Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.10 n.1 Mérida Jan./Mar. 2019
https://doi.org/10.22319/rmcp.v10i1.4350
Technical Notes
Impact of health monitoring of clenbuterol in Guerrero, Mexico: Results from 2011 to 2015
a Universidad Autónoma de Guerrero. Facultad de Ciencias Químico Biológicas. Guerrero, México.
b Secretaría de Salud de Guerrero. Laboratorio Estatal de Salud Pública “Dr. Galo Soberón y Parra”. Guerrero, México.
cCentro de Investigación Científica y de Educación Superior de Ensenada, Baja California. México.
The use of clenbuterol in livestock production entails risks to human health that must be characterized and managed by the agricultural and health authorities. This work evaluated the results of the health surveillance program of this compound in bovine meat products that are commercialized in Guerrero, Mexico. A retrospective analysis of the evolution of the illegal use of clenbuterol and of the record of food poisoning with this chemical was carried out for the 2011-2015 period. The rate of positive samples decreased gradually to be 6.8 % in 2015 and, in general, was lower in comparison with the immediate antecedents; however, it was always higher than the average rates obtained in the rest of the country (Guerrero= 9.5 %, National= 5.8 %). The health jurisdictions with the greatest presence of the drug were Tierra Caliente and the North, with 12.1 and 16.8 %, respectively, as well as in locations with a large population like Acapulco (9.6 %). Few cases of human poisoning were reported in the first three years of the period (3 in 2011, 5 in 2012, and 4 in 2013) and none in the two subsequent years; however, there is an under-registration issue, which may be due to different individual, regional and institutional factors that are discussed further below. The progress observed in this program should be maintained by strengthening health surveillance with the aim to eradicate this illegal activity in the short term.
Key words: Clenbuterol; Food poisoning; Health surveillance
El empleo del clembuterol en la producción de ganado implica riesgos a la salud humana que deben ser evaluados y atendidos por las autoridades agropecuarias y sanitarias. En este trabajo se evaluaron los resultados del programa de vigilancia sanitaria de dicho compuesto en productos cárnicos de bovinos que se comercializan en Guerrero, México. Se realizó un análisis retrospectivo del periodo comprendido entre 2011 y 2015, sobre la evolución de su uso ilegal y el registro de intoxicaciones alimentarias por clembuterol. Los porcentajes de muestras positivas disminuyeron gradualmente hasta quedar en 2015 en 6.8 % y, en general, fueron más bajos en comparación con los antecedentes inmediatos; sin embargo, siempre fueron superiores a los porcentajes promedio del resto del país (Guerrero= 9.5 %, Nacional= 5.8 %). Las jurisdicciones sanitarias con mayor presencia del fármaco fueron Tierra Caliente y Norte, con 12.1 y 16.8 %, respectivamente, así como en lugares con una población grande como Acapulco (9.6 %). Se reportaron pocos casos de intoxicaciones humanas en los tres primeros años (2011= 3, 2012= 5 y 2013= 4) y ninguno en los dos posteriores; sin embargo, hay un sub-registro que puede deberse a diversos factores tanto individuales, regionales e institucionales que se discuten más adelante. El avance observado en este programa ha sido significativo; sin embargo, debe fortalecerse la vigilancia sanitaria con la finalidad de erradicar esta actividad ilegal en el corto plazo.
Palabras clave: Clembuterol; Intoxicación alimentaria; Vigilancia sanitaria
Clenbuterol is an antagonist compound of the beta adrenergic receptor of the vascular, myometrial, and bronchial smooth muscle, used in veterinary medicine as a bronchodilator and tocolytic drug1; in certain countries, its administration is authorized as a treatment for respiratory illnesses in humans. Because this compound acts as a distributing agent in animals by eliminating fats and promoting the synthesis of proteins, it causes accelerated growth of the muscle mass, and is therefore utilized, illegally and indiscriminately, to increase meat production in bovine livestock2-5. The ingestion of foods with clenbuterol residues entails a hazard for consumers, as it can cause serious intoxications associated to such symptoms as palpitations, tachycardia, headache, tremor in the extremities, high blood pressure, anxiety, nervousness, itch, nausea, stomach ache, fever, vomiting, asthenia and muscle weakness, accompanied by alterations of certain hematological (leucocytes) and chemical (electrolytes, glucose) parameters6-10.
In order to protect the population against hazards due to the consumption of foods contaminated with clenbuterol, the Undersecretariat for Food Regulation and Control and Health Promotion, dependent upon the Department of Health of the State of Guerrero, Mexico, has implemented the Food Innocuousness Program (formerly known as Potentially Hazardous Foods Project), since the year 2005, based on the Mexican Official Norm NOM-194-SSA1-200411, which consists of verification visits to various establishments, such as markets, slaughterhouses and supermarkets, where samples of meat products are collected by the “Dr. Galo Soberón y Parra” Public Health Laboratory of the State of Guerrero (LESP-Guerrero) in order to detect clenbuterol, using the Ridascreen Clenbuterol Fast enzyme immunoassay (r-biopharm, Germany)12. The purpose of this study was to evaluate the results of this program in order to determine its effectiveness in reducing the illegal use of clenbuterol in the production of beef in the state of Guerrero.
This is a retrospective, comparative, transversal observational study. The results of the health surveillance program for clenbuterol implemented in Guerrero between the years 2011 and 2015 were analyzed in order to determine the suitability of the sampling scheme and establish whether there was a tendency in the percentages of positive cases through the years, as well as compare the results obtained in the state with the situation across the country. The use of clenbuterol was analyzed at the regional and municipal level, and the number of food intoxications derived from this illegal practice was reported.
Two hundred fifty (250) g samples of liver and muscles (and, occasionally, eyeball and urine samples) of bovine cattle were collected and screened for clenbuterol. The samples were stored in polyethylene bags and plastic jars with a screw cap (for urine samples); they were perfectly identified and transported to LESP-Guerrero at a temperature of 2 to 8º C. The samples were prepared as follows:
A) Liver and muscle. 5 g of the previously ground sample were placed in a 50 ml (CorningTM, USA) centrifuge tube. 25 ml of HCl 0.05 M were then added, and the tube was mechanically shaken during 20 min. It was subsequently centrifuged at 4,000 xg for 15 min at a temperature of 10 to 15 ºC, after which 18 ml of supernatant were transferred to another tube, to which 2 ml of NaOH 0.5 M were added and mixed during 10 min. Finally, 4 ml of a phosphate buffer (KH2PO4 0.5 M pH 3) were added, and the mixture was homogenized and left to stand for another 90 min at 2-8 ºC. The mix was then centrifuged, and 10 ml of supernatant were drawn and passed through a C18 type solid-phase extraction column (R-Biopharm, Germany).
B) Eyeball. The pieces were frozen at -20 ºC during 24 h in order to lyse the tissue. They were subsequently dissected, and the extracted fluid was diluted in distilled water at a ratio of 1:2 and centrifuged at 2 000 xg during 5 min. 20 µl of the supernatant were drawn for the immunoassay.
C) Urine. These samples were analyzed directly without any previous extraction treatment, and they were centrifuged at 2 000 xg during 5 min only when they exhibited turbidity. The adequate volume for the analysis was 30 to 50 ml.
The solid phase extraction was carried out by conditioning the C18 column with 3 ml of methanol, and 2 ml of a rinse buffer (KH2PO4 0.05 M pH 3); the supernatant was applied, and 3 ml of rinse buffer were added. The column was dried and slowly eluted with 1 ml of HPLC-degree methanol (Honeywell Burdick and Jackson, USA). The extract was concentrated at 50 to 60 ºC under a stream of air; the dry residue was reconstituted with 400 µl of HPLC-degree water (Sigma Aldrich, Switzerland).
The extracts were analyzed by means of a competitive enzyme immunoassay (Ridascreen Clembuterol Fast, R-Biopharm, Germany)12 with a reading on a UV-Vis iMark spectrophotometer (Bio-Rad Laboratories Inc, USA) at 450 nm; the clenbuterol concentration was expressed in ng/kg or ppb (parts per billion). According to the norm NOM-194-SSA1-200411, those samples with concentrations above 2 000 ng/kg were considered to be contaminated, detected or positive. Samples with lower results were interpreted as “not detected”. As for the analytical quality of the method, the acceptance criteria described in the reagent’s insert were met12. Likewise, in each run, the commercial controls and samples with known concentrations were analyzed, and the expected values were always obtained. Notably, this Laboratory has been enabled by the Federal Commission of Protection against Health Risks (Comisión Federal para la Protección contra Riesgos Sanitarios, COFEPRIS), as the third laboratory authorized to conduct analyses for health regulation and control, as it fulfills the technical and quality management requirements established in the norm NMX-EC-17025-IMNC-200613.
The results of the program were statistically analyzed by means of a correlation study (Pearson’s r coefficient) between the number of collected specimens and such variables as the population and the dressed beef output of the visited municipalities. The percentage of contaminated samples was subsequently calculated according to the Health Jurisdiction (HJ)> of the studied provenance and year; graphs were developed on which the annual percentages of contaminated samples in Guerrero were related to the nationwide percentages, as well as with the number of persons intoxicated during the studied period. The results calculated for the HJs and the municipalities were depicted in thematic maps. These analyses and graphs were developed using the SPSS version 21 (IBM Corporation, USA) and ArcGIS version 9.3 (ESRI, USA) software.
According to the analysis of the results, the sample collection was not uniform as to the quantity, as in 2011 more meat products were analyzed than in any other year (n= 172), while the number of analyzed meat products diminished substantially, to 91 in 2012, and 105 in 2013. This variable showed a recovery in 2014 and a progression in 2015 (Table 1). This behavior may be due to the fact that the budget allotted to the operation of the health surveillance program is different every year, and the activities to which it is destined involve expenses; this affects the planning of the inspection visits to supermarkets, slaughterhouses and meat retail shops directly. Furthermore, the presence of samples contaminated with clenbuterol leads to the redesigning of strategies with the aim to obtain better results in inhibiting this illegal activity in livestock production. This entails emphasizing surveillance, particularly in those localities where there have been positive cases, and relegating to a second place those where this problem has not been observed. The application of this risk-based approach renders the program effective and reduces the use of financial and human resources.
Table 1: Contaminated / collected samples and percentage of contaminated samples by year (annual %) and health jurisdiction (HJ)
| 2011 | 2012 | 2013 | 2014 | 2015 | Total | HJ (%) | |
|---|---|---|---|---|---|---|---|
| Tierra Caliente | 4/25 | 0/9 | 6/26 | 2/24 | 1/23 | 13/107 | 12.1 |
| North | 4/22 | 2/12 | 0/10 | 6/19 | 4/30 | 16/95 | 16.8 |
| Center | 2/36 | 1/8 | 0/19 | 0/21 | 4/32 | 7/116 | 6.0 |
| Mountain | 0/9 | 2/4 | 0/11 | 0/11 | 1/7 | 3/42 | 7.1 |
| Costa Grande | 4/28 | 1/16 | 0/11 | 0/13 | 0/24 | 5/92 | 5.4 |
| Costa Chica | 0/17 | 0/12 | 0/8 | 5/20 | 0/24 | 5/81 | 6.2 |
| Acapulco | 8/35 | 1/30 | 0/18 | 2/21 | 1/21 | 12/125 | 9.6 |
| Annual, % | 12.8 n=172 | 7.7 n=91 | 5.7 n=105 | 11.6 n=129 | 6.8 n=161 | 9.3 n=658 |
In terms of the sample types, there was a certain equality in the amount collected from the liver (n= 329) and the muscles (n= 310) (Table 2). The Work Instruction14 of the Sanitary Commission of COFEPRIS establishes that, in the course of inspection visits to slaughterhouses, butcher shops and supermarkets, the meat products must be collected by triplicate: a sample for analysis at the State Laboratory, a second sample to be kept by the Undersecretariat for Health Regulation, and a third one, for the owner of the regulated establishment -in case they should express any inconformity regarding the result-, to be subjected to analysis at some other authorized laboratory in the country (so that a final dictum may be issued by the corresponding health authority).
Table 2: Percentage of samples in which the presence of clenbuterol was detected, by simple type and corresponding year
| Year | Type of sample | Result | Total | % | |
|---|---|---|---|---|---|
| Not detected | Detected | ||||
| 2011 | Liver | 89 | 15 | 104 | 16.9 |
| Muscle | 61 | 7 | 68 | 11.5 | |
| 2012 | Liver | 53 | 7 | 60 | 13.2 |
| Muscle | 31 | 0 | 31 | 0 | |
| 2013 | Liver | 37 | 1 | 38 | 2.7 |
| Muscle | 56 | 4 | 60 | 7.1 | |
| Eyeball | 6 | 1 | 7 | 16.7 | |
| 2014 | Liver | 53 | 4 | 57 | 7.6 |
| Muscle | 61 | 11 | 72 | 18.0 | |
| 2015 | Liver | 67 | 3 | 70 | 4.5 |
| Muscle | 74 | 5 | 79 | 6.8 | |
| Urine | 9 | 3 | 12 | 33.3 | |
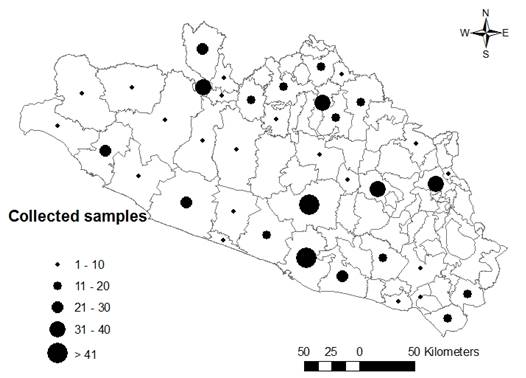
The collection was similar in all regions except for the Mountain, where only 42 samples were gathered (Table 1). The difficult access to the localities may be an important factor limiting the operation of the program in this area of the state, all the more because the samples must be transported and preserved at a temperature of 2 to 8 ºC, and since the transportation times to the laboratory are usually long, the cold chain may be broken at some point, damaging the quality of the sample; this is another factor that may influence the small number of samples collected in this region. A total of 39 (48.1 %) out of the 81 municipalities that make up the state were visited, with a focus on those with the largest population and greatest touristic, economic and political importance: Acapulco (n= 119), Chilpancingo (n= 73), Iguala (n= 39), Tlapa (n= 31), Ciudad Altamirano (n= 34), and Zihuatanejo (n= 29) (Figure 1). An analysis of the correlation between the number of inhabitants of the visited municipalities and the amount of meat products collected in these yielded a high correlation between the two variables (r= 0.912, P<0.001); this is logical, as health surveillance must be increased in direct proportion with the growing number of consumers, for purposes of prevention and hazard control. There is no relationship between the levels of dressed beef production in the municipalities and the samples obtained in them (r= -0.045); i.e. the sample collection was not influenced by the high output of certain municipalities. Based on this, it is recommended broadening the coverage of the sampling in the municipalities and regions in order to increase the likelihood of detecting positive cases and, thus, have elements for making decisions that may contribute to attain better results in health surveillance for clenbuterol.
The percentage of positive samples, i.e. the relationship between the amount of meat products contaminated with clenbuterol and the total number of meat products collected exhibited a downward tendency through the years (Table 1). This reduction was gradual, except for the year 2014, when there was a slight increase (11.6 %). Eventually, this percentage diminished to approximately half of the initial one (2011= 12.8 % vs 2015= 6.8 %). This indicates an important success of the surveillance program for clenbuterol in terms of health risk reduction for beef consumers in the state of Guerrero. The global percentage (9.3 %) was lower than that obtained by Chávez et al (15 in a similar study conducted during the years 2005-2010, in which the average annual percentage was 13.1 %, with almost 24 % of the samples contaminated with this chemical in 2005. However, these figures always were above the national average (Guerrero mean= 9.5 %, national mean= 5.8 %; P= 0.1) (Figure 2). In some cases, like that of the year 2011, the percentage of positive samples in Guerrero was twice the percentage of the rest of the country (12.8 vs 5.1 %, respectively); therefore, the goal should be to reverse this behavior in the short or medium term in order to reduce the contamination levels to those of the rest of the country.
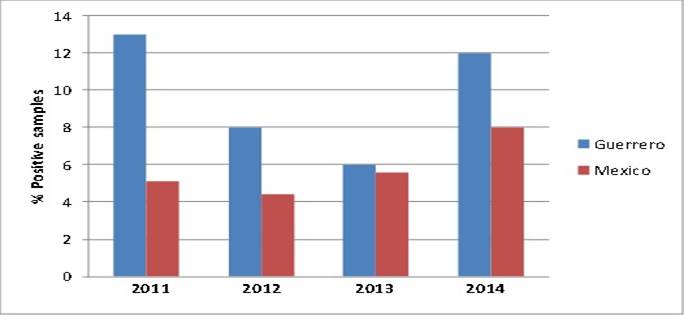
Figure 2: Comparison between the percentages of positive samples in Guerrero and at a national level during 2011-2014
The eyeball and urine were not the samples of choice in the sampling scheme of the program. However, as shown in Table 1 clenbuterol was more successfully detected in these biological samples, as only seven eyeball specimens were required to find residues of the drug in one; likewise, the compound of interest was found in up to three of the nine urine samples tested. This is due to the pharmacokinetics of the molecule, since high concentrations-and therefore a high permanence- of clenbuterol have been reported in the retina after the chemical has been administered therapeutically16. Likewise, this agent is excreted slowly through the urine, where it may be found up to the ten days after the exposure17,18, whereas in the blood it can only be detected within five days after the intake19. Despite the evidence presented in this and other studies16,20, collection of eyeball samples has not been the procedure of choice because the condition of obtaining three specimens from the same animal as indicated in the Work Instruction of COFEPRIS14 cannot be met (for logical reasons). Furthermore, urine samples are obtained only in special operations of the health authority of the slaughterhouses, and this activity is not very frequent, as it carried out only when a public complaint has been filed or when there is a history of introduction of contaminated meat into these establishments.
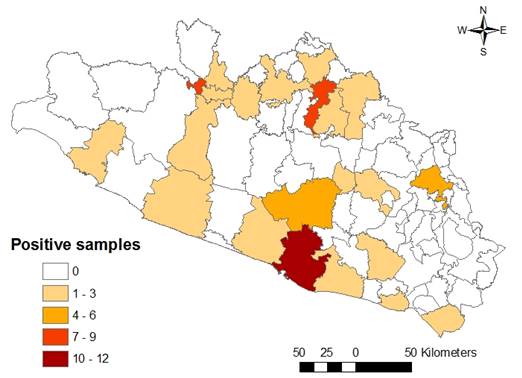
In the regional analysis, the main HJs that contributed higher percentages of positive samples were the North and Tierra Caliente -with 16.8 and 12.1 %, respectively-, where the presence of this chemical was constant in almost all the studied years. In Acapulco, despite the intense surveillance (more samples were collected there than in any other HJ), positive cases also occurred repeatedly, with 9.6 %, although to a lesser extent than in the other two jurisdictions. In the remaining HJs, the situation was more controlled, and positive cases occurred only sporadically. Clenbuterol was detected in 22 municipalities, notably in those with a larger population, like Acapulco, Chilpancingo, Iguala, Ciudad Altamirano, and Tlapa, where the largest number of contaminated samples were found (Figure 3).
There are some records of intoxication in humans due to consumption of meat products contaminated with clenbuterol: three cases occurred in 2011, five in 2012, and four in 2013; no other cases were registered in the remaining years (Figure 4). Six of these cases were detected in Acapulco, three in Iguala, two in Chilpancingo, and one in Chilapa, which are the municipalities that exhibited the largest number of positive samples, with the exception of Chilapa, where only one case, directly related to an intoxication, occurred in 2012. As with many health issues in Mexico and other countries, food intoxications with clenbuterol are underreported, possibly due to a lack of information at the first level of healthcare for recognizing the symptoms that may imply the need for a deeper study of the cases in order to attain an adequate diagnosis21; furthermore, people do not seek treatment at the healthcare centers because they lack economic resources or because of a shortage of institutional medical services. Therefore, it estimates that the real number of cases is larger.

Figure 4: Percentage of contaminated samples (bars) and cases of intoxication (dotted line) in each studied year
Because this chemical contaminant is still present in foods, it is important to maintain and strengthen monitoring by increasing the number of inspection visits to establishments, as well as to follow up on the cases of contaminated samples through the traceability chain, in order to locate the livestock producers and make them aware of the negative impact of these inadequate livestock production practices on human health.
Finally, intersectoral coordination is required to fight this problem; every institution must design, within the scope of its authority and responsibilities, an integral scheme to promote good production practices from the initial stages, preventing livestock breeders from seeking a better output through the use of substances that are dangerous for the consumers. Likewise, when there is sufficient evidence of the illegal use of clenbuterol, acts of authority must be executed to the ultimate consequences in compliance with the laws in force, based on the fact that this activity poses a threat to public health and must therefore be penalized without reserve or exceptions.
In the light of these results, and in comparison with the previous years, it is only fair to mention the work carried out by the Department of Health of Guerrero in relation to this phenomenon, whereby the number of positive cases and, therefore, of food intoxications, has been substantially reduced. Nevertheless, the surveillance program must be kept in operation with the aim to eradicate the illegal use of clenbuterol in order to protect the population from the related health hazards.
Acknowledgements
The authors wish to express their gratitude to the Undersecretariat for Food Regulation and Control and Health Promotion for the operation of the health surveillance program for clenbuterol in Guerrero; to their colleagues Roberto Huante and Diego Morán for their technical assistance, and to Dr. Hugo Saldarriaga for the revision of the manuscript and for his valuable contributions.
REFERENCES
1. Sillence MN, Mathews ML, Badran TW, Pegg GG. Effects of clenbuterol on growth in underfed cattle. Austr J Agric Res 2000;51:401-406. [ Links ]
2. Sakai N, Sakai M, Mohamad-Haron DE, Yoneda M, Ali-Mohd M. Beta-agonist residues in cattle, chicken and swine livers at the wet market and the environmental impacts of wastewater from livestock farms in Selangor State, Malaysia. Chemosphere 2016;165:183-190. [ Links ]
3. Kearns CF, McKeever KH, Malinowski K, Struck MB, Abe T. Chronic administration of therapeutic levels of clenbuterol acts as a repartitioning agent. J Appl Physiol 2001;91:2064-2070. [ Links ]
4. Peters AR. b-agonists as repartitioning agents: a review. Vet Rec 1989;124:417-420. [ Links ]
5. Mitchell GA, Dunnavan G. Illegal use of β-adrenergic agonists in the United States. J Anim Sci 1998;76:208-211. [ Links ]
6. Brett J, Dawson AH, Brown JA. Clenbuterol toxicity: a NSW poisons information centre experience. Med J Aust 2014;200(4):219-221. [ Links ]
7. Hoey AJ, Matthews ML, Badran TW, Peg GG, Sillence MN. Cardiovascular effects of clenbuterol are β2-adrenoreceptor-mediated in steers. J Anim Sci 1995;73:1754-1765. [ Links ]
8. Brambilla G, Cenci T, Franconi F, Galarini R, Macri A, Rondoni F, Strozzi M, Loizzo A. Clinical and pharmacological profile in a clenbuterol epidemic poisoning of contaminated beef meat in Italy. Toxicol Lett 2000;114(1-3):47-53. [ Links ]
9. FAO/WHO Expert Committee on Food Additives. Residues of some veterinary drugs in animals and foods. Monographs prepared by the Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Geneva. 1992. [ Links ]
10. Hoffman RJ, Hoffman RS, Freyberg CL, Poppenga RH, Nelson LS. Clenbuterol ingestion causing prolonged tachycardia, hypokalemia, and hypophosphatemia with confirmation by quantitative levels. Clin Toxicol 2001;39(4):339-344. [ Links ]
11. Diario Oficial de la Federación. NOM-194-SSA1-2004, Productos y Servicios. Especificaciones sanitarias en los establecimientos dedicados al sacrificio y faenado de animales para abasto, almacenamiento, transporte y expendio. Especificaciones sanitarias de productos. http://www.salud.gob. mx/unidades/cdi/nom/194ssa104.html . Consultado 25 Mar, 2016. [ Links ]
12. R-Biopharm AG. Enzyme immunoassay for the quantitative analysis of Clenbuterol and other β-agonists. http://www.r-biopharm.com/products/food-feed-analysis/residues/hormones-and-anabolics/clenbuterol/item/ridascreen-clenbuterol-fast . Accessed Apr 27, 2015. [ Links ]
13. Instituto Mexicano de Normalización y Certificación. NMX-EC-17025-IMNC-2006 Requisitos generales para la competencia de los laboratorios de ensayo y de calibración. México. 2006. [ Links ]
14. Comisión Federal para la Protección contra Riesgos Sanitarios. Comisión de Operación Sanitaria. Instrucción de trabajo para la vigilancia sanitaria del clembuterol en productos cárnicos. México, DF. Secretaría de Salud. 2008. [ Links ]
15. Chávez LA, Diaz JA, Pérez B, Alarcón MA. Tendencia de 2005 a 2010 de los niveles de clembuterol en muestras de bovinos en Guerrero, México. Rev Mex Cienc Pecu 2012;3(4):449-458. [ Links ]
16. Smith DJ, Paulson GD. Distribution, elimination, and residues of [14C] clenbuterol HCl in Holstein calves. J Anim Sci 1997;75(2):454-461. [ Links ]
17. Harkins JD, Woods WE, Lehner AF, Fisher M, Tobin T. Clenbuterol in the horse: urinary concentrations determined by ELISA and GC/MS after clinical doses. J Vet Pharma Therapeutics 2001;24:7-14. [ Links ]
18. Dave M, Sauer MJ, Fallon RJ. Clenbuterol plasma pharmacokinetics in cattle. Analyst 1998;123:2697-2699. [ Links ]
19. Yang YG, Song LX, Jiang N, Xu XT, Di XH, Zhang M. Pharmacokinetics of ambroxol and clenbuterol tablets in healthy Chinese volunteers. Int J Clin Exp Med 2015;8(10):18744-18750. [ Links ]
20. Li L, Tang C, Zhao Q, Zhang K. The potential of various living tissues for monitoring clenbuterol abuse in food-producing Chinese Simmental beef cattle. J Anal Toxicol 2016;40(1):72-77. [ Links ]
21. Wu ML, Deng JF, Chen Y, Chu WL, Hung DZ, Yang CC. Late diagnosis of an outbreak of leanness-enhancing agent-related food poisoning. Am J Emerg Med 2013;31(10):1501-1503. [ Links ]
Received: January 14, 2017; Accepted: November 29, 2017











 text in
text in