taught to drink milk food from a bucket with a nipple10,41. This behaviour would be similar to that produced when an animal suckles its mother's udder.
In 1928 it had already been observed, by using rumen fistulae, that milk flowed through the groove to omasum and abomasum using a mechanism of nipple for feeding calves, however when drinking directly from a bucket lots of ingested milk passed to the rumino-reticular cavity10. A few years later, in 1942, Wise et al25, working with 17-56 d-old calves, also found more milk in the rumen and reticulum when they were fed using a regular bucket (open) than when they did it with a nipple bucket, located slightly elevated10. In examining the effect of closure of the reticular groove in the absorption of a combination of sulfamethoxazole-trimethoprim, using calves 6 wk of age, trained to suck through a nipple-bucket with teat, they found that the maximum plasma concentration and persistence sulfamethoxazole time is 7.5 times higher and 6.9 times longer when administered by teat bucket if catheter is used50, while the trimethoprim is only detected in plasma if calves are fed from buckets with nipple, which is justified in both cases by the closure of the reticular groove, with the consequent passage of drugs, given orally, to the abomasum, resulting an increased availability of these drugs50.
Mineral salts
Various substances have been tested to manipulate the reflex of the reticular groove in ruminants; in some cases applied parenterally and in many others orally administered.
Copper salts
Several authors believe that oral copper salts remain the most effective method. According to Pochón10, after reviewing multiple researches, in ovine the most effective is copper sulphate21,25,29,30,32,36,38,39,44, but other salts as copper acetate and copper chloride21,32, zinc sulphate21,30 are also capable of causing the closure. In adult cattle its effectiveness has also been proven24, while they have not been particularly effective when used in calves10,33 nor goats51.
According to some authors21 copper sulphate solution at 10 % is the most potent agent to stimulate the reticular groove reflex due to stimulation of oropharyngeal receptors in adult sheeps. Copper sulphate at 10 % has also been employed38 to cause the closure of the reticular groove and to study electromyographically the forestomaches motility in adult sheep. Prior to electromyographic study, they administered a solution at 20 % of glucose orally, before and after application of copper sulphate, and found that all the sheep responded positively to stimulation, altering their glycaemia significantly. To activate the reflex of groove Nicholson and Belkhiri18 used oral copper sulphate (1 g in 10 ml of water), but later inhibited the reflex with the application of clonidine.
In an attempt to test the effect of the administration of copper sulphate and cobalt sulphate in the stimulation of the reticular groove Sargison et al34 used recently fed sheep (10 in each group), 10 mo old, who were administered barium sulphate as a contrast medium and polyethylene spheres impregnated with barium as solid radiopaque markers and studied under radioscopy. However, others52 were not able to consistently stimulate the reticular groove with copper sulphate (20 ml at 10 %, via per os).
Salts of sodium
Conversely, sodium salts (chloride, sulphate, bicarbonate, acetate, etc.) appear to have a greater effect in cattle while in sheep they are rarely effective32,53. De Vuyst29 caused a reflex of reticular groove closure in adult cattle with a 10 % sodium bicarbonate solution, whereas if this solution was administered via catheter, surpassing the oral mucosa, reflex did not occur. A similar effect was found in experimental research carried out with a 7 % sodium sulphate solution, with a solution of 0.1 % eosin as a marker, which revealed that any food administered afterwards passed through the reticular groove, which means the adult ruminant high quality nutrients could pass to the abomasum through the reticular groove, avoiding destruction by ruminal fermentation29. Several researchers had previously managed to close the reticular groove in cattle, with high effectiveness25,30,36,53.
Mikhail et al26 obtained a significant increase in glycaemia in goats by oral glucose administration after stimulating the reticular groove with 1.5 ml of saturated NaCl solution or 10.5 ml of 1.5 %, NaCl solution applied intravenously. The application of sodium bicarbonate or copper sulphate in calves orally, by oesophageal catheter, fails to stimulate the closure of the reticular groove33, but perhaps may also be influenced by the discomfort caused to the animal when using this method, which may result in an inability to close the groove10. Bakker54 administered solutions of hypertonic dextrose, chloride hypertonic saline, hypertonic sodium bicarbonate and magnesium sulphate, and none of them was able to trigger the reticular groove reflex, so he concluded that previous studies that had encouraged closure by using various salts, especially NaCl and HCO3Na, could not be repeated and were caused by the closure of the reticular groove as a result of hypovolemia, rather than the closing of this structure itself.
Glucose solutions
Glucose in concentrations between 5 and 10 % may able to cause the closure of the reticular groove24, however Riek53 was unable to achieve this effect. The administration of sugar solutions was not enough to stimulate the closure of the reticular groove when were administered orally in goats26, perhaps because the animals used in the experience still keep the reflex of the reticular groove closure51.
Other salts
In 1997 and 1999 Smith et al55,56, found that zinc (zinc sulphate and zinc acetate) also stimulates the reflex of reticular groove in sheep, and that this effect depends on concentration of the zinc solution.
Vasopressin
There are many experiences in which vasopressin has been used to trigger this effect20,26,48,57. In some cases, administration has been exogenous, in others endogenous release in thirsty and dehydrated animals, or after intravenous administration of sodium chloride solutions, has taken place.
Water deprivation in goats causes an increase in blood levels of vasopressin (ADH) as a response to thirst51. Endogenous vasopressin release, after the stimulation of osmoreceptors by hypertonic sodium chloride, is the cause of the closure of the reticular groove and the resulting increase in blood glucose58.
Mikhail et al26 determined the influence of thirst, administration of sodium chloride in the common carotid artery and vasopressin in the closure of the reticular groove in adult goats. NaCl solutions induce endogenous vasopressin secretion, responsible for the effect on the reticular groove; water deprivation for 48 h also led to the passage of large amounts of water to the abomasum, with hyperglycaemia if water was administered orally with glucose26.
Encinas et al37 studied the pharmacokinetics of a nonsteroidal anti-inflammatory, sodium meclofenamate, used in sheep for treatment and prophylaxis of allergic diseases and mastitis. This product was orally and intravenously administered to sheep, and determined the influence of reticular groove closure in drug bioavailability. To stimulate the reticular groove, they employed as a pre-treatment solution lysine-vasopressin (0.3 IU/kg iv PV) or 0.9 % NaCl iv 10 min before oral glucose (as a surrogate marker) and sodium meclofenamate. They noted that in sheep to whom lysine-vasopressin was administered, the reticular groove closed in all cases, whereas with 0.9 % NaCl, stimulation only occurred in 2 of 6 sheep. Of course the plasma concentration and removal rate of sodium meclofenamate after oral administration was influenced by the state of the reticular groove, while differences in bioavailability between pretreatments were not found.
Vasopressin has also been used with positive results in adult cows with acute phosphorus deficiency, to whom phosphate was orally administered. It was determined that the solution was diverted into the abomasum with a faster absorption59.
Reflex inhibition
Reflex inhibition can also be interesting in some practices with sheep. Inhibition can be achieved administering a local anaesthetic in the oral cavity, with atropine intravenous injections, acting at the level of the efferent pathway12,60 and with metoclopramide (0.2 mg/kg)61 which act as an antagonist of dopamine through the cholinergic system. Domperidone has a similar effect although not with the same access intensity in CNS that metoclopramide62. Some research18 state that norepinephrine acts on groove motility through a cholinergic mechanism.
Nicholson and Belkhiri18 used clonidine at doses of 2 and 4 (g/kg iv in adult sheep as a reticular groove reflex inhibitory by its agonist action to (-2 adrenoreceptor, causing inhibition of ruminorreticular motility by acting on the CNS. Orally administered copper sulphate was employed to enable the reflex of the reticular groove. Clonidine at doses of 2 (g/kg produced a decrease in reticular motility, complete halt during 10-50 minutes in those animals receiving 4 (g/kg. When subsequently used a (-2 antagonist idazoxan at 0.1 mg/kg, prior to application of clonidine to prevent inhibition of the groove closure, they observed an increase in the peak concentration of the marker used (xylose), more significant if only idazoxan was used. Hexamethonium exerts a nodal locking effect similar to vagotomy, avoiding the contraction of the reticular groove10,13.
Use in veterinary medicine
The ability to control this reflex is of great interest in the oral administration of various drugs, to treat certain diseases, as well as in the more efficient use of some food resources. There are many experimental protocols in which it has been demonstrated that treatment with glucose solutions26,42,63-65, with NSAIDs such as meclofenamate and acetaminophen37,66,67, with certain antibiotics such as chloramphenicol57 or sulfamethoxazole-trimethoprim50, and some antiparasitic are generally ineffective due to product degradation by ruminal microflora or, conversely because of too fast circulation through the rumen68-70. It is therefore of great interest to stimulate furrow closure in the first case and suppressing it in the later.
No doubt the therapeutic efficacy of certain substances would considerably improve if these products could go through the stomach directly and reach the abomasum. It has even been postulated that the management of reticular groove could be used to obtain higher economic returns handling cattle feed23,29,42,44,62.
Some researchers have made use of vasopressin at different concentrations for stimulating the reticular groove in ruminants, facilitating the treatment of diseases such as ketosis, diarrhoea and ovine pregnancy toxaemia26,28,42,64. So, some authors63,71 obtained better results in the treatment of diseases such as nonspecific diarrhea bovine or primary ketosis in animals with whom vasopressin was used to stimulate the closure of the reticular groove than in those in the control group. El-Hamamsy et al64 and González-Montaña et al28 used lysine-vasopressin to stimulate the closure of the reticular groove and demonstrated the efficacy for increasing glucose following administration of a glucose solution orally, since glucose went directly to the abomasum avoiding unwanted rumen fermentations.
According Ranzini Rodrigues et al23 performance of calves receiving a source of non-degradable protein is, most of the time, higher than that of animals that do not receive it72-74 due to the higher flow amino acids that reach the small intestine23. Therefore, an effective way to avoid protein degradation as it passes through the rumen, thereby preventing the loss of essential dietary amino acids, would be to provide protein sources through the reticular groove.
Other authors have demonstrated the effectiveness of using the reflex of the reticular groove in the administration of various liquid supplements in calves (milk, skim milk, soybean bran suspension, fish meal and whey) compared to those receiving concentrated41,47. Also Standaert et al75 found increased milk production in cows that were fed with casein through the reticular groove compared to those receiving the same amount of protein, but in a solid form.
Conversely, in some cases a reflex inhibition of reticular groove is important from the viewpoint of the administration of various drugs. So management of reticular groove is particularly relevant when used in conjunction with antiparasitic40,76,77. Mc Ewan and Oakley40 attributed the failure of certain anthelmintic to the closure of the reticular groove in calves, by reducing the time of exposure to certain parasites such as nematodes. In calves ranging between 125 and 205 kg, the groove is still functional, that is the reason for necropsy evidence of ruminal bypass in half the animals, and that is why antiparasitic efficacy is reduced. The effectiveness of fenbendazole is variable against inhibition of Ostertagia ostertagi larvae in the calf76,77.
Other antiparasitics studied by stimulating the reticular groove are benzimidazole68, oxfendazole69 or some coccidiostats as the medium chain fatty acids (MCFAs)70,78.











 text in
text in 



