Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.10 n.2 Mérida Apr./Jun. 2019
https://doi.org/10.22319/rmcp.v10i2.4526
Review
Hydroxycinnamic acids in animal production: pharmacokinetics, pharmacodynamics and growth promoting effects. Review
a Centro de Investigación en Alimentación y Desarrollo A.C. Laboratorio de Ciencia y Tecnología de la Carne, (CIAD A.C.), Carretera a la Victoria km. 0.6. Hermosillo, Sonora 83304, México.
b Universidad Autónoma de Baja California. Instituto de Ciencias Agrícolas, Baja California, México.
c Centro de Investigación en Alimentación y Desarrollo A.C. CIAD A.C. Laboratorio de Nutrición Animal, Hermosillo, Sonora, México.
d Centro de Investigación en Alimentación y Desarrollo A.C. CIAD A.C. Laboratorio Bioenergética y Genética Molecular. Hermosillo, Sonora, México.
Use of natural source additives in animal production is increasingly important because they potentially promote growth in ways similar to synthetic compounds, such as anabolic hormones and antibiotics, but without risks to animal or consumer health or degrading meat quality. Encompassing a wide variety of compounds extracted from different plant parts, natural origin additives can be administered as essential oils, mixtures of compounds or isolated compounds to function as medicinal remedies or dietary supplements. Phenolic compounds are widely used and include hydroxycinnamic acids, present in a variety of vegetables, fruits and grains. These acids exhibit interesting bioactivities such as antioxidant, antimicrobial, prevention of cardiovascular diseases and immunomodulation. Use of hydroxycinnamic acids in animal production is currently limited but may hold promise in promoting growth. Before this can occur further research is needed on their pharmacokinetics and pharmacodynamics, posology, exposition period and effects, as well as their possible metabolic routes and biotransformation in animal organisms. This review covers inclusion of hydroxycinnamic acids in livestock diets, their pharmacokinetic properties and pharmacodynamics, and findings on their effects in promoting growth and improving meat quality.
Key words: Hydroxycinnamic acids; Ruminants; Monogastrics; Pharmacokinetic; Pharmacodynamic; Growth promoter
El uso de aditivos de origen natural en producción animal ha tomado gran importancia en el sector pecuario, debido a su potencial de promover el crecimiento de una forma similar a los compuestos sintéticos como hormonas y antibióticos, pero sin causar posibles daños a la salud del animal, del consumidor o detrimento en la calidad de la carne. En los aditivos de origen natural existe una amplia variedad de compuestos, que son extraídos de distintas partes de las plantas, donde se toman ciertos aceites esenciales, mezclas de compuestos o compuestos aislados para utilizarse como remedios medicinales o suplementos alimenticios. Dentro de estos extractos, se encuentran los ácidos hidroxicinámicos, presentes en una gran variedad de vegetales, frutas y granos; los cuales presentan interesantes propiedades bioactivas como son, antioxidantes, antimicrobianos, preventivos de enfermedades cardiovasculares e inmunomoduladores. El uso de este tipo de aditivos en producción animal aún es limitado, pero se sugiere que su inclusión puede ser favorable como una estrategia para promover el crecimiento; sin embargo, dos aspectos importantes a estudiarse en los ácidos hidroxicinámicos es su farmacocinética y farmacodinamia, y a partir de allí establecer las condiciones de dosis, períodos de uso y efectos, además las posibles rutas y biotransformaciones que pueden ocurrir en el organismo animal. Esta revisión discute sobre la inclusión de ácidos hidroxicinámicos en dietas de animales de engorda, propiedades farmacocinéticas y farmacodinamias, y los hallazgos como promotores de crecimiento y sus efectos en la calidad de la carne.
Palabras clave: Ácidos hidroxicinámicos; Rumiantes; Monogástricos; Farmacocinética; Farmacodinamia; Promotor de crecimiento
Introduction
Use of synthetic growth promoters in animal production results in better feedlot weight gain, and higher lean meat yields1. However, they are known to have negative repercussions which can affect some meat quality parameters2,3, and to pose an intoxication risk due to retention of synthetic compound residues in the organs and meat4-7. Due to their potential risks use of these compounds has been restricted in the European Union and many Asian countries4. This constitutes a limiting factor for meat-exporting countries that use this technology which can lead to substantial financial losses. The meat industry has responded by searching for safe alternatives for promoting growth in livestock.
A promising alternative is the use of natural vegetal-source compounds, better known as phytochemicals (PC). These are non-nutritional secondary metabolites used by plants to protect themselves against microorganisms, pests and herbivores. Classification of PC is complex because it can be based on their properties (e.g. biological function), origin, purity, or chemical structure (e.g. polyphenols, isoprenoids, essential oils and phytoestrogens)8,9. Phytochemicals can be administered as whole portions of a plant (e.g. roots, leaves, bark), their by-products, or as bioactive compounds in essential oils, isolated compounds or mixtures of compounds10.
After being used for years in humans as alternative medicine and remedies for chronic conditions most PC are classified as generally-recognized-as-safe (GRAS)7,11. They are beginning to find a role in animal production systems as a way to fight infections and improve animal health status, and thus attain optimal development throughout the growth stages. This may allow the eventual replacement of routinely applied synthetic compounds such as antibiotics, hormones, and β-adrenergic agonists12.
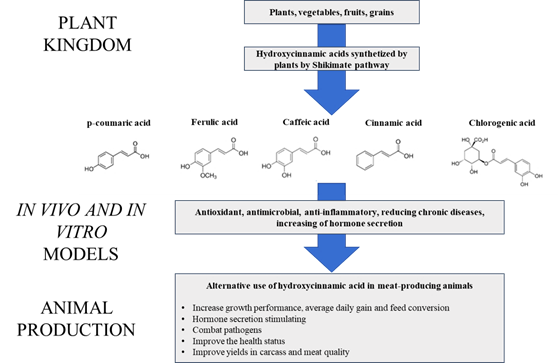
Among the PC are the hydroxycinnamic acids (HA), a group of phenols present in the fruits, roots, grains and seeds of plants. The best known of the PC are caffeic acid, ferulic acid, p-coumaric acid, sinapic acid and chlorogenic acid13. Used to fight disease and illness in humans, these acids can also be added to animal feed or administered separately to affect physiological changes that can contribute to growth14. Recent studies report improved growth performance, animal health and meat quality when HA are exogenously supplemented in animal diets15,16,17.
Understanding the mechanism for this action will require research into HA pharmacokinetics (i.e. absorption, distribution, metabolism and excretion), average time of efficacy, bioavailability and pharmacodynamics. In addition, information is needed on the direct relationship between HA and their action sites, biotransformation and physiological modifications, and how they are changed during metabolism18.
The most controversial aspect of HA use, and that of most natural additives, is their posology and the possible routes involved in growth, muscle deposition and nutrient utilization. These are needed before HA can be suggested as possible alternatives to synthetic growth promoters. This review addresses the possible absorption pathways of HA, their biotransformation and the metabolic changes they experience when added to growth diets in animals with the purpose of promoting growth without adversely affecting meat quality.
Hydroxycinnamic acids: definition, sources and properties
Hydroxycinnamic acids (HA) are derived from cinnamic acid and are common in plants and fruits in the form of organic acids or glycoside esters, or attached to proteins and other cell wall molecules such as cellulose, xylans and lignin13,19. Plentiful in plants, they are secondary metabolic products known to be used in defense against pathogens and insects8,20. They are synthesized via the shikimate pathway in which the amino acid phenylalanine is the precursor to HA. Recent research has addressed their potential bioactive effects and benefits in humans and animals when administered as nutritional supplements. Reported bioactive properties include antioxidant, antimicrobial, prevention of chronic diseases such as cancer and atherosclerosis, and growth promotion in animals21,22,23.
The HA can be extracted from plant cell walls by alkaline and enzymatic methods24,25,26. Their basic structure is a phenylpropanoid, with caffeic acid being the most common in nature27.
The hydroxyl groups present in the aromatic ring of HA makes antioxidant activity their main attribute22. This activity has been demonstrated in both in vivo and in vitro models aimed at preventing or treating diseases related to oxidative stress, such as cancer, diabetes, cardiovascular disorders and inflammatory diseases13,28,29,30. There is increasing interest in their antimicrobial capacity since they are known to inactivate or eliminate pathogenic bacteria and can modify the intestinal microflora, possible improving nutrient use and reducing disease incidence by promoting optimal immune system functioning31,32,33. These capacities highlight their wide range of possible applications in growing animals; initially they could replace synthetic growth promoters but their bioactive properties could provide additional advantages.
Hydroxycinnamic acids as additives in growing animals
Increasing research is being done on HA supplementation in livestock systems and their potential biological activities. This responds to changing perspectives among meat consumers who now demand healthier products from natural sources with the purpose intent of avoiding any health risks and impacts on meat quality caused by ingestion of synthetic compounds.
When used as additives HA can act in diverse ways, including as antibiotics, ionophores, antioxidants, anti-inflammatories, anabolics or flavor enhancers. In most cases they exercise these activities without compromising animal health or meat quality (Figure 1)34,35. Unlike some synthetic substances, which can only be used for limited periods and/or in a specific growth phase, the HA and other PC compounds are not apparently limited to a specific phase, nor do they cause damage from residual effects36,37,38.
Hydroxycinnamic acids in growth performance and carcass quality tests
Limited research has been done on inclusion of isolated HA in growth performance tests and there is still insufficient evidence to claim productive benefits in animals10,39. Ferulic acid (FA) has been tested recently in growing animals in search of a possible growth-promoting effect, but its effects have been contradictory and inconsistent and no action mechanism has been identified.
In pigs receiving 100 mg FA/kg feed for 28 d no improvements were observed in productive performance or primary carcass cuts40. Lambs supplemented with 300 mg FA/d for 34 d exhibited no differences in productive performance versus a control, although carcass rib eye area did improve with FA (control= 16.61 vs FA= 18.0 cm2), possibly an indication of increased muscle tissue deposition41.
In a study in which two FA doses (5 and 10 mg/kg live weight/d) were administered in finishing heifers, daily weight gain was higher in the FA treatments (1.02 and 1.24 kg/d, respectively) than in the control (0.93 kg), and feed conversion improved by up to 20 %. Carcass yield in the FA treatments increased by 1.64% over the control, and rib eye area was greater in the 5 mg FA treatment than in the control (85.61 vs 82.12 cm2)42.
A study in which 15 mg FA/kg feed was supplemented in finishing pigs found dorsal fat thickness to be similar between treatments using the β-agonist ractopamine (9.60 mm) and FA (9.67 mm)43. This suggests possible activation of hormone-sensitive lipase (responsible for lipolysis) in subcutaneous fat, with much of the resulting energy redirected to other metabolic functions such as muscular deposition. Ferulic acid has also been reported to increase synthesis of endogenous hormones, including prolactin and growth hormones, which may translate into greater muscle deposition44. However, more research is needed at the cellular level and of blood metabolites to determine if FA acts as an anabolic-type growth promoter.
Pure cinnamic acid has not yet been studied in vivo in growing animals. However, in an in vitro study cinnamic acid was found to be recognized in the 3T3-L1 cells of adipocytes, to stimulate the AMPk activation and to improve insulin sensitivity, possible altering the fatty acids profile45. Cinnamaldehyde is not a HA but is present in sources similar to cinnamic acid; indeed, in animals cinnamic acid can be synthesized from this compound. In one study supplementation with cinnamaldehyde (400 and 800 mg/d) in beef cattle for 28 d improved daily weight gain (2.18 and 2.08 kg [respectively] vs 1.97 kg control), and rib eye area was greater than in the control (89.5 vs 86.3 cm2)46. The authors suggest that cinnamaldehyde modifies microbial populations and the volatile fatty acids profile, which can improve nutrient digestion, reduce methane gas production and thus allow rerouting of this energy to muscle growth. However, high doses of cinnamaldehyde (1,600 mg/d) can decrease ruminal fermentation and thus diminish availability of protein from microbes and feed, compromising animal nutrition47.
In ruminants, HA such as cinnamic acid, p-coumaric acid and ferulic acid may be lost in the ruminal fluid by absorption and utilization by rumen microorganisms, or hydrogenation by specific bacteria, thus limiting growth48. In contrast, other authors claim that the phenolic monomers in forage can be released and absorbed in the gastrointestinal tract, possibly providing benefits to the animal49,50.
Tests of FA in vitro and in mice have shown it to have possible fat reducing activities caused by an adipocyte dysfunction involving lower growth of preadipocytes to the detriment of fatty acids and cholesterol in the liver and plasma51,52. Both caffeic acid and chlorogenic acid inhibit enzymes responsible for synthesis of fatty acids such as fatty acid synthase and 3-hydroxy-3-methylglutaryl CoA53,54.
The revised literature suggests that HA supplementation in cattle has a growth promoting effect. However, more research is needed to confirm this promoter activity since some studies report the contrary. For example, in two studies of ruminal culture employing 0.2 % p-coumaric acid and chlorogenic acid, the phenolic monomers in the forage or those added to the diet negatively affected the rumen, acting as antimicrobials in cellulolytic populations, and limiting use of energy from forage structural carbohydrates50,55. However, the HA in forage lignin exhibit digestibility in different sections of the gastrointestinal tract primarily the rumen, abomasum and ileum suggesting the presence of various interactions, both positive and negative, between HA and the biological processes of digestion and metabolism in ruminants50.
Based on studies of FA in pigs and cattle in which doses and times have been tested, low doses (5 mg/kgLW/d) for periods not greater than 30 d can be suggested for supplementation with HA when seeking a growth promoter effect. That said, each monomer can act, absorb and metabolize in different ways, meaning that it is vital to closely monitor animal health status when supplementing HA for productive purposes.
Changes in meat quality in animals receiving hydroxycinnamic acids as supplements
Oxidation and microbial growth are the principal causes of reductions in meat quality since they diminish its nutritional, sensory, functional and health properties for the consumer. This generates a breakdown in the animal production chain and consequently, significant financial losses for the meat industry56,57.
With the aim of improving the quality and stability of meat and meat products the industry has tested both synthetic and natural antioxidants58,59,60. Using the animal’s metabolism, additives are included in the diet to reduce oxidation processes, formation of volatile compounds and microbial deterioration in the meat, while maintaining its nutritional quality and extending its shelf life31,61.
A wide variety of compounds and mixtures are used in animal diets to exert a protective effect on meat; one common example is vitamin E62,63,64. Although little research has been done on HA in animal diets these compounds are known to have high antioxidant capacity, especially ferulic acid, caffeic acid and p-coumaric acid. They can therefore be seen as possible nutritional supplements aimed at preventing lipid oxidation in meat by inhibiting formation of primary and secondary products (e.g., malondialdehyde - MDA)17,40,65,66.
Phytogenic substances are known to improve the quality of pork and beef67,68; for example, administration of FA administered at 5 or 6 mg/kgLW/d for 30 d in beef cattle diets retarded lipids oxidation17,69. Values less than 1 mg MDA/kg meat were recorded at up to d 10 of storage under refrigeration and metmyoglobulin formation was lower in the supplemented treatments than in the control, confirming a protective effect against oxidation of polyunsaturated fatty acids and myoglobin protein17,69. In another study a mixture of FA (100 mg/kg feed) and vitamin E (400 mg/kg feed) were found to have a protective effect when added to diets for finishing pigs, resulting in lower muscle tetrabutylammonium (TBA) values and lower meat hardness than in the control40.
It is important to consider, however, that supplementation of PC for long periods or at high doses can cause a pro-oxidant effect in meat and accelerate fatty acids and protein oxidation. For example, supplementation with FA in beef cattle at 6 mg/kgLW/d for 60 d17 or 10 mg/kgLW/d for 30 d69 prior to slaughter, produced more than 2 mg MDA/kg meat beginning on d 3 of storage and formation of up to 30% myoglobin after 7 d of storage. This pro-oxidant effect of FA after long-term or high-dose supplementation may be due to accumulation of high levels of FA in the muscle, providing a stimulus for oxidation onset. High concentrations of antioxidants are known to affect the stability of trace metals, possibly altering myoglobin stability and leading to its oxidation70,71.
During animal growth in commercial livestock systems vitamin E is commonly used during the finishing phase and prior phases to maintain color stability in meat and retard its oxidation during storage58,60. Hydroxycinnamic acids (HA) can exert a similar benefit as well as a growth promoter effect in animal metabolism and HA deposition in muscle. They can thus provide a double benefit or even be used as an adjunct to vitamin E. This synergistic combination has been tested in pigs in a study in which a mixture of FA (100 mg/kg feed) and vitamin E (400 mg/kg feed) halved MDA content in the Longissimus dorsi muscle and increased rib eye area compared to a control (44.70 vs 37.17 cm2)40. Clearly, certain combinations of compounds can provide benefits for livestock producers.
Pharmacokinetics of hydroxycinnamic acids in animal production
Pharmacokinetics research on PC, particularly those on use of HA in growing livestock, are still limited and inconclusive. However, some reports do show that supplementation or intake of these compounds allow them to reach the portal system and thus attain bioavailability in the organism72-76.
Pharmacokinetics refers to the route taken by a drug or compound in an organism, from intake to excretion, including absorption rates in different organs. A substance’s pharmacokinetic will indicate to what degree, if any, it is used by the organism. Whether in a pure form or in combination others, it has been proposed that HA are absorbed to some extent in the stomach and in a greater proportion in the intestine, thus reaching the bloodstream and eventually exerting physiological changes such as reducing oxidation in tissues such as the liver and muscle23,77. However, absorption rates of these compounds in the gastrointestinal tract and their ability to reach to the bloodstream may vary due to enzymes, microorganisms in the rumen or intestine, stress factors, animal species and biotransformations such as glycosylation or sulfation72,78.
Many HA are quite small, meaning they can cross the gastrointestinal barrier by passive diffusion, mainly in the stomach and small intestine, and go on to be absorbed and deposited in different organs with the help of transporters such as albumin54. Once absorbed these compounds subsequently change polarity, becoming more hydrophilic, and are excreted in their glycosylated form in the urine79. Discovering a possible route and the pharmacokinetics of HA in growing animals is complex and most studies have employed murine models to this end33,80. Absorption varies in response to species, diet, physiology, health status and genetics, among other factors. Understanding which HA are most effective and/or more bioavailable in the organism can be aided by reviewing reports for ruminant and monogastric models.
Hydroxycinnamic acid pharmacokinetics in ruminants
The metabolism and kinetics of PC, including HA, in ruminants is very complex. Several modifications occur mainly in the rumen; indeed, the first action site for HA modification is the rumen. It is here that microbial populations and the anaerobic environment cause rapid hydrogenation of phenolic compounds, followed by dehydroxylation and subsequent biotransformation into phenylpropionic acid. This phenylpropionate is then absorbed in the bloodstream for transport to the liver, transformation by β-oxidation and finally excretion in a glycosylated form or as a free acid81.
Some pharmacokinetic studies have addressed the route and modification of ferulic acid, caffeic acid and cinnamic acid in ruminants. Two studies of FA supplemented in sheep and lactating cows found that it was absorbed within the first 5 h post-administration. Sampling was done at shorter intervals in cows and showed that FA may experience rapid absorption since levels increased at baseline and during the first 6 h post-administration but then returned to baseline levels at fourteen hours. It is also possible that a portion of the compound was not modified in the rumen and was subsequently absorbed in low concentrations72,73.
A wide variety of dietary origin phenolic compounds are present in the rumen fluid, with 3-phenylpropionic acid being the most abundant (50 to 80 %), and cinnamic acid being a minor component (7 %)49,82. The structure of HA in the rumen depends on rumen microorganism profile and HA dose; an approximately 0.4 % HA supplementation level in the diet can impair animal growth and diet utilization55). Degradation of forage, particularly lignin, can also be compromised by HA release, especially FA, since it limits growth of cellulolytic bacteria. Due to its stronger ester bonds, release of p-coumaric acid occurs at lower levels than FA50,83. Rumen cellulolytic bacteria are responsible for degrading phenolic compounds through hydrogenation of the HA side chain, which limits their bioavailability. Future research needs to focus on the different microorganism species in the rumen and how they generate significant changes in administered HA. It would also be of interest to quantify changes in microbial populations and volatile fatty acids, which are important in the use of nutrients in ruminants72,84.
Biotransformation of HA in the rumen can be prevented by encapsulating or saponifying the compounds, allowing them to reach target tissues and exert any bioactive effects. Encapsulation involves formation of small lipid particles (i.e. nano- and micro-particles) capable of storing and stabilizing bioactive substances such as salts, amino acids, proteins or PC. The encapsulating substance needs to protect the bioactive substances from interaction with the environment and control their release at a specific site or soft tissue in the organism85,86. Due to the complexity of the rumen bacterial community and its importance in nutrient use, different studies have focused on encapsulation as a way of directing compounds to target tissues, or of ensuring that a compound is used only by specific bacterial populations through controlled release. For example, substances such as resveratrol, fumaric acid, probiotics, conjugated linoleic acid, and ionophores, among others, have been used to reduce methane emission by changing the rumen bacterial population or stabilizing the intestinal microbiota86-89.
Hydroxycinnamic acid pharmacokinetics in monogastrics
In monogastrics phenolic compounds more easily preserve their structure and therefore experience a lower degradation-transformation rate. It is thus more probable that they can exercise some effect, mainly as antioxidants, because their rapid absorption and entrance into the bloodstream can prevent free radical generation by oxidative stress23,40. Hydroxycinnamic acids have also been reported to be antimicrobial agents in the intestinal microbiota or against pathogenic species, and anti-inflammatory agents that improve nutrient absorption by improving bowel physiology31. Understanding these activities, however, requires identifying the initial structure of the HA administered and all its subsequent structural changes, which may limit its effects24,78.
In monogastric animals such as pigs, cinnamic acid derives from cinnamaldehyde present in the feed, which later oxidizes into cinnamic acid in the stomach and small intestine. Average estimated life for this compound in this animal ranges from three to 5 h post-administration. Certain HA may already be circulating in the bloodstream, but transporters are needed to convey them to the intended target tissue. Serum albumin is one of the principal metabolite carriers in the organism and has recently been shown to have affinity for chlorogenic acid, ferulic acid and cinnamic acid; this could be of interest for investigating its affinity in organs such as the liver, kidneys, intestine and muscle tissue54.
Studies with caffeic acid and ferulic acid have shown that, much like cinnamic acid (approximately 90 % absorption), these compounds are rapidly absorbed in the stomach and small intestine25. Caffeic acid is rapidly absorbed within the first two hours post-feeding, but, due to its non-ionized form, can also undergo passive absorption in the stomach53.
After absorption in the organism HA can be found intact in the plasma or urine, but also in conjugated forms such as glucuronide, sulfates or sulfa-glucuronides. However, depending on their interest in these metabolites, intestinal microbial populations can transform HA. Monocarboxylic acid transporters responsible for absorption of some phenolic acids (including HA) may be present in different tissues24, and could be involved in transport of absorption processes in target tissues such as the liver, fat or muscle.
Hydroxycinnamic acid bioavailability
When substances such as drugs or dietary compounds are administered to an organism they are subject to a series of mechanisms that alter their structure and reduce compound bioavailability and consequently any possible biological activity. Bioavailability can therefore be defined as the percentage or fraction of a compound available in an intact form that reaches the target tissue, considering any changes this compound may have experienced as it passes through each stage of the digestive process90.
Most phenolic compounds, including HA, have beneficial effects but exhibit very low bioavailability when included in diets. This may be because these compounds are embedded in the polymeric matrices of arabinoxylans, pectins and xyloglucans, limiting their potential action in the organism. In addition, microbial changes produced in the gastrointestinal tract can produce conjugated forms of HA(24, 91,92). Most studies focused on the use of plants and plant extracts in livestock involve a large number of phenolic compounds, including HA. This makes it difficult to determine which of these compounds is responsible for any observed improvement in animal growth, health status or metabolic changes. True in vivo availability is actually limited and possible benefits are attributed to mixtures of compounds and conjugated forms rather than to individual compounds31,34,36,93; in vitro studies are therefore needed to test isolated HA.
Studies in ruminants report that in the ileum FA (4 mg/ml) and p-coumaric acid (9 mg/ml) are released from forage. These levels are notably higher than in the rumen (< 1.0 mg/ml), possibly due to the complexity of the matrix and the enzymes and microorganisms that structurally modify these monomers50,83.
Encapsulation is a promising strategy for improving the probability that HA arrive at target tissues. Some studies using other matrix-immersed compounds have shown that they can exert significant effects on animal metabolism. In one study, dairy cows were administered an encapsulated cinnamaldehyde and gallic acid mixture (300 mg/d) for 15 d, which increased total rumen volatile fatty acids concentration vs the control (108.9 mmol/L vs 98.3 mmol/L) and improved milk production (3 kg/d more than control)94. The authors attribute this increase largely to modification of rumen microbial populations which raised AGV by reducing methane gas generation, thus allowing more efficient use of the energy in the feed. A different encapsulated mixture of cinnamaldehyde (100 g/t feed) and thymol (150 g/t feed) administered in pigs improved daily weight gain vs a control (0.45 vs 0.37 g/d) and lowered the rate of diarrhea by 50 %95. This was due to optimal modulation of intestinal microbiota, particularly a reduction in E. coli populations, which improved animal immune system functioning.
Encapsulation has also been used with other molecules with efficient results. For instance, encapsulating zinc (100 ppm) in a 10 % lipid covering helps to mitigate the symptoms of colibacillosis in weaned pigs96. Protecting probiotic cultures by encapsulation is known to improve nutrient digestibility and absorption, improve immune system function and prevent infections in both ruminant and monogastric species97,98,99. Design of HA encapsulation systems is likely to prove a valuable technique in administering these compounds in animals and thus clarifying their route of action and their effects.
Pharmacodynamic of hydroxycinnamic acids in growing animals
Pharmacodynamics is the study of a compound’s action at specific sites and different levels (e.g. sub-molecular, molecular, cellular, tissue, organ or organism) using in vivo and/or in vitro models, and different techniques and instruments to identify its effective action in the organism100. Hydroxycinnamic acids employ different mechanisms and cause modifications at various biological levels which can be translated into benefits for the organism such as better growth performance or maintenance of oxidative status. However, what evidence exists for their pharmacodynamics is inconclusive and for many it is non-existent.
Very few reports are currently available on HA pharmacodynamics in growing animals aimed at understanding their growth promoter effect. As an additive in diets for cattle FA has exhibited interesting effects in vitro and in vivo, be it in a pure form or as a diet ingredient14,81,92. In vivo, FA has been reported to affect enzyme and hormone profiles in both ruminants and pigs (Table 1)44,72,73.
Table 1 Pharmacodynamics of hydroxycinnamic acids in fattening animals and in vitro tests
| Species | Additive | Site of action | Response | Author |
|---|---|---|---|---|
| Heifers | Ferulic acid (100 mg and 500 mg) | Plasma | Increase in the concentration of prolactin and growth hormone | (44) |
| Ruminant | 0.1%, 0.2% of p-coumaric, ferulic and synaptic acid | Rumen | The cellulolytic population in rumen is not modified; only p-coumaric acid presents a reduction of bacteria responsible for fiber degradation | (55) |
| Pigs | Ferulic acid (100 mg/kg feed) | Plasma | Increase of antioxidant enzymes GPx11 and NFE2L2-ARE2, and reduction of malonaldehyde concentration in blood | (40) |
| Pigs | Ferulic acid (150 mg/kg) | Ear | Increase in the synthesis of the hemo-oxygenase-1 enzyme and free radicals reduction | (101) |
| Pigs | Plant extracts including hydroxycinnamic acids | Plasma | Increase Insulinic Growth Factor-1 (IGF-1) | (102) |
| In vitro | Cinnamic acid | Adipocytes | Activation of AMPk3, responsible for the activation of lipolytic and lipogenic enzymes in the cell | (45) |
1GPx1= Glutathione peroxidase-1; 2 NFE2L2-ARE= nuclear factor, erythroid 2 like 2.3AMPk= AMP-activated protein kinase.
One study of FA supplementation in cows found an increase in growth hormone and serum prolactin concentrations, suggesting possible alteration of the pituitary gland and consequently greater muscle protein deposition44. An evaluation of changes in rumen microbial populations in response to 0.1 % and 0.2 % concentrations of ferulic acid, sinapic acid and p-coumaric acid found ferulic and sinapic acid to have little effect on the cellulolytic bacteria responsible for fiber degradation, indicating these acids did not limit bacterial viability and maintained normal fiber degradation levels55. However, p-coumaric acid exhibited a pronounced ability to traverse the cell wall of cellulolytic bacteria and protozoans, exercising an antimicrobial effect that limited nutrient digestibility and lowered microbial protein concentration.
When FA was supplemented in finishing pigs it increased activity of the antioxidant enzymes GPX1 (glutathion peroxidase 1) and NFE2L2 (nuclear factor [erythroid-derived 2]-like 2)-ARE, but without significant changes in productive performance and carcass yields40. A characteristic effect of synthetic β-adrenergic agonist compounds is reduction of dorsal fat deposition. In a recent study43, FA supplementation in finishing pigs caused a similar effect, reduction of dorsal fat, possibly due to stimulation of hormone-sensitive lipase (not evaluated), which is responsible for fatty acids degradation and redirection of the energy from fat to muscle deposition. A neuroprotective effect has been reported with supplementation of FA in pigs, attributable to its ability to eliminate free radicals and regulate the cytoprotective enzyme heme oxygenase-1 (HO-1) in confined animals subjected to constant noise101.
Overall this literature review highlights the limited extent of research on the pharmacodynamics of HA in growing livestock. Most studies have been done with rats, and much more data is needed on the direct effects of HA in ruminants and monogastric species to better understand their growth promoting mechanisms.
Conclusions and implications
Use of hydroxycinnamic acids in animal diets is not currently common practice. However, they hold promise since their application is known to result in positive changes in animal growth and meat quality. Very little research is yet available on the metabolism of hydroxycinnamic acids when supplemented in animal diets. Future use of these compounds depends on studying these beneficial effects and the metabolic pathways activating or inhibiting them. Additional variables also need study such as toxicity, allergic effects, antioxidants in meat and production costs. Some research has been done on the pharmacokinetics and biotransformation of isolated hydroxycinnamic acids, mainly in rats or in vitro models. Very little information is available on p-coumaric acid, chlorogenic acid and sinapic acid in animal models, and some reports suggest they have negative effects on growth. Growth performance tests in various animal models using low doses of hydroxycinnamic acids such as ferulic acid could help to determine if these effects are general. Overall, more accurate and comprehensive research is needed on the action of hydroxycinnamic acids in the animal production chain.
Literatura citada:
1. Mersmann HJ. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J Anim Sci 1998;76(1):160-172. [ Links ]
2. Dávila-Ramírez JL, Avendaño-Reyes L, Macías-Cruz U, Torrentera-Olivera NG, Zamorano-García L, Peña-Ramos A, González-Ríos H. Effects of zilpaterol hydrochloride and soybean oil supplementation on physicochemical and sensory characteristics of meat from hair lambs. Small Rumin Res 2013;114(2):253-257. [ Links ]
3. Avendaño L, Torres V, Meraz F, Pérez C, Figueroa F, Robinson P. Effects of two b-adrenergic agonists on finishing performance, carcass characteristics, and meat quality of feedlot steers. J Anim Sci 2006;84:3259-3265. [ Links ]
4. Vallejos A, Zaragoza JC, Parres JA. Intoxicación por clembuterol”, Sistema Nacional de Vigilancia Epidemiológica. 2007;18:24. [ Links ]
5. Smith D. The pharmacokinetics, metabolism, and tissue residues of beta-adrenergic agonists in livestock. J Anim Sci 1998;76(1):173-194. [ Links ]
6. Pulce C, Lamaison D, Keck G, Bostvironnois C, Nicolas J, Descotes J. Collective human food poisonings by clenbuterol residues in veal liver. Vet Hum Toxicol 1991;33(5):480-481. [ Links ]
7. Kuiper H, Noordam M, van Dooren-Flipsen M, Schilt R, Roos A. Illegal use of beta-adrenergic agonists: European Community. J Anim Sci 1998;76(1):195-207. [ Links ]
8. Lopez-Romero JC, Ansorena R, Gonzalez-Aguilar GA, Gonzalez-Rios H, Ayala-Zavala JF, Siddiqui MW. In: Plant secondary metabolites, Vol 2: Stimulation, extraction, and utilization. Chap 5: Applications of plant secondary metabolites in food systems. 1rst ed. Waretown, NJ, USA: Apple Academic Press; 2016:195-232. [ Links ]
9. Gottlieb OR. Phytochemicals: differentiation and function. Phytochemistry 1990;29(6):1715-1724. [ Links ]
10. Meskin MS, Bidlack WR, Davies AJ, Omaye ST. Phytochemicals in nutrition and health.1rst ed. Florida, USA: Chemical Rubber Company press; 2002. [ Links ]
11. Alemanno A, Capodieci G. Testing the limits of global food governance: the case of ractopamine. Eur J Risk Regul 2012;(12):400-407. [ Links ]
12. Patra AK, Saxena J. Dietary phytochemicals as rumen modifiers: a review of the effects on microbial populations. Antonie Leeuwenhoek 2009;96(4):363-375. [ Links ]
13. Chen JH, Ho C-T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agric Food Chem 1997;45(7):2374-2378. [ Links ]
14. Scalbert A, Andres-Lacueva C, Arita M, Kroon P, Manach C, Urpi-Sarda M, Wishart D. Databases on food phytochemicals and their health-promoting effects. J Agric Food Chem 2011;59(9):4331-4348. [ Links ]
15. Jiang J, Xiong YL. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci 2016;120:107-117. [ Links ]
16. Waghorn GC, McNabb WC. Consequences of plant phenolic compounds for productivity and health of ruminants. Proc Nutr Soc 2003;62(02):383-392. [ Links ]
17. González-Ríos H, Dávila-Ramírez J, Peña-Ramos E, Valenzuela-Melendres M, Zamorano-García L, Islava-Lagarda T, Valenzuela-Grijalva N. Dietary supplementation of ferulic acid to steers under commercial feedlot feeding conditions improves meat quality and shelf life. Anim Feed Sci Technol 2016;222:111-121. [ Links ]
18. Labarca J. Nuevos conceptos en farmacodinamia: ¿debemos repensar cómo administramos antimicrobianos? Rev Chilena de Infectol 2002;19:S33-S37. [ Links ]
19. Niño-Medina G, Carvajal-Millán E, Lizardi J, Rascon-Chu A, Marquez-Escalante JA, Gardea A, Martinez-Lopez AL, Guerrero V. Maize processing waste water arabinoxylans: Gelling capability and cross-linking content. Food Chem 2009;115(4):1286-1290. [ Links ]
20. Maeda H, Dudareva N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol 2012;63:73-105. [ Links ]
21. Kroon PA, Williamson G. Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agric 1999;79(3):355-361. [ Links ]
22. Razzaghi-Asl N, Garrido J, Khazraei H, Borges F, Firuzi O. Antioxidant properties of hydroxycinnamic acids: a review of structure-activity relationships. Curr Med Chem 2013;20(36):4436-4450. [ Links ]
23. Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci 2008;86(Suppl 14):E140-E148. [ Links ]
24. Lafay S, Gil-Izquierdo A. Bioavailability of phenolic acids. Phytochem Rev 2008;7(2):301-311. [ Links ]
25. El-Seedi H, El-Said A, Khalifa S, Göransson U, Bohlin L, Borg-Karlson A-K, Verpoorte R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J Agric Food Chem 2012;60(44):10877-10895. [ Links ]
26. Asaff TA, De La Torre MM, Macias OR. Proceso para la recuperación de ácido ferúlico.. 2004. https://www.google.com/patents/WO2004110975A1?cl=en . Consultado Nov 8, 2016. [ Links ]
27. Crozier A, Jaganath IB, Clifford MN. Phenols, polyphenols and tannins: an overview. 494 Plant secondary metabolites: Occurrence, structure and role in the human diet. Oxford, 495 UK: John Wiley & Sons; 2006. [ Links ]
28. Rocha LD, Monteiro MC, Teodoro AJ. Anticancer properties of hydroxycinnamic acids-A Review. Canc Clin Onc 2012;1(2):109. [ Links ]
29. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004;74(17):2157-2184. [ Links ]
30. Alam MA, Subhan N, Hossain H, Hossain M, Reza HM, Rahman MM, Ullah MO. Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr Metab 2016;13(1):27. [ Links ]
31. Valenzuela-Grijalva NV, Pinelli-Saavedra A, Muhlia-Almazan A, Domínguez-Díaz D, González-Ríos H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J Anim Sci Technol 2017;59(1):8. [ Links ]
32. Hong J-C, Steiner T, Aufy A, Lien T-F. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest Sci 2012;144(3):253-262. [ Links ]
33. Zhao Z, Moghadasian MH. Bioavailability of hydroxycinnamates: a brief review of in vivo and in vitro studies. Phytochem Rev 2010;9(1):133-145. [ Links ]
34. García-González R, López S, Fernández M, González JS. Effects of the addition of some medicinal plants on methane production in a rumen simulating fermenter (RUSITEC). Int Cong Series 2006;1293(0):172-175. [ Links ]
35. Busquet M, Calsamiglia S, Ferret A, Kamel C. Plant extracts affect in vitro rumen microbial fermentation. J Dairy Sci 2006;89(2):761-771. [ Links ]
36. Hashemi SR, Davoodi H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet Res Commun 2011;35(3):169-180. [ Links ]
37. Sørum H, Sunde M. Resistance to antibiotics in the normal flora of animals. Vet Res 2001;32(3-4):227-241. [ Links ]
38. Aarestrup FM, Jenser LB. Use of antimicrobials in food animal production. In: Simjee S. 528 foodborne diseases. Series Infectious Disease. Human Press Inc. Totowa NJ, USA. 529 2007:405-417. [ Links ]
39. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 2003;78(3):517S-520S. [ Links ]
40. Li Y, Li L, Li J, Zhang L, Gao F, Zhou G. Effects of dietary supplementation with ferulic acid or vitamin E individually or in combination on meat quality and antioxidant capacity of finishing pigs. Asian-Australas J Anim Sci 2015;28(3):374. [ Links ]
41. Macías-Cruz U, Perard S, Vicente R, Álvarez F, Torrentera-Olivera N, González-Ríos H, Soto-Navarro S, Rojo R, Meza-Herrera C, et al. Effects of free ferulic acid on productive performance, blood metabolites, and carcass characteristics of feedlot finishing ewe lambs. J Anim Sci 2014;92(12):5762-5768. [ Links ]
42. Peña-Torres EF. Efecto de la suplementación de ácido ferúlico y ferulato de etilo en el comportamiento productivo y calidad de la carne de bovinos [tesis maestría]. Hermosillo, Sonora, México: Centro de Investigación en Alimentación y Desarrollo; 2014. [ Links ]
43. Herrera RH, Castillo MLA, Torres AJA. Methods to accelerate muscle development, 604 decrease fat deposits, and enhance feeding efficiency in pigs. Methods to accelerate 605 muscle development, decrease fat deposits, and enhance feeding efficiency in pigs. U.S. 606 patent published in February 24, 2011, Num 20110046224. 607 607 http://www.faqs.org/patents/app/20110046224 ; 2009. Accessed Feb 26, 2017. [ Links ]
44. Gorewit R. Pituitary and thyroid hormone responses of heifers after ferulic acid administration. J Dairy Sci 1983;66(3):624-629. [ Links ]
45. Kopp C, Singh SP, Regenhard P, Müller U, Sauerwein H, Mielenz M. Trans-cinnamic acid increases adiponectin and the phosphorylation of AMP-activated protein kinase through G-protein-coupled receptor signaling in 3T3-L1 adipocytes. Int J Mol Sci 2014;15(2):2906-2915. [ Links ]
46. Yang W, Ametaj B, Benchaar C, He L, Beauchemin K. Cinnamaldehyde in feedlot cattle diets: Intake, growth performance, carcass characteristics, and blood metabolites. J Anim Sci 2010;88:1082-1092. [ Links ]
47. Cardozo P, Calsamiglia S, Ferret A, Kamel C. Effects of alfalfa extract, anise, capsicum, and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high-concentrate diet. J Anim Sci 2006;84(10):2801-2808. [ Links ]
48. Chesson A, Stewart CS, Wallace RJ. Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria. Appl Environ Microbiol 1982;44(3):597-603. [ Links ]
49. Borneman WS, Akin D, VanEseltine W. Effect of phenolic monomers on ruminal bacteria. Appl Environ Microbiol 1986;52(6):1331-1339. [ Links ]
50. Jung H-JG, Fahey GC, Merchen NR. Effects of ruminant digestion and metabolism on phenolic monomers of forages. Br J Nutr 1983;50(3):637-651. [ Links ]
51. Adam A, Crespy V, Levrat-Verny MA, Leenhardt F, Leuillet M, Demigné C, Rémésy C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J Nutr 2002;132(7):1962-1968. [ Links ]
52. Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem 2008;109(4):691-702. [ Links ]
53. Olthof MR, Hollman PC, Katan MB. Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 2001;131(1):66-71. [ Links ]
54. Li S, Huang K, Zhong M, Guo J, Wang WZ, Zhu R. Comparative studies on the interaction of caffeic acid, chlorogenic acid and ferulic acid with bovine serum albumin. Spectrochim Acta A Mol Biomol Spectrosc 2010;77(3):680-686. [ Links ]
55. Akin DE. Forage cell wall degradation and ρ-coumaric, ferulic, and sinapic acids. Agron J 1982;74(3):424-428. [ Links ]
56. Karre L, Lopez K, Getty KJ. Natural antioxidants in meat and poultry products. Meat Sci 2013;94(2):220-227. [ Links ]
57. Falowo AB, Fayemi PO, Muchenje V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res Int 2014;64:171-181. [ Links ]
58. Chan W, Hakkarainen K, Faustman C, Schaefer D, Scheller K, Liu Q. Dietary vitamin E effect on color stability and sensory assessment of spoilage in three beef muscles. Meat Sci 1996;42(4):387-399. [ Links ]
59. Faustman C, Chan W, Schaefer D, Havens A. Beef color update: the role for vitamin E. J Anim Sci 1998;76(4):1019-1026. [ Links ]
60. Morrissey PA, Buckley DJ, Galvin K, Decker E, Faustman C, Lopez-Bote CJ. Antioxidants 636 in muscle foods: nutritional strategies to improve quality. Vitamin E and the oxidative 637 stabilities of pork and poultry. 1rst ed. USA: John Wiley & Sons; 2000:263-287. [ Links ]
61. Cox S, Gupta S, Abu-Ghannam N. Effect of different rehydration temperatures on the moisture, content of phenolic compounds, antioxidant capacity and textural properties of edible Irish brown seaweed. Food Sci Tech 2012;47(2):300-307. [ Links ]
62. Bloomberg BD, Hilton GG, Hanger KG, Richards CJ, Morgan JB, VanOverbeke DL. Effects of vitamin E on color stability and palatability of strip loin steaks from cattle fed distillers grains. J Anim Sci 2011;89(11):3769-3782. [ Links ]
63. Yang A, Lanari M, Brewster M, Tume R. Lipid stability and meat colour of beef from pasture-and grain-fed cattle with or without vitamin E supplement. Meat Sci 2002;60(1):41-50. [ Links ]
64. McDowell L, Williams S, Hidiroglou N, Njeru C, Hill G, Ochoa L, Wilkinson N. Vitamin E supplementation for the ruminant. Anim Feed Sci Technol 1996;60(3):273-296. [ Links ]
65. Brenes A, Viveros A, Goñí I, Centeno C, Sayago-Ayerdy S, Arija I, Saura-Calixto F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult Sci 2008;87(2):307-316. [ Links ]
66. Cho J, Kim H, Kim I. Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers. Livest Sci 2014;160:82-88. [ Links ]
67. Biquan Z, Lu C, Lianqiang C. Effects of phytogenic feed additives on growth performance, carcass, meat quality and pork antioxidative capacity in finishing pigs. J China Feed 2011;14:007. [ Links ]
68. Descalzo A, Sancho A. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci 2008;79(3):423-436. [ Links ]
69. Peña E, González-Ríos H, Islava T, Valenzuela-Melendres M, Peña-Ramos A, Zamorano L, Pinelli A, Dávila-Ramírez JL. 0896 Ferulic acid in diets of heifers and its effect on the oxidative stability of meat stored in refrigeration. J Anim Sci 2016;94 (Suppl 5):431-432. [ Links ]
70. Faustman C, Sun Q, Mancini R, Suman SP. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci 2010;86(1):86-94. [ Links ]
71. Jiménez CIE, Martínez EYC, Fonseca JG. Flavonoides y sus acciones antioxidantes. Rev Fac Med UNAM 2009;52(2). [ Links ]
72. Soberon M, Cherney D, Cherney J. Free ferulic acid uptake in ram lambs. J Anim Sci 2012;90(6):1885-1891. [ Links ]
73. Soberon M, Cherney J, Liu R, Ross D, Cherney D. Free ferulic acid uptake in lactating cows. J Dairy Sci 2012;95(11):6563-6570. [ Links ]
74. Marcucci MC, Ferreres F, Garcıa-Viguera C, Bankova V, De Castro S, Dantas A, Valente P, Paulino N. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol 2001;74(2):105-112. [ Links ]
75. Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med 2010;31(6):446-467. [ Links ]
76. Aura AM. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem Rev 2008;7(3):407-429. [ Links ]
77. Schoonmaker J. Novel feed additives for beef cattle. 76th Minnesota Nutrition Conference. Prior Lake, MN; 2015:130-142. [ Links ]
78. Flachowsky G, Lebzien P. Effects of phytogenic substances on rumen fermentation and methane emissions: A proposal for a research process. Anim Feed Sci Technol 2012;176(1-4):70-77. [ Links ]
79. Acamovic T, Brooker J. Biochemistry of plant secondary metabolites and their effects in animals. Proc Nutr Soc 2005;64(03):403-412. [ Links ]
80. Chen Y, Ma Y, Ma W. Pharmacokinetics and bioavailability of cinnamic acid after oral administration of Ramulus Cinnamomi in rats. Eur J Drug Metab Pharmacokinet 2009;34(1):51-56. [ Links ]
81. Chesson A, Provan GJ, Russell WR, Scobbie L, Richardson AJ, Stewart C. Hydroxycinnamic acids in the digestive tract of livestock and humans. J Sci Food and Agric 1999;79(3):373-378. [ Links ]
82. Martin A. The origin of urinary aromatic compounds excreted by ruminants 2. The metabolism of phenolic cinnamic acids to benzoic acid. Br J Nutr 1982;47(01):155-164. [ Links ]
83. Kondo T, Ohshita T, Kyuma T. Comparison of phenolic acids in lignin fractions from forage grasses before and after digestion by sheep. Anim Feed Sci Technol 1994;47(3-4):277-285. [ Links ]
84. Besle J-M, Jouany J-P, Cornu A. Transformations of structural phenylpropanoids during cell wall digestion. FEMS Microbiol Rev 1995;16(1):33-52. [ Links ]
85. Lajoie MS, Cummings KR. Encapsulated dietary fatty acid salt products for ruminants. 566 Encapsulated dietary fatty acid salt products for ruminants. Google Patents; 1999. 567 567 https://www.google.com/patents/US5874102 . Accessed Jun11, 2017. [ Links ]
86. Augustin MA, Sanguansri L, Lockett T. Nano‐and micro‐encapsulated systems for enhancing the delivery of resveratrol. Ann New York Acad Sci 2013;1290(1):107-112. [ Links ]
87. Wood T, Wallace R, Rowe A, Price J, Yáñez-Ruiz D, Murray P, Newbold C. Encapsulated fumaric acid as a feed ingredient to decrease ruminal methane emissions. Anim Feed Sci Technol 2009;152(1):62-71. [ Links ]
88. Soto L, Frizzo L, Avataneo E, Zbrun M, Bertozzi E, Sequeira G, Signorini M, Rosmini M. Design of macrocapsules to improve bacterial viability and supplementation with a probiotic for young calves. Anim Feed Sci Technol 2011;165(3):176-183. [ Links ]
89. Chandler TL, Fugate RT, Jendza JA, Troescher A, White HM. Conjugated linoleic acid supplementation during the transition period increased milk production in primiparous and multiparous dairy cows. Anim Feed Sci Technol 2017;224:90-103. [ Links ]
90. Gessner D, Ringseis R, Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr 2016;1-24. [ Links ]
91. Turner AL, Shewry PR, Lovegrove A, Spencer JPE. Release of covalently bound 587 hydroxycinnamate, ferulic acid, from whole-grain. Proc Nutr Soc 2015;74: E113. [ Links ]
92. Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem 2002;50(7):2161-2168. [ Links ]
93. Vasta V, Luciano G. The effects of dietary consumption of plants secondary compounds on small ruminant products quality. Small Rumin Res 2011;101(1):150-159. [ Links ]
94. Blanch M, Carro MD, Ranilla MJ, Viso A, Vázquez-Añón M, Bach A. Influence of a mixture of cinnamaldehyde and garlic oil on rumen fermentation, feeding behavior and performance of lactating dairy cows. Anim Feed Sci Technol 2016;219(Suppl C):313-323. [ Links ]
95. Li SY, Ru YJ, Liu M, Xu B, Péron A, Shi XG. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest Sci 2012;145(1):119-123. [ Links ]
96. Kwon CH, Lee CY, Han SJ, Kim SJ, Park BC, Jang I, Han JH. Effects of dietary supplementation of lipid‐encapsulated zinc oxide on colibacillosis, growth and intestinal morphology in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim Sci J 2014;85(8):805-813. [ Links ]
97. Zhang L, Li J, Yun TT, Li AK, Qi WT, Liang XX, Wang YW, Liu S. Evaluation of pilot-scale microencapsulation of probiotics and product effect on broilers. J Anim Sci 2015;93(10):4796-4807. [ Links ]
98. Jiao PX, Wei LY, Walker ND, Liu FZ, Chen LY, Beauchemin KA, Yang WZ. Comparison of non-encapsulated and encapsulated active dried yeast on ruminal pH and fermentation, and site and extent of feed digestion in beef heifers fed high-grain diets. Anim Feed Sci Technol 2017;228(Suppl C):13-22. [ Links ]
99. Meng QW, Yan L, Ao X, Zhou TX, Wang JP, Lee JH, Kim IH. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. J Anim Sci 2010;88(10):3320-3326. [ Links ]
100. Lees P, Cunningham F, Elliott J. Principles of pharmacodynamics and their applications in veterinary pharmacology. J Vet Pharmacol Ther 2004;27(6):397-414. [ Links ]
101. Fetoni AR, Mancuso C, Eramo SLM, Ralli M, Piacentini R, Barone E, Paludetti G, Troiani D. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience 2010;169(4):1575-1588. [ Links ]
102. Liu G, Wei Y, Wang Z, Wu D, Zhou A, Liu G. Effects of herbal extract supplementation on growth performance and insulin-like growth factor (IGF)-I system in finishing pigs. J Anim Feed Sci 2008;17(4):538-547. [ Links ]
Received: June 12, 2017; Accepted: April 26, 2018











 text in
text in