Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.6 n.4 Mérida Oct./Dec. 2015
Articles
Response of IFN-γ and IL-4 in mice inoculated with rabies virus recombinant G protein
a Centro Nacional de Investigaciones Disciplinarias en Microbiología Animal (CENID-MA), INIFAP, México D.F. México.
b Universidad Autónoma de Morelos, Cuernavaca, Morelos, México.
c Departamento de Biotecnología. INIA. Madrid, España.
d Departamento de Genética y Bioestadística. FMVZ-UNAM. México.
The aim of this study was to compare the performance of IFN-γ and IL-4 in mice inoculated with recombinant rabies virus G protein that was expressed either in transgenic maize or baculovirus. For this purpose, groups of mice were inoculated as follows: Group 1, inactivated rabies vaccine im; Group 2, plant-derived recombinant G protein orally; Group 3, baculovirus-expressed G protein im; Group 4, baculovirus-expressed G protein orally; and group 5, untransformed maize orally. The levels of IFN-γ and IL-4 and specific antibodies were evaluated every 15 d. A challenge was performed at d 60 post-inoculation (pi). Groups 1 and 3 promoted the best humoral response. On the other hand, the results showed that the same groups showed the best levels of IFN-γ at d 10 pi; while the pick of IL4 was detected at d 15 pi. For the survival study, 83 % of the mice immunized with inactivated vaccine, maize or im baculovirus extracts survived viral infection. The recombinant rabies G protein expressed in baculovirus promoted IFN-γ and antibodies in a similar way to the inactivated rabies vaccine. In spite of this, mice fed with transgenic maize survived to the challenge at the same percentage than group 3.
Keywords: Rabies; Baculovirus; Maize; IFN-γ; IL-4
El objetivo de este estudio fue comparar el comportamiento de IFN-γ e IL-4 en ratones inoculados con proteína G recombinante del virus de la rabia, que se expresa en maíz transgénico o baculovirus. Para este propósito, grupos de ratones se inocularon de la siguiente manera: Grupo 1, vacuna contra el virus de la rabia inactivado vía im; Grupo 2, proteína G recombinante derivada de plantas por vía oral; Grupo 3, proteína G expresada en baculovirus vía im; Grupo 4, proteína G expresada en baculovirus por vía oral; Grupo 5, maíz no transformado por vía oral. Los niveles de IFN-γ e IL-4 y los anticuerpos específicos se evaluaron cada 15 días. El desafío se realizó a los 60 días post inoculación (pi). Los grupos 1 y 3 promovieron una mejor respuesta humoral. Por otro lado, los resultados demostraron que los mismos grupos mostraron los mejores niveles de IFN-γ en el día 10 pi; mientras que la IL-4 se detectó en el día 15 pi. Para el estudio de supervivencia, el 83 % de los ratones inmunizados con vacuna inactivada, maíz y los inoculados im con extractos de baculovirus sobrevivieron la infección viral. La proteína G de la rabia recombinante expresada en baculovirus promovió IFN-γ y los anticuerpos de una manera similar a la vacuna de rabia inactivada. A pesar de esto los ratones alimentados con maíz transgénico sobrevivieron al desafío en el mismo porcentaje que el grupo 3.
Palabras clave: Rabia; Baculovirus; Maíz; IFN-γ; IL-4
Introduction
A large number of the anti-rabies vaccines have been developed using different systems, including baculovirus and plants1-7. One of the major advantages of the baculovirus-insect cell expression system is the high yield of recombinant protein produced per liter of cell culture8. Although the expression of the rabies virus G protein in different plant systems has been reported7,9 the development of an oral rabies vaccine that has a reduced cost of production and distribution will be very useful, especially for developing countries where the disease is endemic in humans and domestic animals. Glycoprotein is the main antigen of rabies virus, it is a transmembrane protein, consisting of a cytoplasmic domain, a transmembrane domain and an ectodomain exposed as trimmers. The ectodomain is involved in the induction of neutralizing antibodies and cytotoxic and helper T lymphocytes10,11. Many studies have demonstrated that crude extracts, purified recombinant antigen expressed in plants, or the whole plant can induce local or systemic immune responses1,7,12. In the literature there are few studies that have evaluated the type of cytokines induced by new generation rabies vaccines13.
The aim of this study was to compare the response of IFN-γ and IL-4 in mice inoculated with recombinant rabies virus G protein (rGpRV) that was expressed either in transgenic maize or baculovirus-infected insect cells. For this propose the transformation and regeneration of maize plants for the production of the transgenic corn used for immunization was performed as described previously14.
Materials and methods
Recombinant proteins
While for recombinant baculovirus, the fragment encoding the soluble glycoprotein of rabies virus was amplified by RT-PCR. (SuperScriptTM One-Step RT-PCR, Invitrogen, Carlsbad, CA, USA) using a haematophagous bat CASS-88 strain virus. The primers: 5’-GTGTGGATCCTATGAAATT CCCCATC-3’ and 3’-GAGACTCTAGATCCCCAGTTA GGGAG-5’ (BamHI and XbaI restriction sites included in the primers are underlined) were used to amplified gene G. The PCR product was inserted into a BamHI and XbaI-digested pFastBacMel-B2 vector under the control of the polyhedrin constitutive promoter as described previously15.
For the detection of the recombinant G glycoprotein in both expression systems, it was performed a Western blot using a hyper-immune rabbit serum prepared in our laboratory14. Protein concentrations were determined using the Bradford method (Bio-Rad, USA) and the expression level of rGpRV was determined by densitometry using a standard curve of bovine serum albumin. Gels were stained with Coomassie blue and the signals were scanned and quantified by Kodak 1D Image Analysis Software (East Kodak Company, New Haven, USA).
Evaluation of immune response
For evaluating immune response 21-d-old CD1 male mice (n= 90) were purchased from Bioterio México (Mexico, DF) and maintained under clean conditions with food and water ad libitum. All animal experiments were approved and performed according to the guidelines of the Mexican Official Standard NOM-062-ZOO-1999. Animals were divided into five groups: Group 1 received an intramuscular (im) dose of commercial inactivated rabies vaccine (Derriplus, Pronabive, México, DF); Group 2, mice were fed one gram of maize seeds that contained 100 μg of rabies virus G protein; Group 3 received an im dose of 100 μg G protein recovered from insect cells infected with BacGrabMel; Group 4 mice were orally inoculated with 100 μg G protein expressed by BacGrabMel; Group 5 mice were fed untransformed maize. The remaining mice were bled before immunization and at 10, 15, 30, 45 and 60 d post inoculation (dpi) to detect specific anti-G antibodies. At 60 dpi, each mouse was im challenged with 106.1 LD50/mL of CASS-88 rabies strain. Animals showing signs of rabies were euthanized (15 d), and their brains were removed and tested for rabies by the fluorescent antibody test (FAT)16. Sera were tested for rabies virus neutralizing antibody (VNA) titres using the rapid fluorescent focus inhibition test (RFFIT), as previously described17.
Cellular response
On the other hand, 12 mice from each group were used for cytokine detection. Three mice from each group were humanely euthanized at 5, 10, 15 and 20 d after immunization and spleens were removed to measure cytokine secretion. First, a kinetic test was prepared to determine the best time for splenocyte proliferation. Therefore, splenocyte suspensions were prepared and dead cells were removed using Lympholyte® cushion (Cedarlane, Ontario, Canada). The optimum time (72 h) to collect post-vaccination spleen culture supernatants was determined by a time-curve13. Supernatants were harvested and assayed for the presence of IL-4 and IFN-γ using ELISA ReadySET-Go systems (eBioscience, San Diego CA, USA) according to the manufacturer’s instructions. Briefly, the Maxisorp® ELISA plates were coated with 100 μL/well of capture, incubated overnight at 4 °C and washed three times. Wells were blocked with assay diluents and incubated at room temperature for 1 h. Standards were diluted at appropriate concentrations adding 100 μL/well of top standard concentration to the appropriate wells. A total of 100 μL were added per well of each sample and the plates were incubated at room temperature for 2 h. Subsequently, detection antibody was added. Finally, Avidin-HRP and substrate solution were added and were incubated at room temperature for 15 min. Plates were read at 450 nm.
Statistical analysis
Repeated analysis of variance (ANOVA) was used to test for differences in all the outcomes among groups receiving different immunizations regimens. The levels of IFN-γ and IL-4 were analyzed by two-way ANOVA with Dunnett’s and Bonferroni multiple comparisons of means. The level of significance used was 0.05. Statistical calculations were carried out using IBM’s SPSS 19 ® statistical package.
Results
Transformed and non-transformed plant extract protein and Sf9 cells transfected with BacGrabMel were evaluated by western blot. Immunoassay demonstrated the successful expression of the 65 kDa G protein monomer (Figure 1). This band was not detectable in negative controls. By densitometry it was determined that one gram of maize contained 50 μg of rabies glycoprotein. Meanwhile, using baculovirus it was determined that 2 to 7 mg of recombinant protein could be obtained per litre of supernatant from cells infected with the recombinant baculovirus; IFN-γ was detected in the splenocytes of mice from all immunized groups from 5 d after vaccination until 20 d post-immunization. The levels of IFN-γ were similar in animals immunized with the inactivated vaccine (group 1) to those that were im immunized with the baculovirus-expressed G protein (group 3) and significantly higher (P<0.01) than those in the orally immunized groups (groups 2 and 4) (Figure 2A). Likewise, although to a lesser extent, IL-4 induction was also observed in mice from all the inoculated groups. The maximum concentration of IL-4 was detected 15 d post-immunization, and the levels were similar in all vaccinated animals (Figure 2B). As expected, IFN-γ and IL4 were not detected in the control unimmunized mice (group 5).
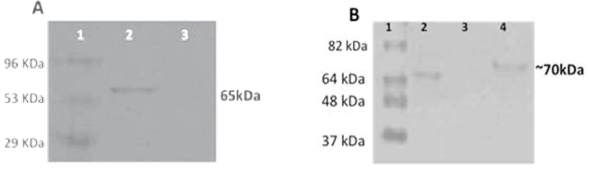
The recombinant glycoprotein was detected in both expression systems. A) Lane 1 molecular weight markers; lane 2 BacGrabMel Sf9 infected cellular extracts; lane 3 Sf9 uninfected cellular extracts. B) Lane 1 molecular weight markers; lane 2 rabies virus G protein purified from BHK-21 cells; lane 3 untransformed maize tissue extracts; lane 4 maize plant tissue extracts transformed with the G gene.
Figure 1 Western blot identification of the rabies virus G protein in two eukaryotic expression systems: baculovirus (A) and maize (B).
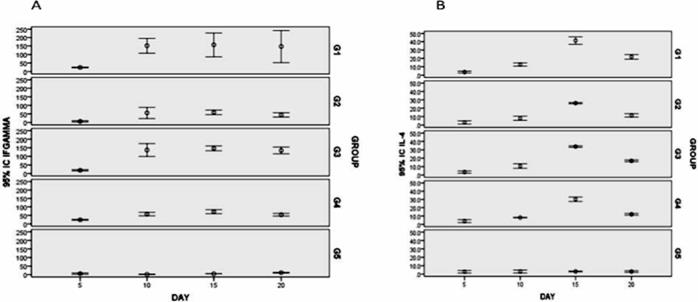
Immunized with the inactivated commercial vaccine (G1), maize by the oral route (G2), protein derivate of baculovirus inoculated im (G3), protein derivate of baculovirus by the oral route (G4) and mice fed with untransformed maize (G5). For INF-y results are expressed as the means ±95% I.C. For IL-4 results are expressed as pg/ml (P<0.001).
Figure 2 A) Levels of INF-g in immunized mice. B) Quantification of IL-4 cytokine by ELISA.
Figure 3, shows specific neutralizing antibodies in vaccinated groups, they were detected from 15 dpi until the day of challenge (60 dpi). No specific antibodies were detected in animals from the control group (group 5). Mice in group 3, immunized by the im route with the baculovirus expressed G protein, elicited antibody levels similar to those in group 1 until 30 d postimmunization. These levels were significantly higher (P<0.01) than those from the other two immunized groups (2 and 4). All (100 %) mice immunized with the inactivated vaccine survived to challenge done at d 60 pi, 83 % of the mice from group 2 (edible plant-derived rGpRV) and group 3 (baculovirus-expressed G protein administered im) survived the infection. In contrast, none of the unvaccinated mice (0 %) or those orally inoculated with the recombinant G protein (0 %) were protected (Figure 4). Presence of rabies virus was demonstrated by the FAT test in brains of all dead animals.
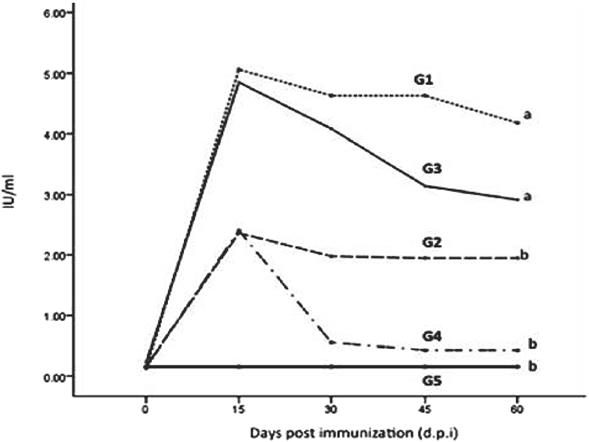
Mice were immunized with inactivated commercial vaccine (G1), maize by the oral route (G2), protein derivate of baculovirus inoculated im (G3), protein derivate of baculovirus by the oral route (G4) and mice fed with untransformed maize (G5). The results are expressed as international units by millilitre (IU/ ml), at different times after immunization (P<0.01).
Figure 3 Neutralizing antibody levels induced by recombinant rabies glycoprotein (rGpRV) expressed in different expression systems in mice.

Mice immunized with inactivated commercial vaccine (G1) 100%; maize by the oral route (G2) 88.8%; glycoprotein derivate of baculovirus inoculated im (G3) 88.8%; protein derivate of baculovirus by the oral route (G4) 0% and mice fed with untransformed maize (G5) 0%.
Figure 4 Survival rates of immunized mice challenged with the virulent CASS-88 rabies virus strain.
Discussion
Recombinant protein production in mammalian and insect cell lines and transgenic plants and animals are being progressively incorporated into production activities18. The aim of this study was to analyse the response of IFN-γ and IL4 in inoculated mice with rabies virus G protein (GpRV) expressed in baculovirus and maize. In this paper, it was only used the soluble region of the GpRV in order to obtain a soluble protein in the two expression systems. In previous studies it has been shown that the soluble glycoprotein maintains antigenic and immunogenic properties inducing virus neutralizing antibodies and thereby eliciting complete protection against challenge19. Western blot analysis showed the correct expression of glycoprotein in both maize and baculovirus. However, the molecular weight of the recombinant protein expressed in maize was slightly higher. This likely reflects different post-translational modifications, as has been previously observed9. The domain of GpRV has three potential N-linked carbohydrate sites at Asn37, Asn247, and Asn319, of which the latter two are efficiently core glycosylated20. In the case of plants, in general, the signal peptide is cleaved according to the same rules in plants and in mammals. Additionally, an oligosaccharide precursor to the Asn residue of a tripeptide Asn-X-Ser/Thr of the protein is the same in plant cells21. Baculovirus permits multiple post-translational modifications which are similar or identical to those occurring in mammalian cells, such as oligomerization, phosphorylation, glycosylation, and proteolytic cleavage8.
The expressed G protein conserved its native immunogenic properties as demonstrated by immune reactivity against a specific antiserum. Western blot analysis indicated the generation of recombinant glycoprotein rabies virus with antigenic and immunogenic properties. The results of mice immunized by im or oral dosage indicated that the presence of cytokines (IFN-γ and IL4) results in a tendency to develop a Th1 cell response after immunization with both recombinant proteins tested, as evidenced by the detection of IFN-γ up to d 20 post-vaccination. Similar results were observed by Tesoro-Cruz et al15,22 using a DNA rabies vaccine and Hu et al23 with a traditional vaccine with adjuvant. This result supports the hypothesis of Drings24, who suggested that subunit vaccines (such as edible or baculovirus-derived vaccines) preferentially develop a Th1 response.
However, after 15 d, a slight increase in the concentration of IL-4, a typical cytokine of Th2 response, was observed. This response is related to the presence of antibodies. IL-4 detection has previously been reported by Perrin et al25 after administration of a naked DNA rabies vaccine in dogs. Moreover, the production of IFN and high antibody titres in sheep vaccinated with a recombinant adenovirus and with an edible vaccine that express the GpVR have been demonstrated2.
Immunization of mice with each of the recombinant proteins tested, either produced in plants or in insect cells, induced protective specific antibodies when they were administered by the oral or im routes, respectively, as immunized animals were protected against challenge with a lethal dose of rabies virus. These results are consistent with those obtained in previous works in which GpRV was expressed in different plant systems7.
In this research it was observed that the level of neutralizing antibodies is related with protection; however, these began to descend at 45 dpi. Concerning the protection assay, in the case of groups 2 and 3, the protection levels were higher than the minimal requirement established by Mexican regulations (NOM-067-ZOO-2007), in which more than 80 % protection is sufficient for classical vaccines. The protection levels (83 %) observed in mice fed the transgenic maize expressing G protein may be due to partial degradation by the gastric juices, although in other cases, this effect was not observed. In this study, the challenged was at d 60 to evaluate the presence of long-term antibodies using a single dose without adjuvants or booster, as this would be very useful in livestock. Moreover, it is worth highlighting that the animals of group 2 immunized orally with maize showed a higher protection compared to group 4, which had been immunized by the same route but with baculovirus-derived protein. It is possible that this is due to the protein used in group 4 which was administered in a solution, exposing the protein to the action of both proteases and pH present in the gastric system, which could have degraded this protein faster than when expressed in maize. The latter would have served as a protector of the recombinant protein.
Conclusions and implications
This study demonstrates that G protein derived from either plant or baculovirus-infected insect cells protects against a lethal challenge with rabies virus at a level similar to that of an inactivated vaccine; both systems may be a good alternative as a thermostable vaccine, which would be very useful to prevent and control the disease in tropical and subtropical countries. Based on this evidence, it was conclude that the recombinant rabies G protein expressed in baculovirus promoted IFN-γ and antibodies in a similar way to the inactivated rabies vaccine. In spite of this, mice fed with transgenic maize survived to the challenge at the same percentage than group 3. These antigens promote both Th1 and Th2 responses.
Acknowledgements
Thank to Drs. Juan Carlos Saiz, José Angel Martínez Escribano and Carmen Nuñez from INIA-Spain for their contribution to express the recombinant baculovirus. This research was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT) grant (G34635B) .
REFERENCES
1. Arntzen C. Plant-derived vaccines and antibodies: potential and limitations. Vaccine 2005;23:1859-1865. [ Links ]
2. Bouet-Cararo C, Contreras V, Fournier A, Jallet C, Guilbert JM, Dubois E, et al. Canine adenoviruses elicit both humoral and cell-mediated immune responses against rabies following immunization of sheep. Vaccine 2011;29:1304-10. [ Links ]
3. Briggs JD, Dreesen WD, Wunner HW: Vaccines. In: Rabies. Jackson AC, Wunner HW editors. USA: Academic Press; 2002:371-400. [ Links ]
4. Liu X, Yang Y, Sun Z, Chen J, Ai J, et al. A recombinant rabies virus encoding two copies of the glycoprotein gene confers protection in dogs against a virulent challenge. PLoS ONE 2014;9:e87105. [ Links ]
5. Paolazzi CC, Perez O, de Filippo J. Rabies vaccine. Developments employing molecular biology methods. Mol Biotechnol 1999;11:137-147. [ Links ]
6. Rojas-Anaya E, Loza-Rubio E, Olivera-Flores MT, Gomez-Lim M. Expression of rabies virus G protein in carrots (Daucus carota). Transgenic Res 2009;18:911-919. [ Links ]
7. Loza-Rubio E, Rojas-Anaya E. Vaccine production in plant systems an aid to the control of viral diseases in domestic animals: a review. Acta Vet Hung 2010;58:511-522. [ Links ]
8. Liu X, Wu X, Li L, Liu Z, Wang Z. Use of baculovirus expression systems for generation of virus-like particles: Success and challenges. Protein Express Purif 2013;90:104-116. [ Links ]
9. Yusibov V, Streatfield SJ, Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccine 2011;1:313-321. [ Links ]
10. Macfarlan RI, Dietzschold B, Koprowski H. Stimulation of cytotoxic T-lymphocyte responses by rabies virus glycoprotein and identification of an immunodominant domain. Mol Immunol 1986;23:733-741. [ Links ]
11. Wojczyk B, Shakin-Eshleman SH, Doms RW, Xiang ZQ, Ertl HC,Wunner WH, Spitalnik, SL. Stable secretion of a soluble, oligomeric form of rabies virus glycoprotein: influence of Nglycan processing on secretion. Biochemistry 1995;34:2599-2609. [ Links ]
12. Aliahmadi A, Rahmani N, Abdollahi M. Plant derived human vaccines; an overview. Inter J Pharma 2006;(2):268-279. [ Links ]
13. Feria-Romero IA, Chavez-Rueda K, Orozco-Suárez S, Blanco-Favela F, Calzada-Bermejo F, Chávez-Sánchez L, et al. Intranasal anti-rabies DNA immunization promotes a Th1-related cytokine stimulation associated with plasmid survival time. Arch Med Res 2011;42:563-571. [ Links ]
14. Loza-Rubio E, Rojas-Anaya E, López J, Olivera-Flores MTJ. Induction of a protective immune response to rabies in sheep after oral immunization with transgenic maize. Vaccine 2012;30:5551-5556. [ Links ]
15. Jiménez de Oya N, Galindo I, Escribano-Romero E, Blázquez AB, Alonso-Padilla J, Halaihel N, et al. Expression and immunoreactivities of hepatitis E virus genotype 3 open Reading frame-2 (ORF-2) recombinant proteins expressed in insect cells. Food Environmen Virol 2009;1:77-84. [ Links ]
16. Dean DJ, Ablseth MK, Atanasiu. The florescent antibody test. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva, Italy: WHO; 1996:80-95. [ Links ]
17. Smith JS, Yager PA, Baer GM, Meslin FX, Kaplan MM, Koprowski H. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus neutralizing antibody. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva, Italy: WHO; 1996:181-191. [ Links ]
18. Ferrer-Miralles N, Domingo-Espín J, Corchero JL, Vázquez E, Villaverde A. Microbial factories for recombinant pharmaceuticals. Microb Cell Fact 2009;24:17. [ Links ]
19. Gupta PK, Sharma S, Walunj S, Chaturvedi Raut AA, Patial S, Rai A, Pandey KD, Saini M. Immunogenic and antigenic properties of recombinant soluble glycoprotein of rabies virus. Vet Microbiol 2005;108:207-214. [ Links ]
20. Shakin-Eshleman SH, Remaley AT, Eshleman JR, Wunner WH, Spitalnik SL. N-linked glycosylation of rabies virus glycoprotein. Individual sequons differ in their glycosylation efficiencies and influence on cell surface expression. J Biol Chem 1992;267:10690-10698. [ Links ]
21. Gomord V, Faye L. Posttranslational modifications of therapeutic proteins in plants. Curr Opin Plant Biol 2004;7:171-181. [ Links ]
22. Tesoro-Cruz E, Hernández-González R, Alonso-Morales R, Aguilar-Setién A. Rabies DNA vaccination by intranasal route in dogs. Dev Biol (Basel) 2006;125:221-31. [ Links ]
23. Hu, X, Liu R, Zhu N. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology 2013;218:1524-1528. [ Links ]
24. Drings A. Towards a vaccine against the European Lyssaviruses a structural and immunological approach [PhD thesis]. Freien Universitäl Berlin. 1998. [ Links ]
25. Perrin P, Jacob Y, Tordo N. DNA-based immunization against Lyssaviruses. Intervirology 2000;43:302-311. [ Links ]
Received: January 06, 2015; Accepted: March 18, 2015











 text in
text in 


