Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.14 n.1 Texcoco Jan./Feb. 2023 Epub June 19, 2023
https://doi.org/10.29312/remexca.v14i1.3115
Articles
24-Epibrasinolide (24-EBL) as rooting inducer of blueberry cuttings on different substrates
1Instituto de Investigaciones Agropecuarias y Forestales-Universidad Michoacán de San Nicolás de Hidalgo. Tarímbaro, Michoacán, México. CP. 58880. Tel. 443 3223500, ext. 5219. (1243772g@umich.mx; luis.lopez.perez@umich.mx; boquera@umich.mx).
2Facultad de Agrobiología ‘Presidente Benito Juárez’-Universidad Michoacán de San Nicolás de Hidalgo. Uruapan, Michoacán, México. CP. 60170. Tel. 452 5236474. (martha.elena.pedraza@umich.mx).
3Centro Regional Morelia-Universidad Autónoma Chapingo. Morelia, Michoacán, México. CP. 58170. Tel. 443 3161489. (rebollaralviter@gmail.com).
Brassinosteroids are steroidal phytohormones that have been widely used in agriculture, few studies on the effect they can have in inducing root formation in blueberry (Vaccinium corymbosum) cuttings have been conducted. The objective of this research was to evaluate the effect of 24-epibrasinolide (24-EBL) at different concentrations (0, 20, 40 and 60 μg L-1) and of the substrate on the rooting of cuttings, a mixture of river sand and tezontle (ST), peat (Peat Moss®) and perlite (PMP), a mixture of coconut fiber and perlite (CFP), peat (Peat Moss®) (PM) and coconut fiber (CF). The variables evaluated 30 days after the establishment of the experiment were percentage of survival (%SC), percentage of rooted cuttings (%RC), number of roots per cutting (NR), main root length (MRL) and total length of the root system (TLR). The data obtained were subjected to analysis of variance and comparison of means with the Tukey test. The results showed that blueberry cuttings with 24-EBL at a concentration of 60 μg L-1 in ST substrate have 84.7% of survival and 67.76% of rooted cuttings. Cuttings exposed to 24-EBL at a concentration of 40 and 60 μg L-1 inserted in substrate of ST mixture had a longer root length and more roots per cutting compared to other substrates. It is concluded that 24-EBL induces root formation in cuttings of blueberry plants in ST substrate.
Keywords: blueberry; brassinosteroid; cuttings; substrate
Los brasinoesteroides son fitohormonas esteroidales que se han utilizado ampliamente en la agricultura, se han realizado pocos estudios sobre el efecto que pueden tener para inducir la formación de raíces en esquejes de arándano (Vaccinium corymbosum). El objetivo de esta investigación fue evaluar el efecto del 24-epibrasinolide (24-EBL) a distintas concentraciones (0, 20, 40 y 60 µg L-1) y del sustrato sobre el enraizamiento de esquejes, se utilizó mezcla de arena de río y tezontle (AT), turba (Peat Moss®) y perlita (PMP), mezcla de fibra de coco y perlita (FCP), turba (Peat Moss®) (PM) y fibra de coco (FC). Las variables evaluadas a los 30 días después de establecido del experimento, fueron porcentaje de supervivencia (%SE), porcentaje de esquejes enraizados (%ER), número de raíces por esqueje (NR), longitud de raíz principal (LRP) y longitud total del sistema radicular (LTR). Los datos obtenidos se sometieron a análisis de varianza y comparación de medias con la prueba de Tukey. Los resultados mostraron que los esquejes de arándano con 24-EBL a una concentración de 60 µg L-1 en sustrato de AT presentan 84.7% de supervivencia y 67.76% de esquejes enraizado. Los esquejes expuestos a 24-EBL a una concentración 40 y 60 µg L-1 insertada en sustrato de mezcla de AT presentaron una mayor longitud de raíz y más raíces por esqueje en comparación con otros sustratos. Se concluye que el 24- EBL induce la formación de raíces en esquejes de plantas de arándano en sustrato de AT.
Palabras clave: arándano azul; brasinoesteroide; esquejes; sustrato
Introduction
Blueberry (Vaccinium corymbosum) is one of the berries of greatest economic relevance in Mexico and worldwide, it is characterized by the content of phenolic compounds with antioxidant activity (Rodríguez et al., 2019). In 2020, Mexico ranked sixth in production with 50 293 t, 2.6% more than the previous year. The production of this berry has had an exponential growth in the last 10 years, registering an average annual growth rate of 25.1% (SIAP, 2021).
One of the main methods of propagation of blueberry is by rooting cuttings (Villegas et al., 2019). There are several elements that affect the rooting capacity of cuttings (Cárdenas and López, 2011), among which are the type of substrate, exogenous and endogenous growth regulators, nutrition and physiological condition of mother plants, type of cutting, rooting season and environmental conditions (A’saf et al., 2020).
In the propagation by cuttings, different types of substrates of mineral and organic origin are used, the use of peat stands out, which is made from decomposed plant materials, has good water retention and aeration, the instability in its structure and its high cation exchange capacity interferes with plant nutrition (Braha and Rama, 2018). Coconut fiber is another substrate that is frequently used, it is a fiber composed of cellulose and lignin, it is characterized by having low electrical conductivity, retaining moisture, being resistant to bacteria and presenting high compaction when in powder form, which generates aeration problems, for this reason it is combined with other substrates (Putrino et al., 2020).
On the other hand, river sand has good granulometric characteristics that range from 0.5 to 2 mm in diameter, a desirable sand for the production of plants in containers should mainly have particles of medium and coarse size. The use of sands with a wide (dispersed) distribution of particle sizes is undesirable, as it could result in a substrate with low aeration capacity. The use of sand should be restricted to less than one third of the total volume of the substrate, as it can result in a high weight per volume (bulk density), which is not recommended, its water retention capacity is medium and its cation exchange is zero (Luna et al., 2021).
Perlite and tezontle are considered inert substrates that have mineral origin. Volcanic rocks that have been subjected to ignition temperatures undergo modifications in their physical structure, generating light and porous rocks, which favor the infiltration of water in downward movement. Due to these characteristics, when mixing mineral and organic substrates, an ideal ratio of moisture retention, nutrient concentration and drainage is achieved. This allows good aeration of the roots (Bannoud and Bellini, 2021).
According to Pagani et al. (2015), the interaction of the composition of the substrate with environmental conditions such as humidity, temperature, nutrients administered by irrigation and the presence of hormones generates good conditions for rooting, taking into consideration that its texture and structure are maintained.
One of the most common practices to induce root formation is the use of growth regulators and the most used are auxins (Blythe et al., 2004) and recently brassinosteroids (BR) (Serna et al., 2012), which are considered the sixth phytohormone, are steroidal substances that stimulate plant growth when applied exogenously (Vazquez et al., 2019). These were discovered in 1960 and are currently used for various purposes in agriculture.
The different analogues of brassinosteroids have shown effects on the primary and secondary metabolism of several crops, such as tomato, cucumber and grape, where an increase in leaf area, stem elongation, pollen tube growth, reorientation of cellulose microfibrils in stem and leaves was observed, (Hussain et al., 2020), induction of the formation of conductive tissue in roots, changes in photomorphogenesis activity and cell division can also stimulate or inhibit rhizogenesis and participate in signaling multiple biochemical reactions of phosphorylation and methylation of kinase complexes in the cell membrane (Bergonci et al., 2014).
However, so far not enough studies showing the effect of brassinosteroids on the root development of blueberry cuttings using different substrates have been developed. Therefore, the objective of this study was to evaluate the effect of three different concentrations of 24-Epibrasinolide (24-EBL) and five substrate mixtures on the rooting and survival of cuttings of blueberry plants in greenhouses.
Materials and methods
Location and description of the experimental area
The experiment was carried out in a greenhouse of the Institute of Agricultural and Forestry Research (IIAF) for its acronym in Spanish, of the Michoacan University of San Nicolás de Hidalgo located in the Morelia-Zinapécuaro highway, km 9.5, 58880, Tarímbaro, Michoacán. With geographical coordinates: 19.7681622, - 101.1513041. Elevation 1 860 m.
Plant material
The plant material consisted of cuttings between 7 and 10 cm in length with three axillary buds that were obtained from lateral branches, selected during a pruning of healthy 2-year-old plants of Vaccinium corymbosum variety Biloxi. These stem sections were disinfected by immersion in sodium hypochlorite at 35 ppm for 1 min, then rinsed five times with deionized water and immersed in solution with fungicide (LucavFlow® 10 ml L-1) for 1 min, finally rinsed 10 times with deionized water.
The basal part of the cuttings (2 cm) was placed for 2 h in a solution of 24-Epibrasinolide (24-EBL) and a control solution [dimethyl sulfoxide (DMSO) + polyoxyethylenesorbitan monolaurate surfactant agent (Tween 20) + distilled water], then they were transplanted into cubic black plastic containers of 1 L capacity containing the substrate mixtures, prior to planting, the substrates were irrigated with deionized water until drained and then the cuttings were planted, the containers were irrigated daily at field capacity for 30 days until the end of the experiment.
Conditions in the greenhouse
The greenhouse where the experiment was installed is of the curved roof type with milky white plastic of gauge 720, which provides 30% shade and allows maintaining a uniform temperature inside the greenhouse of 27 ° C on average during the day and a relative humidity of 47%.
Experimental design
A completely randomized experimental design with factorial arrangement was used, with 4 repetitions in each pot, the experimental unit consisted of a cutting. Twenty treatments were generated from the combination of the two factors, factor substrate: types of substrates Sphagnum® Peat Moss (PM); mixture of PM + perlite in a ratio 2:1 (V/V) (PMP); commercial coconut fiber (Power Forteco) (CF); mixture of CF + perlite in a ratio 2:1 (V/V) (CFP) and mixture of river sand + tezontle in a ratio 1:1 (V/V) (ST) and the factor concentration of 24-EBL (0, 20, 40, 60 μg L-1).
Phytohormone preparation
The solutions of 24-EBL at 20, 40 and 60 μg L-1 were obtained by dilutions with distilled water of a stock solution of 24-Epibrasinolide (24-EBL) at a concentration of 0.01 g L-1. This solution was prepared by dissolving 10 mg of 24-EBL (Sigma-Aldrich Epibrassinolide, ≥85%) in 1 ml of dimethyl sulfoxide (DMSO), then 25 ml of double distilled water and 0.1% (V/V) of polyoxyethylenesorbitan monolaurate (Tween 20) as surfactant were added, the final volume was adjusted to 1 L with double distilled water.
Physical and chemical characterization of substrates
The substrates were disinfected with sodium hypochlorite solution at 35 ppm and washed three times with deionized water, placed in black plastic containers of cubic design with a capacity of 1 L. The electrical conductivity (EC) of the substrate was determined with the saturation extract method (Vargas et al., 2008). The concentration of N in the substrates was performed by the microKjeldahl method. P, K, Ca and Mg were determined after wet digestion and quantified by atomic absorption spectrophotometry (Varian ICP-AES Plasma 96).
These analyses were carried out in triplicate at the laboratory of the Center for Innovation and Agrifood Development of Michoacán (CIDAM) for its acronym in Spanish, located in the city of Morelia, Michoacán. Bulk density (Db) was determined by the graduated cylinder method (Acosta et al., 2014) and real density (Dr) by fluid pycnometry (Díaz et al., 2012). From these determinations, the total porosity of the substrate was obtained using the equation Pt (%) = 100 (1-Db/Dr). The determination of moisture retention was performed by the hanging column method (Crespo et al., 2018) and from this measurement, air porosity (Pa) was obtained. These tests were done in the Laboratory of Plant Nutrition at the Institute of Agricultural and Forestry Research (IIAF) for its acronym in Spanish.
Variables evaluated
The variables evaluated were percentage of survival of cuttings, number of rooted cuttings (those that had a main root of 2 mm in length), number of roots per cutting, main root length and total length of the root system. To analyze the root system, photographs of the rooted cuttings were taken for each treatment and the images were processed with the Root System Analyser software - analysis of images and sequences of plant roots, of the University of Vienna (Leitner et al., 2014). The analyses were performed 30 days after (DAE) the experiment was established.
Results and discussion
Characterization of substrates
The substrates used in this work presented the following characteristics: Peat Moss, it is a substrate with almost zero salinity effects (EC) of 0.5 dS m-1, a high concentration of N of 0.84 g kg-1, low in P with 5.2 ppm, poor in K with 15 ppm, poor in Ca with 10 ppm and poor in Mg 7 ppm, a high moisture retention of 64.5%, these results are similar to those reported by Gómez et al. (2013).
Perlite was not characterized in this study, the reference values indicated in the supplier’s technical sheet (Multiperl® Hotyicola) were taken, which indicates the following characteristics: expanded volcanic siliceous rock with a pH: 7, it has a total pore volume of 95%, the approximate density is 0.12 kg L-1.
Regarding the results obtained for the mixture of PMP, it is a substrate with very low salinity effects (EC) of 0.1 dS m-1, as mentioned by Sánchez et al. (2008), a high N concentration of 1 g kg-1, low in P with 4 ppm, very low in K with 3 ppm, limited in Ca with 3 ppm, a high moisture retention of 60%. The coconut fiber presented a mean concentration of N, high P content, very high in K, slightly high in Ca and Mg, similar results reported by Pardo and Pardo (2008) (Table 1).
Table 1 Chemical characteristics of the substrates evaluated in the rooting of blueberry cuttings.
| Substrate | EC (dS m-1) | Total N | P | K | Ca | Mg |
| PM | 0.5 | 0.84 | 5.2 | 15 | 10 | 7 |
| PMP | 0.1 | 1 | 4 | 3 | 3 | 2 |
| CF | 3.9 | 0.51 | 20 | 594 | 19 | 25 |
| CFP | 3 | 0.34 | 12.1 | 713 | 58 | 42 |
| ST | 2.7 | 0.61 | 6.9 | 698 | 49 | 28 |
PM= Sphagnum® Peat Moss; PMP= mixture of PM + perlite in a ratio 2:1 (V/V); CF= commercial coconut fiber (Power Forteco); CFP= mixture of CF + perlite in a ratio 2:1 (V/V); ST= mixture of river sand + tezontle in a ratio 1:1 (V/V); EC= electrical conductivity; N= nitrogen (g kg-1); P= phosphorus; K= potassium; Ca= calcium; Mg= magnesium (ppm).
The mixture of river sand and tezontle, with almost zero salinity effects (EC), because it has a mineral origin, has a very poor content of organic matter in nutrients, as mentioned by Luna et al. (2021), this mixture is extremely low in N and P, very high in K, slightly high in Ca and Mg. Regarding the physical characteristics of this mixture, it presented a high bulk density, percentage of water retention of 43.3% (Table 2).
Table 2 Physical characteristics of the substrates evaluated in the rooting of blueberry cuttings.
| Substrate | Bulk density (g cm-3) | Real density (g cm-3) | Moisture retention (%) | Air porosity (%) | Total porosity (%) |
| PM | 0.27 | 2.12 | 64.5 | 12.3 | 76.8 |
| PMP | 0.18 | 1.18 | 46.4 | 31.6 | 78 |
| CF | 0.24 | 2.18 | 58.2 | 13.5 | 71.7 |
| CFP | 0.21 | 2.45 | 51.7 | 26.5 | 58 |
| ST | 0.48 | 2.24 | 43.3 | 20.5 | 63.8 |
PM= Sphagnum® Peat Moss; PMP= mixture of PM + perlite in a ratio 2:1 (V/V); CF= commercial coconut fiber (Power Forteco); CFP= mixture of CF + perlite in a ratio 2:1 (V/V); ST= mixture of river sand + tezontle in a ratio 1:1 (V/V).
Substrate effect
The rooting capacity of cuttings from Ericaceae plants is limited because blueberries have lower nutrient requirements than most crops and thrives in acidic soils (pH of 4.5-5.5) with limited availability of essential nutrients such as nitrate-nitrogen (NO3 -N), phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) (Braha and Rama, 2018). When evaluating the effect of the concentration of 24-EBL and the type of substrate separately and the interaction of the factors, significant differences (p= 0.05) were found in the variables evaluated in blueberry (Vaccinium corymbosum) cuttings, these results are shown in (Table 3).
Table 3 Survival percentage (%SC), rooting percentage (%RC), number of roots (NR), main root length (MRL), total length of the roots (TLR) of cuttings of blueberry (V. corymbosum) variety Biloxi by substrate, brassinosteroid concentration and factor interaction.
| Treatment | Variable | ||||
| %SC | % RC | NR | MRL (mm) | TLR (mm) | |
| Substrate | |||||
| PM | 16.84 d | 13.48 d | 1.29 c | 2.72 d | 6.3 d |
| PMP | 27.18 c | 25.08 b | 2.64 b | 3.32 c | 6.57 d |
| CF | 28.42 c | 21.74 c | 1.96 c | 3.79 c | 7.06 c |
| CFP | 33.25 b | 26.52 b | 2.76 b | 4.82 b | 8.56 b |
| ST | 48.88 a | 39.11 a | 4.29 a | 6.06 a | 10.98 a |
| Brassinolide (μg L-1 24-EBL) | |||||
| 0 | 5.11 d | 4.35 d | 1.02 d | 2.76 cd | 7.15 d |
| 20 | 19.75 c | 15.21 c | 2.94 c | 3.9 c | 7.5 c |
| 40 | 44.05 b | 36.12 b | 4.06 b | 5.25 b | 8.05 b |
| 60 | 54.75 a | 44.32 a | 6.36 a | 7.65 a | 8.89 a |
| Interaction (substrate*brassinolide) | |||||
| PM * 0 | 10.97 c | 1.3 d | 1.27 d | 1.09 c | 3.91 d |
| PM * 20 | 13.25 c | 14.38 d | 1.98 d | 2.61 b | 6.21 c |
| PM * 40 | 23.75 b | 19 bc | 2.96 bc | 2.81b | 6.44 c |
| PM * 60 | 28.51 b | 23.18 bc | 2.73 c | 2.97 b | 6.67 c |
| PMP * 0 | 2.8 c | 2.24 d | 1.92 d | 1.19 c | 4.33 c |
| PMP * 20 | 15.5 c | 12.4 d | 2.97 bc | 3.5 b | 6.09 c |
| PMP * 40 | 40.39 b | 32.32 b | 2.98 bc | 4.09 b | 7.15 c |
| PMP * 60 | 50 b | 40.44 b | 3.4 b | 4.4 b | 7.75 bc |
| CF * 0 | 4 c | 4.03 d | 1.13 d | 1.17 c | 3.74 d |
| CF * 20 | 15.8 c | 13.06 d | 3.06 b | 3.21 b | 6.91 c |
| CF * 40 | 42.3 b | 38.58 b | 3.14 b | 3.41 b | 7.13 c |
| CF * 60 | 52.6 b | 44.66 b | 3.22 b | 3.51 b | 7.47 bc |
| CFP * 0 | 7 c | 5.28 d | 1.16 d | 1.67 c | 8.24 b |
| CFP * 20 | 18.2 bc | 14.56 c | 3.26 b | 4.71 b | 8.41 b |
| CFP * 40 | 50.1 b | 40.08 b | 3.99 b | 4.91 b | 8.63 b |
| CFP * 60 | 57.7 b | 56.16 a | 3.77 b | 6.01 a | 8.97 b |
| ST * 0 | 11.1 c | 8.92 c | 1.87d | 1.31 c | 6.56 c |
| ST * 20 | 36 b | 28.8 b | 3.56 b | 5.5 ab | 8.86 b |
| ST * 40 | 63.21 b | 50.96 a | 3.79 b | 6.05 a | 10.91 ab |
| ST * 60 | 84.57 a | 67.76 a | 4.89 a | 7.39 a | 11.59 a |
| Significance | |||||
| Substrate | 0.003* | 0.021* | 0.015* | 0.002* | 0.001* |
| 24-EBL | 0.029* | 0.042* | 0.013* | 0.009* | 0.003* |
| Interaction | |||||
| Substrate x 24-EBL | 0.001* | 0.04* | 0.025* | 0.005* | 0.008* |
Substrates= sphagnum® Peat Moss (PM), mixture of Sphagnum® Peat Moss and perlite in a ratio 2:1 (V/V) (PMP); commercial coconut fiber (Power Forteco) (CF); a mixture of commercial coconut fiber (Power Forteco) and perlite in a ratio 2:1 (V/V) (CFP) and a mixture of river sand and tezontle in a ratio 1:1 (V/V) (ST). 24-EBL= 24 epibrasinolide. Tukey used to compare differences in means; significance at p= 0.05. ns= not significant; *= significant. Means in the same column followed by the same superscript letter are not significantly different.
In the percentage of survival of cuttings, the mixture of ST had the highest mean of 48.88%, the PM presented the lowest mean of 16.84%, while the mixtures of PMP and CFP did not show significant differences (p< 0.05), with these results the effect that perlite has on the mixture of substrates was observed, these results are similar to the studies reported by De-Boodt and Verdonck (1972), where they indicate that the values of aeration porosity are higher in perlite with 37.79%, it is a value that is considered favorable, in this research the mixture of perlite and peat (Peat Moss®) had an aeration of 31.6%.
On the other hand, the results obtained in the present research support the suitability of the coconut fiber substrate for blueberry propagation, as reported by Machado et al. (2014), when mixed with perlite. It is not recommended to use this substrate at 100% due to the chemical characteristics of coconut fiber, with high concentrations of K, Na and Cl, it can cause stress on plants and delay rooting.
The effect of the interaction of factors in the rooting of cuttings was observed at day 30, most studies reported that the rooting of blueberry cuttings occurs in a time of 55 to 60 days, with a success percentage of 40 to 52% (Castro and Guzmán, 2013). Pego et al. (2019) reported that some species of Ericaceae root better in mixture of peat and river sand; however, with the results obtained in the present work, the mixture of river sand and tezontle presented good physicochemical properties for the rooting of blueberry, this mixture maintains adequate ventilation in the rooting environment.
Effect of the brassinosteroid 24-EBL
When the effect of 24-EBL on the percentage of survival of cuttings was analyzed, a significant effect of this factor was observed, depending on the concentration, when the cuttings were treated with 60 μg L-1, this variable was 54.75%, while with 0 μg L-1 it was only 5.11%. ST treatments with 20 and 40 μg L-1 presented 36% and 63.7% respectively.
Regarding the percentage of rooted cuttings and the number of roots per cutting, the concentration of brassinolide was a significant factor (p= 0.042 and p= 0.013 respectively). When a concentration of 60 μg L-1 24- EBL was used, the percentage of rooting was 67% in the ST mixture, in this same mixture with 40 μg L-1, 60% of the cuttings rooted, while with the concentration of 20 μg L-1, 28% of the cuttings rooted and without the brassinolide only 8% rooted (Table 3).
These results are similar to those presented by Ahammed et al. (2017) in Arabitodpsis, where the effect of brassinolides as bioactive products that potentiate and induce early physiological mechanisms thanks to intermembrane receptors called BRASSINOSTEROID INSENSITIVE1 (BRI1), BRI1-LIKE 1 (BRL1) and BRL3 was observed. BRI1 binds to brasinolide (BL), the most active form of brassinolides, and leads to ligand-mediated activation of the BRL1 receptor, resulting in transphosphorylation events, which also involve coreceptors, such as BRI1 ASSOCIATED KINASE1 (BAK1) (Ayub et al., 2020), these results indicate that cuttings of blueberry variety Biloxi have these receptors and that when they are in the presence of 24-EBL, these are activated triggering cascades of signals that induce the formation of root tissue.
It was observed that the concentration of 24-EBL and the number of roots have a direct relationship, at a higher concentration of 24-EBL, the number and length of roots increase (Table 3). Exogenous application of different brassinolide analogues is known to be used to improve seed germination and induce growth promotion of hypocotyls, cotyledons, leaf blades, main and lateral root elongation (Hussain et al., 2020); nevertheless, until now they had not been used as root-inducing agents in blueberry cuttings.
The combination of the concentration of 40 μg L-1 with CFP and CF substrates did not show significant differences. At a concentration of 20 μg L-1, the substrates CF, PM, CFP and PMP did not present significant differences and showed values of 50% to 34% less with respect to the treatment of ST (Figures 1A, 1B).
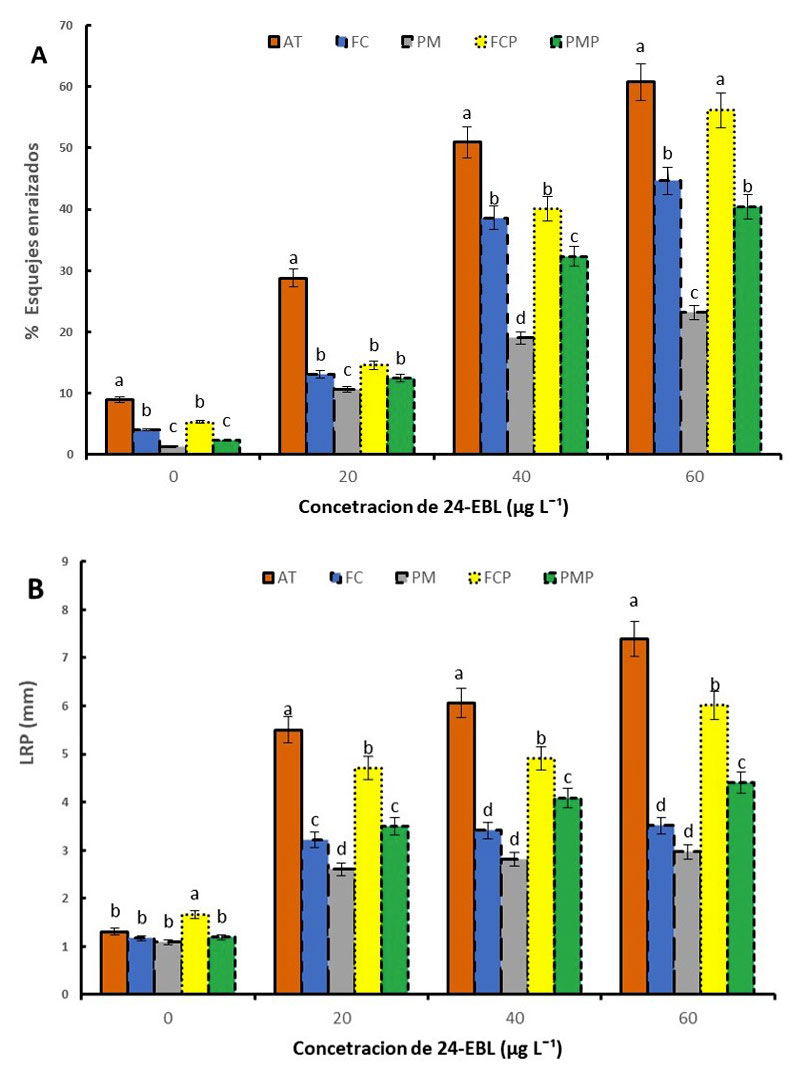
Figure 1 A) Percentage of rooted cuttings; and B) main root length (LRP) of blueberries, propagated on different substrates and treated with 24-EBL. Substrates= sphagnum® Peat Moss (PM); mixture of Sphagnum® Peat Moss and Perlite in a ratio 2:1 (PMP); coconut fiber c (Power Forteco) (FC); mixture of coconut fiber (Power Forteco) and perlite in a ratio 2:1 (FCP) and a mixture of river sand and tezontle in a ratio 1:1 (V/V) (AT). Columns with different letters indicate significant differences (Tukey p< 0.05). Means ± standard error.
Mouchel et al. (2006) mention that there is a synergism between exogenous brassinolides and other plant phytohormones, such as auxins and ABA (abscisic acid), which are responsible for the induction of primary roots. Specifically, 24-EBL also regulates root development through an interaction with auxin in a dose-dependent manner (Sun et al., 2020).
In this study, it was determined that Biloxi roots well in substrate of river sand and tezontle (80%), is acceptable in mixtures of coconut fiber and perlite (50%), peat (Peat Moss) and perlite (52%). For propagation by blueberry cuttings, it is not recommended to use substrates such as peat (Peat Moss) and coconut fiber at 100%, it is recommended to use them in mixture with perlite or river sand.
Blueberry rooting is commonly performed under in vitro conditions using auxins as a root inducer (Hung and Trueman, 2012). The presence of 24-EBL was effective for root formation in blueberry cuttings as shown in the present study, using an alternative substrate to those that are frequently used for propagation decreases cost by directly rooting cuttings under non-aseptic conditions using commercial substrates and solutions of brassinolides at low concentrations.
Conclusions
The combination of river sand and tezontle with 60 μg L-1 of brassinolides was the treatment with the highest percentage of rooted cuttings, number of roots and root length. The use of the brassinolide 24-EBL is a good biotechnological tool for the induction of the root system in blueberry (Vaccinium corymbosum) variety Biloxi.
Acknowledgements
To CONACYT and the Michoacan University of San Nicolás de Hidalgo
REFERENCES
A’saf, T. S.; Ajlouni, M. G.; Ayad, J. Y.; Othman, Y. A. and Hilaire, R. 2020. Performance of six different soilless green roof substrates for the mediterranean region. The Science of the Total Environment. 730:2-10. https://doi.org/10.1016/j.scitotenv.2020.139182. [ Links ]
Acosta-Durán, C.; Vázquez-Benítez, N.; Villegas-Torres, O.; Vence, L. B. y Acosta-Peñaloza, D. 2014. Vermicomposta como componente de sustrato en el cultivo de Ageratum houstonianum Mill. y Petunia hybrida E. Vilm. En contenedor. Bioagro. 26(2):107-114. https://www.redalyc.org/pdf/857/85731100005.pdf. [ Links ]
Ahammed, G. J.; He, B. B.; Qian, X. J.; Zhou, Y. H.; Shi, K.; Zhou, J. Z.; Yu, J. Q. and Xia, X. J. 2017. 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environmental Pollution. 229:922-931. https://doi.org/10.1016/j. envpol.2017.07.076. [ Links ]
Ayub, R. A. and Pereira, A. B. 2020. Brassinosteroid combined with indolbutyric acid in blueberry micropropagation. J. Agric. Sci. 14(5):59-65. https://doi.org/10.5539/jas.v14n5p59. [ Links ]
Bannoud, F. and Bellini, C. 2021. Adventitious rooting in populus species: update and perspectives. Front. Plant Sci. 12:1-22. https://doi.org/10.3389/fpls.2021.668837. [ Links ]
Bergonci, T.; Ribeiro, B.; Ceciliato, P. H. O.; Guerrero, A. J. C.; Silva, F. M. C. and Moura, D. S. 2014. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J. Exp. Bot. 65(8):2219-2230. https://doi.org/10.1093/jxb/eru099. [ Links ]
Blythe, E. K.; Sibley, J. L.; Ruter, J. M. and Tilt, K. M. 2004. Cutting propagation of foliage crops using a foliar application of auxin. Sci. Hortic. 103(1):31-37. https://doi.org/10.1016/j.scienta.2004.04.011. [ Links ]
Braha, S. and Rama, P. 2018. Impact of the shoot maturity level on rooting, acclimatisation of green and semi-hardwood cuttings of the blueberry (Vaccinium corymbosum L.) cv Bluecrop stimulated with Indol butyric acid and naphthalene acetic acid. Agric, Forest. 64(3):79-87. https://doi.org/10.17707/AgricultForest.64.3.07. [ Links ]
Cárdenas-Navarro, R. y López-Pérez, L. 2011. Propagación vegetativa de rosa: efecto del sustrato, luminosidad y permanencia de la hoja. Sci. Agropec. 2(4):203-211. https://doi.org/10.17268/sci.agropecu.2011.04.02. [ Links ]
Castro-Restrepo, D. y Álvarez-Guzmán, J. A. 2013. Micropropagación clonal de tres genotipos mortiño, Vaccinium meridionale sw., por proliferación de yemas axilares. Actualidades biológicas. 35(99):145-160. [ Links ]
Crespo-Crespo, G. M. R.; González-Eguiarte, D. R.; Rodríguez-Macías, R.; Ruiz-Corral, J. A. y Durán-Puga, N. 2018. Caracterización química y física del bagazo de agave tequilero compostado con biosólidos de vinaza como componente de sustratos para cultivos en contenedor. Rev. Internac. Contamin. Ambien. 34(3):373-382. https://doi.org/10.20937/rica.2018.34.03.01. [ Links ]
De-Boodt, M. and Verdonck, O. 1972. The physical properties of the substrates in horticulture. Acta Hortic. 26:37-44. https://doi.org/10.17660/actahortic.1972.26.5. [ Links ]
Díaz, L. A.; Fischer, G. and Pulido, S. P. 2012. La fibra de coco como sustituto de la turba en la obtención de plántulas de uchuva (Physalis peruviana L.). Rev. Colomb. Cienc. Hortíc. 4(2):153-162. https://doi.org/10.17584/rcch.2010v4i2.1236. [ Links ]
Gómez-Merino, F. C.; Trejo-Téllez, L. I.; García-Albarado, J. C. y Morales-Ramos, V. 2013. Lulo (Solanum quiroense Lamarck). Como nuevo elemento del paisaje en México: germinación y crecimiento en sustratos orgánicos. Rev. Mexic. Cienc. Agríc. 4(5):877-887. http://www.scielo.org.mx/scielo.php?script=sci-abstract&pid=S200709342013000900002&lng=es&nrm=iso . [ Links ]
Hung, C. D. and Trueman, S. J. 2012. Cytokinin concentrations for optimal micropropagation of Corymbia torelliana and C. citriodora. Australian Forestry. 75(4):233-237. https://doi.org/10.1080/00049158.2012.10676407. [ Links ]
Hussain, M. A.; Fahad, S.; Sharif, R.; Jan, M. F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B. S.; Sun, C.; Gu, L.; Ahmad, I.; Jiang, Z. and Hou, J. 2020. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regulation. 92(2):141-156. https://doi.org/10.1007/s10725-020-00647-8. [ Links ]
Leitner, D.; Felderer, B.; Vontobel, P. and Schnepf, A. 2014. Recovering root system traits using image analysis exemplified by two-dimensional neutron radiography images of lupine. Plant Physiol. 164(1):24-35. https://doi.org/10.1104/pp.113.227892. [ Links ]
Luna-Fletes, J. A.; Cruz-Crespo, E. and Can-Chulim, A. 2021. Pumice stone, tezontle and nutritive solutions in the cultivation of cherry tomato. Terra Latinoam. 39:1-12. https://doi.org/10.28940/terra.v39i0.781. [ Links ]
Machado, R. M. A.; Bryla, D. R. and Vargas, O. 2014. Effects of salinity induced by ammonium sulfate fertilizer on root and shoot growth of highbush blueberry. Acta Hortic. 1017(49):407-414. https://doi.org/10.17660/actahortic.2014.1017.49. [ Links ]
Mouchel, C. F.; Osmont, K. S. and Hardtke, C. S. 2006. BRX mediates feedback between brassinosteroid levels and auxin signalling in root growth. Nature. 443(7110):458-461. https://doi.org/10.1038/nature05130. [ Links ]
Pagani, A.; Molinari, J.; Lavado, R. and Benedetto, A. 2015. Behavior impatient Wallerana Hook. F in alternative pot substrates: Mechanisms involved and research perspectives. J Plant Nutr. 38(14):2185-2203. https://doi.org/10.1080/01904167.2014.988357. [ Links ]
Pardo-Giménez, A. and Pardo-González, J. E. 2008. Evaluation of casing materials made from spent mushroom substrate and coconut fibre pith for use in production of Agaricus bisporus (Lange) Imbach. Rev. Investing. Agrar. 6(4):683-690. https://doi.org/10.5424/sjar/2008064-361. [ Links ]
Pego, R. G.; Fiorini, C. V. A.; Machado, A. F. L. and Gomes, M. V. S. 2019. Propagation of Streptosolen jamesonii (Benth) miers by stem cutting treated with IBA in different substrates. Ornamental Hortic. 25(1):26-33. https://doi.org/10.14295/oh.v25i1.1184. [ Links ]
Putrino, F. M.; Tedesco, M.; Bodini, R. B. and Oliveira, A. L. 2020. Study of supercritical carbon dioxide pretreatment processes on green coconut fiber to enhance enzymatic hydrolysis of cellulose. Bio. Technol. 309:1-7. https://doi.org/10.1016/j.biortech.2020.123387. [ Links ]
Rodriguez, S. C.; Vincent, C. and Rufus, I. 2019. Blueberry IPM: past successes and future challenges. Ann. Rev. Entomol. 64(1):95-114. https://doi.org/10.1146/annurev-ento-011118-112147. [ Links ]
Sánchez-Córdova, T.; Aldrete, A.; Cetina-Alcalá, V. M. and López-Upton, J. 2008. Caracterización de medios de crecimiento compuestos por corteza de pino y aserrín. Madera y Bosques. 14(2):41-49. [ Links ]
Serna, M.; Hernández, F.; Coll, F. and Amorós, A. 2012. Brassinosteroid analogues effect on yield and quality parameters of field-grown lettuce (Lactuca sativa L.). Sci. Hortic. 143:29-37. https://doi.org/10.1016/j.scienta.2012.05.019. [ Links ]
SIAP. 2021. Sistema de Información Agroalimentaria y Pesquera, S. Panorama agroalimentario. gob.mx. https://www.gob.mx/siap/documentos/panorama-agroalimentario-2021. [ Links ]
Sun, L.; Feraru, E.; Feraru, M. I.; Waidmann, S.; Wang, W.; Passaia, G.; Wang, Z. Y.; Wabnik, K. and and Kleine-Vehn, J. 2020. PIN-LIKES coordinate brassinosteroid signaling with nuclear auxin input in Arabidopsis thaliana. Current Biol. 30(9):1579-1588. https://doi.org/10.1016/j.cub.2020.02.002. [ Links ]
Vargas-Tapia, P.; Castellanos-Ramos, J. Z.; Sánchez-García, P.; Tijerina-Chávez, L.; López-Romero, R. M. y Ojodeagua-Arredondo, J. L. 2008. Caracterización física, química y biológica de sustratos de polvo de coco. Rev. Fitotec. Mexic. 31(4):375-375. [ Links ]
Vázquez, M. N.; Guerrero, Y. R.; Noval, W. T.; González, L. M. and Zullo, M. A. T. 2019. Advances on exogenous applications of brassinosteroids and their analogs to enhance plant tolerance to salinity: A review. Austr. J. Crop Sci. 13(1):115-121. https://search.informit.org/doi/10.3316/informit.338566792578080. [ Links ]
Villegas-Monter, A.; Castro-Garibay, S. L. y Castro-Garibay, S. L. 2019. Enraizamiento de estacas en tres cultivares de arándano (Vaccinium corymbosum L.). AgroProductividad. 12(3):63-68. https://doi.org/10.32854/agrop.v0i0.1328 [ Links ]
Received: October 01, 2022; Accepted: January 01, 2023











 text in
text in 


