Introduction
Studies have suggested a physiological role of vitamins C and E in cattle reproduction1,2. An improvement in cattle fertility after vitamin E supplementation has been reported3,4. This vitamin may improve fertility by a direct antioxidant effect on follicle and embryo development5 or by influencing follicular cell apoptosis and proliferation6.
Vitamin C is necessary to re-activate antioxidant activity of vitamin E7,8. The effect of vitamin C on reproductive function is mediated by its participation in collagen synthesis, hormone secretion and its antioxidant properties9. It has been suggested that several injections of vitamin C before and after estrus can improve fertility in repeat breeder cows10. Unfortunately, there is little research that evaluates the effect of this vitamin on dairy cattle reproductive performance. Recent studies analyzing the impacts of vitamin C on fertility are lacking, researchers may have lost interest in evaluating reproductive responses of cattle to this vitamin because it is thought that bovines do not need vitamin C supplementation11.
It is known that pregnancy rate in cows is improved when 3,000 mg of vitamin C and 3,000 IU of vitamin E are injected at the same time on the expected day of preovulatory follicle emergence, combined with subsequent injections of vitamin C at estrus detection and 2 d after artificial insemination (AI)12. The first injection of these vitamins aimed to affect follicle development6,13 and possibly oocyte quality. The second injection of vitamin C was administered to emulate the natural rise of this vitamin during estrus in cattle14. The third dose of vitamin C was injected to influence corpus luteum functionality15,16. Thus, based on previous experience, the hypothesis tested in this study was that cows injected with 6,000 mg of vitamin C and 6,000 IU of vitamin E before and after synchronized estrus will have a higher pregnancy rate than cows injected with 3,000 mg of vitamin C and 3,000 IU of vitamin E.
Material and methods
All the technical and animal management procedures in this study were performed following the guidelines of the Canadian Council on Animal Care17.
Animals, treatments and experimental design
The experiment was performed at the dairy cattle research farm of the Universidad Autónoma Chapingo, México. Lactating 4.6 ± 0.35-yr-old Holstein dairy cows (n= 44) with an average of 163.4 ± 20.0 d in milk and in a herd with historical record of 22 L d-1 cow-1 were assigned randomly to one of three treatments: 1) Control: n= 15, cows were not injected with vitamins; 2) VCE3: n= 15, cows received a single i.m. injection of 3,000 IU of vitamin E ((±)α-tocopherol®, Sigma-Aldrich) on d-5 (d 0 is the day of intravaginal device removal) and s.c. injections of 3,000 mg of vitamin C (ascorbic acid®, Q.P., Reasol) on d-5, immediately after estrus detection and 2 d after artificial insemination; 3) VCE6: n= 14, cows were treated as in VCE3, but the doses of vitamins E and C were increased to 6,000 IU and 6,000 mg, respectively. The experimental design was completely random, and the experimental unit was one cow.
Estrus synchronization and breeding
The follicular wave of the cows was synchronized with an intravaginal device containing 1.0 g of progesterone (Sincrogest®, Ourofino Agronegocio, Sao Paulo, Brazil), inserted intravaginally for 8 d, and an i.m. injection of 250 µg of GnRH analogue (GnRH®, Sanfer) at intravaginal device insertion. Corpus luteum regression was induced by i.m. injection of 500 µg of cloprostenol (Celosil®, MSD Animal Health) at intravaginal device removal. Once the intravaginal device was removed, the animals were monitored constantly (at least every 2 h) by direct observation for signs of standing estrus (a cow was considered in estrus when accept mounting by another cow). The cows were artificially inseminated 12 h after estrus detection with a single dose (approximately 20 x 106 spermatozoa) of semen from a single bull of proven fertility.
Nutrition and feeding
The animals received a diet providing 1,117 IU of vitamin E (51.5 kg d-1 cow-1 of a total mixed ratio: fresh alfalfa (21.9 kg), corn silage (21.9 kg) and commercial concentrate (7.7 kg), named Ganadero 18, Productos Agropecuarios Tepexpan, S.A. de CV with protein 18%, fat 4%, fiber 12%. Vitamin E content in diet was determined by high performance liquid chromatography18.
Reproductive parameters
The measured reproductive parameters were diameter of the preovulatory follicle, time to estrus after intravaginal device removal, area of the corpus luteum (CL), pregnancy rate and blood concentrations of estradiol and progesterone. The diameter of the preovulatory follicle and area of CL were measured by real time ultrasonography (Aloka Prosund 2, equipped with 7.5 MHz linear-array transducer, Hitachi Aloka Medical, Ltd Japan) performed by the same technician. Diameter of the preovulatory follicle was calculated by averaging horizontal and vertical measurements immediately after estrus detection, while the area of CL was calculated directly in the ultrasound 9 d after AI. The pregnancy test was performed by ultrasonography 30 and 45 d after AI. Blood samples were collected from the coccygeal vein, using tubes containing sodium heparin as anticoagulant (BD Vacutainer®), immediately after estrus detection and 9 d after AI. The blood samples were centrifuged at 3,000 rpm for 10 min and plasma was separated and stored at -20 °C until the day of analysis for estradiol and progesterone concentrations determination by ELISA (Estradiol- and Progesterone-Elisa, DRG Instruments, GmbH, Germany).
Statistical analysis
The statistical analysis was performed on variables collected only from cows that exhibited estrus. The number of cows that exhibited estrus for each of the treatments were Control=14, VCE3=13 and VCE6=14. A normality test was run in PROC CAPABILITY on the residuals of the final model in each variable. When residuals did not satisfy the normality test, the data was subjected to logarithmic transformation. The statistical model included the fixed effect of treatment. In addition, days in milk and age of the cow were included in the final model only when statistical significance was found. The results are presented as mean ± standard error (SE). In all cases, P≤0.05 was considered significant. The data were analysed by PROC GLM, except for pregnancy rate, and the means were compared by the Tukey Test. The pregnancy rate at 30 and 45 d were analysed by PROC GLIMMIX considering a binary distribution and using the link function logit. The SAS statistical package was used for all analyses.
Results
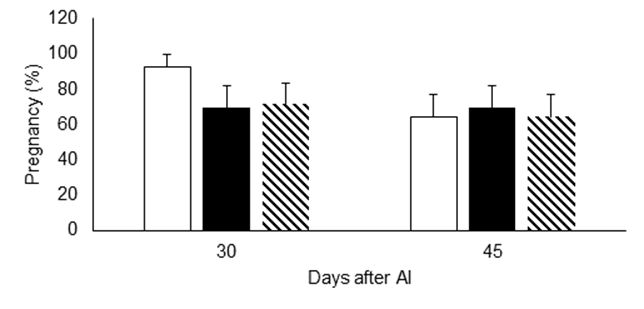
The impacts of injecting increased doses of vitamins C and E on ovarian structure development and hormone concentrations in dairy cattle are shown in Table 1. Overall, cows supplemented with the highest doses of vitamins C and E tended to have a smaller (P=0.06) preovulatory follicle, but blood concentrations of estradiol were not affected by vitamin injections (P˃0.05). The size of the corpus luteum was not different among treatments. However, cows receiving the lowest dose of vitamins had less (P≤0.05) blood progesterone concentrations than those of the control group and those that received the highest dose of vitamins. Moreover, pregnancy rate 30 and 45 d after AI of cows in the control group showed no differences compared to the groups of cows supplemented with vitamins (Figure 1).
Table 1 Effect of supplementation (mean±SE) with 3,000 mg and 3,000 IU, 6,000 mg and 6,000 IU of vitamins C and E, on ovarian structure size, estrus presentation and hormone concentrations in Holstein dairy cows
| Treatment | ||||
|---|---|---|---|---|
| Variable | Control | VCE3 | VCE6 | P |
| Time to estrus, h | 48.1±5.17 | 55.2±5.36 | 62.1±5.10 | 0.17 |
| Diameter of the preovulatory follicle, mm | 18.9±0.71 | 17.1±0.73 | 16.5±0.69 | 0.06 |
| Plasma estradiol concentrations, pg mL-1 | 37.8±4.19 | 40.1±4.00 | 38.8±3.85 | 0.92 |
| Area of the corpus luteum, cm2 | 6.7±0.52 | 7.3±0.54 | 6.0±0.52 | 0.25 |
| Plasma progesterone concentrations, ng mL-1 | 19.4±2.66 | 10.1±2.55* | 19.2±2.44 | 0.02 |
* Significantly different from other groups (P≤0.05).
VCE3 group supplemented with 3,000 mg of vitamin C and 3,000 IU of vitamin E.
VCE6 group supplemented with 6,000 mg of vitamin C and 6,000 IU of vitamin E.
Discussion
A relationship between size of preovulatory follicle and probability of a cow diagnosed as pregnant after a fixed-time AI has been reported in dairy cattle19. Cows with preovulatory follicles between 13.5 to 17.5 mm are more likely to become pregnant after a fixed-time AI20. A possible explanation for the effect of preovulatory follicle size on pregnancy rate could rely on the degree of oocyte competence. According to results of an in vitro study21, as follicle increases in size from 3 to 15 mm, the oocyte diameter also increases, and larger oocytes have been reported to have greater developmental competence22. Another possibility is that young corpora lutea from larger follicles produce more progesterone than those from small preovulatory follicles23. Supporting the above findings, donor cows with preovulatory follicles larger than 12.5 mm had a higher probability of yielding good quality embryos24, but those with preovulatory follicles larger than 20 mm in size are at risk of pregnancy loss25.
The cows injected with vitamins C and E had preovulatory follicles falling under the threshold at which the probability of pregnancy after AI increases20. Since cows injected with the highest dose of vitamins tend to have a small preovulatory follicles, a similar tendency was expected in estradiol concentrations. However, concentrations of this hormone and pregnancy rate among experimental groups was not different. Results from in vitro studies indicate that vitamin C does not affect follicular estradiol production, but it does affect follicle structure13, and vitamin E improves granulosa cell survival6. Results of the present study agree with those obtained from in vitro studies regarding estradiol production. In addition, previous research found that estradiol concentrations and pregnancy rate are not influenced by preovulatory follicle size in cows showing estrus and ovulating spontaneously26, as in the case of the present study.
The progesterone produced by the corpus luteum after AI is responsible for pregnancy maintenance. Dairy cows with good genetic merit for fertility traits had a larger corpus luteum and produce more progesterone than those with poor genetic merit27. Thus, increasing corpus luteum size and progesterone production might be targeted to improve fertility in dairy cattle. Based on its physiological relevance, vitamin C may be an important asset to influence corpus luteum development. It has been reported that ascorbic acid supports collagen biosynthesis during tissue formation and maturation of corpus luteum15, reaching the highest concentration at mid luteal phase28. In addition, vitamin C concentrations correlate positively with corpus luteum size and progesterone level16. However, corpus luteum size was not affected by vitamin supplementation in this study and progesterone concentrations were minor in cows injected with the lowest doses of vitamins C and E.
The corpus luteum was observed and measured by real time ultrasound, and a positive correlation between its size and functionality is assumed29. However, results from this and other studies are in disagreement. The cows injected with the reduced dose of vitamins regardless of having a similar corpus luteum size, produced less progesterone than the other groups. Similarly previous research did not find a correlation between size of the CL in regression phase and progesterone concentrations in cows30. In addition, others found that after d 8 of the oestrus cycle, the size of the corpus luteum does not determine progesterone concentrations31. This finding supports the results of the present work, as corpus luteum was measured on d 9 of the oestrus cycle. Discrepancies among studies are not known, but three points should be considered when CL measurements and progesterone concentrations are to be analysed at the same time. First, from field experience, sometimes ultrasound practitioners fail to find the transducer position that gives the largest view of the CL; this can produce confounding effects when a relationship with progesterone concentrations is sought. Second, the corpus luteum is a dynamic ovarian structure, which is more easily identified and measured during the mid-luteal stage of the oestrus cycle, but measuring this structure at a very early stage (d 2 to 3 after estrus) of development requires a great deal of experience. Third, when diagnosing the status of the corpus luteum, not only its size but also its echographic appearance should be taken into consideration32.
It was not found studies that attempt to evaluate the effect of increasing doses of vitamin E and C on dairy cattle fertility. Other studies have demonstrated a positive effect on pregnancy rate when supplementing vitamins C14 and E33 separately. The effect is mediated by enhancing follicle cell survival6, oocyte competence, corpus luteum functionality15,16,34 or embryo survival35,36. Despite previous experiences showing an improvement in pregnancy rate in cows injected with vitamins12, the results of the present study do not support such findings. However, it is worth noting that cows injected with the lowest dose of vitamins, despite having lower concentrations of progesterone, were capable of sustaining similar pregnancy rates 30 and 45 d after AI compared with the other evaluated groups.
Progesterone stimulates changes within the uterine environment allowing embryo receptivity and survival37. The concentrations of progesterone required to increase the probability of pregnancy occurrence is not well established. One may argue that higher, rather than lower, concentrations of progesterone are better for getting a cow pregnant. However, researches have suggested a range of milk progesterone concentrations within which embryo survival was maximal38. The existence of a range of progesterone concentrations suitable for pregnancy success is acceptable because a large concentration of progesterone could affect fertility by creating an asynchrony between the uterine environment and the embryo39; while a uterine environment with low progesterone concentrations will fail to induce the changes necessary for hosting the embryo40. Besides progesterone concentration, it is well known that embryo quality affects probability of pregnancy, and good quality embryos are better at achieving not only pregnancy, but also live birth in a uterine environment with variable progesterone concentrations, than those embryos of lower quality41. Therefore, it is possible that cows injected with the lowest dose of vitamins may have had good quality embryos36 capable of surviving and establishing pregnancy in a uterine environment with low progesterone concentrations.
Conclusions and implications
Supplementation with vitamins C and E did not affect preovulatory follicle and corpus luteum size, estradiol production on the day of estrus, or pregnancy rate 30 and 45 d after AI. The supplementation with the highest amount of vitamin C and E did not significantly increased the reproductive parameters measured.











 text in
text in