Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.14 n.1 Texcoco Jan./Feb. 2023 Epub June 19, 2023
https://doi.org/10.29312/remexca.v14i1.2924
Articles
Morphometry of fruit and seed of populations of Ferocactus pilosus from the Highlands of Tamaulipas
1TecNM-Instituto Tecnológico de Ciudad Victoria. Boulevard Emilio Portes Gil #1301, Ciudad Victoria, Tamaulipas, México. CP. 87010. Tel. 834 1532000, ext. 325. (erick-burrin@yahoo.com; crystian.vb@cdvictoria.tecnm.mx).
2Colegio de Postgraduados. Carretera México-Texcoco km 36.5. Montecillo, Texcoco, Estado de México. CP. 56230. (hvaquera@colpos.mx).
3TecNM-Campus Instituto Tecnológico de Roque. Carretera Celaya-Juventino Rosas km 8, Celaya, Guanajuato, México. CP. 38110. (francisco.co@roque.tecnm.mx).
4Universidad Autónoma de Tamaulipas-Centro Universitario Adolfo López Mateos. Ciudad Victoria, Tamaulipas, México. CP. 87149. (wpoot@docentes.uat.edu.mx).
In Mexico, more than 75% of cactus species are subject to anthropogenic pressure and habitat destruction, as is the case of Ferocactus pilosus, so it is important to increase knowledge for their recovery in situ. The objective of the study was to determine how the semiarid environment of the Highlands of Tamaulipas tends to limit the distribution of five populations of F. pilosus and promotes morphological changes that operate in fruits and seeds. The dendrogram defined the similarity between populations II, III and IV of the barrel cactus, due to differences in stoniness and slight humidity condition due to the type of climate and elevation. The analyses Manova and Tukey demonstrated the existence of significant differences in seed number (SN) and seed weight (SW) of barrel cactus. The SN was statistically equal between populations II (674 ±191), III (657 ±221) and IV (643 ±246), while in the same populations, SW was 0.92 ±0.27, 0.9 ±0.3 and 0.88 ±0.34 mg, respectively. Populations II, III and IV of the Mexican fire barrel cacti took better advantage of the environmental conditions of the Highlands of Tamaulipas prevailing in 2013 and 2014, which caused a significant variation of SN and SW in the same populations of F. pilosus and the partial rejection of the hypothesis.
Keywords: barrel cactus; number of seeds; weight of seeds
En México, más de 75% de las especies de cactáceas están sometidas a presión antropogénica y destrucción de su hábitat, como es el caso de Ferocactus pilosus, por lo que es importante aumentar el conocimiento para su recuperación in situ. El objetivo del estudio fue determinar la manera en que el ambiente semiárido del Altiplano de Tamaulipas tiende a limitar la distribución de cinco poblaciones de F. pilosus y promueve cambios morfológicos que operan en frutos y semillas. El dendrograma definió la similitud entre poblaciones II, III y IV de la biznaga, debido a diferencias en pedregosidad y ligera condición de humedad por el tipo de clima y la elevación. El análisis Manova y Tukey demostraron la existencia de diferencias significativas en número de semillas (NS) y peso de semillas (PS) de biznaga. El NS fue estadísticamente igual entre las poblaciones II (674 ±191), III (657 ±221) y IV (643 ±246), mientras que en las mismas poblaciones PS fue 0.92 ±0.27, 0.9 ±0.3 y 0.88 ±0.34 mg, respectivamente. Las poblaciones II, III y IV de la biznaga cabuchera, aprovecharon mejor las condiciones ambientales del Altiplano de Tamaulipas prevalecientes en 2013 y 2014, lo que provocó una variación importante de NS y PS en las mismas poblaciones de F. pilosus y el rechazo parcial de la hipótesis.
Palabras clave: biznaga; número de semillas; peso de semillas
Introduction
The Cactaceae family is distinctive for the evolutionary differences and diversification that characterize it (Linkies et al., 2010). The loss of cactus species in their habitat (Durant et al., 2012) is associated with anthropogenic pressure, predation and dependence on nurse plants, which affects sexual reproductive viability (pollination, fertilization, crossing systems) and establishment (Flores-Martínez et al., 2013; Lara et al., 2016). In the American continent there are about 2000 species of cacti (Jiménez, 2011; Goettsch et al., 2015), more than 30% threatened with extinction (Fitz and Fitz, 2017).
Cacti are important in the structure and dynamics of ecosystems in semiarid areas of Mexico (Jiménez, 2011) and the world, but it is necessary to increase the knowledge we have about these species and their environment, in particular about Ferocactus pilosus from the southeast of the state of Tamaulipas, Mexico. In Mexico, F. pilosus or Mexican fire barrel cactus is distributed in the Chihuahuan Desert associated with a diversity of xerophytic species (INEGI, 2005; 2006), present in shallow and limestone soils, in alluvial fans, valleys, hills or plains (Rzedowski, 2006). F. pilosus is a species at risk of least concern (IUCN, 2016) and under special protection in the Official Mexican Standard NOM-059-SEMARNAT-2010 (SEMARNAT, 2010).
The above justifies the development of studies of conservation and restoration of F. pilosus populations in their habitat, in addition to those related to changes that occur in fruit morphology and seed availability (Lara et al., 2016; Ballesteros-Barrera et al., 2017). The fruit defines aspects of the physical and physiological quality of the seed, although it is an instrument by which the variability and dependence of species on environmental factors is verified in a population (Canazza et al., 2009).
The study hypothesizes that the variations in the physical and climatic conditions imposed by the relief of the Highlands of Tamaulipas cause changes independent of those registered by the reproductive structures of Ferocactus pilosus and its populations. While as an objective it was proposed to determine the morphology of the fruit and its relationship with the physical qualities of the seed of populations of F. pilosus from the Highlands of Tamaulipas.
Materials and methods
The study area, known as the Highlands of Tamaulipas, is located between 23.600°/22.656° north latitude and -100.139°/-99.572° west longitude (INEGI-CONABIO-INE, 2008). The physical characteristics of the areas occupied by each population of Ferocactus pilosus studied are shown in Table 1. On the other hand, the average temperature varies between 16.1 and 22.3 °C and rain, if it occurs in the year, is 310.8 mm, between May and September. In the year prior to the fruit collection (2013), the average temperature was 15.3 °C and rainfall was 61.5 mm (CISECE, 2014; station 28115 Uvalles, Miquihuana).
Table 1 Physiographic location and physical characteristics of growth of the populations of Ferocactus pilosus of the Highlands of Tamaulipas (INEGI, 2017).
| Locality | Municipality | Location | Elevation (m) | Stoniness (%) | Soil | Climate | |
| North latitude | West longitude | ||||||
| I | Villa de Miquihuana | 23 540 | -99 821 | 1 700 | 45 | Litosol | Temperate semi-dry |
| II | Ejido La Pérdida, Miquihuana | 23 541 | -99 841 | 1 580 | 65 | Haplic, Petrocalcic Xerosol | Dry semi-warm |
| III | Ejido Estanque de Los Walle, Miquihuana | 23 567 | -99 860 | 1 554 | 60 | Haplic, Petrocalcic Xerosol | Dry semi-warm |
| IV | Ejido Joya de Herrera, Bustamante | 23 481 | -99 840 | 1 776 | 33 | Haplic, Petrocalcic Xerosol | Temperate semi-humid |
| V | Ejido Magdaleno Cedillo, Tula | 22 841 | -99 923 | 1 085 | 20 | Haplic, Petrocalcic Xerosol | Dry semi-warm |
Multivariate cluster analysis was used to measure the degree of similarity between populations through the Euclidean distance of the dendrogram, through the discrimination of populations with similar correlation coefficients (Núñez-Colín et al., 2004). The study factor analyzed was individuals of F. pilosus from six populations of the Highlands of Tamaulipas.
The sampling of barrel cactus fruits was done between January and February 2014, in quadrants of 100 x 100 m, in individuals with similar age and identified by height (1.2 to 1.8 m) and stems (14 to 23). The count of individuals and fruits sampled varied between populations: I (6 individuals and 23 fruits); II (15 and 90); III (7 and 42); IV (12 and 53); and V (9 and 52). The fruit measurements and the extraction and count of seeds were made in twenty fruits of barrel cactus.
In the fruit, the equatorial diameter (ED), diameter of the peduncle insertion zone (PD) and polar length (PL) were measured with a digital vernier (Mitutoyo, Japan). The fruit volume (FV) was calculated by the criterion ‘barrel geometry’ (Hernández and Treviño, 1998), with the formula:
Where: h = PL, in cm; π = 3.416; r1 = minimum radius (RMn); r2 = maximum radius (RMx) of ED, in cm. After drying, the seed number (SN) was obtained from digitalized images, captured and counted in the MideBMP 4.2 software (Ordiales-Plaza, 2000), while the dry weight of seed (SW) per fruit was determined in the analytical balance (Ohaus, USA).
The variables measured in fruit and seed of F. pilosus were subjected to a Pearson Correlation Analysis; the existence of significant correlation between variables led to the development of the analysis of assumptions of the statistical technique with the test of homogeneity of variances of Levene and the test of normality of Shapiro-Wilks, which were satisfactory where these statistical differences occurred. This result allowed the continuation of the process through a multivariate analysis of variance (Manova), which uses the Type III error in the null hypothesis matrix, and which has global significance tests, such as the multivariate F test and the criteria of Wilk’s Lambda, Pillai’s T and Hotelling-Lawley’s T, mainly.
The determination of significant statistical effects in the Manova led to develop the Tukey mean separation test (α≤ 0.05) to determine the effect of the population of F. pilosus as a factor of variation due to the geographical location and physical characteristics of the localities where the fruits were sampled.
Results and discussion
Despite postulating an environmental stability for vegetation, F. pilosus populations differ markedly in growth and development in the Highlands of Tamaulipas, which has caused morphological changes in seed number (SN) and seed weight (SW). This led to the partial rejection of the proposed hypothesis.
Relationship between populations of Ferocactus pilosus
The degree of affinity between F. pilosus populations is shown in Figure 1, which formed three main groups. The greatest similarity was between group 1 composed of the Mexican fire barrel cacti of populations I and V and group 2 of populations III and IV and these with group 3 of population II. This same answer would have been obtained in the tests of Manova and Tukey.
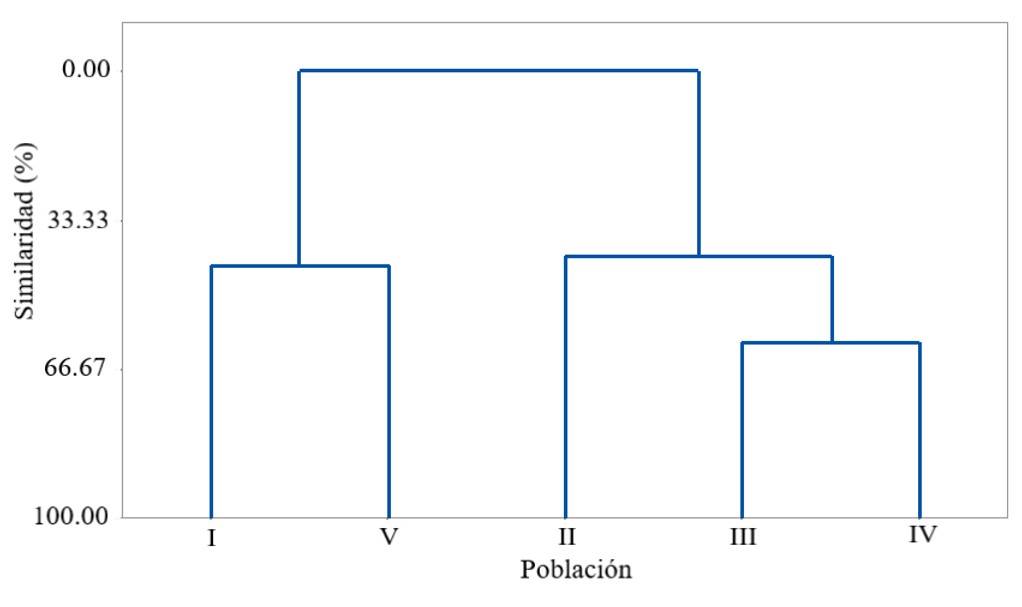
Figure 1 Dendrogram of the populations of the Highlands of Tamaulipas, which shows the existence of variables related to the morphometry of fruit and seed of Ferocactus pilosus (the name of the population is described in Table 1).
However, based on the physical characteristics, populations II and III are similar in the type of climate, soil, geographical location, elevation and surface stoniness. Population IV, on the other hand, has a temperate subhumid climate defined by the higher elevation and lower stoniness and latitudinal geographical location located almost at the height of population II (Table 1).
These characteristics of these populations may have influenced the existence of significant differences in SN and SW of the Mexican fire barrel cactus (Table 2). The relationship that unites these three populations with populations I and V is found in a biotic-type response in which physiological maturity (Ayala-Cordero et al., 2004) and population structure participate (Lara et al., 2016), given the coincidence in age, size and number of stems of the Mexican fire barrel cacti at the time of fruit collection.
Table 2 Tukey’s mean test for seed number (SN) and seed weight (SW) of Ferocactus pilosus populations of the Highlands of Tamaulipas.
| Population | SN | SW (mg) |
| I | 516.96 b | 0.7057 ab |
| II | 673.93 a | 9.9242 a |
| III | 656.88 a | 0.9017 a |
| IV | 643.11 ab | 0.8824 ab |
| V | 563.12 ab | 0.7729 ab |
Mean values with the same literal by variable are statistically equal to each other.
The distribution of F. pilosus in the Highlands of Tamaulipas extends to alluvial soils, where the greatest abundance and diversity of plant species is favored, but it is also located in areas of piedmont and abundant surface stoniness, with a predominance of limestone rocks that retain soil moisture. The results of Tilman (1990) highlight that the interaction between plants is traditionally analyzed in the aerial part, above the ground (energy translated into dry biomass) and the attention to the underground part (supply of water and nutrients), where the root acquires greater importance in environments of low productivity, is low.
The orographic-type shading that normally derives from the Sierra Madre Oriental favors the retention of water in the soil and its availability for plants (Salinas-Rodríguez, 2018). It is known that water deficiency in a succulent stem tends to affect the storage organs, particularly between flowering and seed maturity (Tardieu, 2013). In this sense, Ayala-Cordero et al. (2004) consider as a rule the fact that, in populations of Stenocereus beneckei located in environments restrictive of humidity, a variation in the number and weight of seeds.
The small size (< 1 mm) and the abundant production of seeds of F. pilosus increases its reserve in the soil and dispersion by anemochory (personal observation). Unfortunately, F. pilosus populations are exposed to predators and anthropogenic use of flower buds and fruits, an action that interrupts the reproductive cycle (Alanís-Flores and Velazco-Macías, 2008) and decreases the potential distribution of populations.
This happens in Mexico for F. pilosus, which, despite occupying an extensive area (298 007 km2), has lost 16% of cover at the local level (Ballesteros-Barrera et al., 2017). This requires the search for conservation actions, but importantly to the in-situ recovery of F. pilosus, reproduced under controlled conditions.
Statistical criteria for the selection of variables
The growth and development of the fruit involve internal and external plant factors, such as genetics, hormone content, position and competition of the fruit, as well as availability of water and nutrients, and temperature and luminosity (Fisher et al., 2018). This dependence may have been expressed through the correlation analysis, which reveals a strong positive association between PL vs ED (0.7422; p< 0.0001), PL vs RMx (0.7421; p< 0.0001) and PL vs FV (0.8780; p< 0.0001).
Despite the fact that there was a perfect positive association, although this same condition was maintained between ED vs FV (0.9262; p< 0.0001) and RMx vs FV (0.9262; p< 0.0001), it failed to influence the physical characteristics of the seed, such as SN vs SW (0.9999; p< 0.0001). Based on these results, the Manova was developed. Both SN (F= 3.49; p< 0.0085) as SW (F= 3.47; p< 0.0088) resulted in statistically significant differences when contrasting the populations of Ferocactus involved in the Manova.
In this model, 92% of the variance was explained by the first three characteristic roots, while the Manova procedure meets the multivariate F values and the criteria of Wilk’s Lambda= 0.8087 (F= 1.7; p< 0.0097), Pillai’s T= 0.2049 (F= 1.69; p< 0.0098) and Hotelling-Lawley’s T= 0.2201 (F= 1.7; p< 0.0103). That is, although the value of F varies for each statistic, each corresponding p value is less than α= 0.05; so, the null hypothesis of Manova is rejected and it is concluded that Ferocactus populations have an effect on SN and SW, which are discussed below.
Seed number
The largest number of Mexican fire barrel cactus seeds was recorded in fruits collected from populations II (674 ±191); III (657 ±221); and IV (643 ±246), with respect to the total mean (629), according to Tukey (α≤ 0.05). This answer proves the existence of competition in each population (Raisman and González, 2013), in a restrictive semiarid environment where the requirements are similar but sufficient to form a significant number of seeds in the populations of Mexican fire barrel cactus.
Competition is often associated with the evolution of the strategies employed by the species, such as the nurse plants demanded by the Mexican fire barrel cactus in stages of initial growth and the multiplication of stems, which depends on a mature individual, while competitive ability is determined by phenotypic and genotypic attributes (Grime, 1977), which change among barrel cactus populations. Individuals in populations II, III and IV of F. pilosus showed greater ability to channel their resources into reproductive structures.
A response that manifests itself in a greater variation in the number of seeds. The number of seeds found in F. pilosus is abundant, although lower than those formed by F. histrix, 2 200 seeds per fruit (Del Castillo, 1986; Loza et al., 2012). Normally, seed abundance is accompanied by size reduction, observed in species with r-type reproductive strategy (fluctuating), such as that shown by F. pilosus: mass production of potential individuals (seeds) versus low probability of survival (seedlings).
With development, this species changes its strategy to type k (constant): bulky and long-lived individuals, but their growth is slow, and maturation is late. Therefore, the survival of F. pilosus populations depends on a small number of individuals or their low recruitment capacity (Morláns, 2004). The number of seeds is extremely different between/within cacti, depending on age, number of flowers or fruit size (Santos-Díaz et al., 2010), as well as the high dependence on pollinating agents, so the barrel cactus maintains its functionality and genetic variability at the expense of inbreeding (Stein et al., 2017).
That is, the limited dispersal of F. pilosus seeds may have an impact on the local genetic structure in the short term (Grivet et al., 2009), in view of the reduction of mothers and fathers, a progressive increase in genetic structure and levels of biparental inbreeding, which would explain the decrease in seeds produced, germination rates and vigor (Nora et al., 2011) and low seedling establishment.
The number of F. pilosus seeds showed a behavior similar to that of isolated metapopulation species; that is, they show a simple allometric trajectory in the absence of competition (Torroba et al., 2013), but intraspecific competitive interactions induce morphological variations in certain individuals (Schwinning and Kelly, 2013), as occurred with SN.
Another cause of the specific variation of the seed of F. pilosus is the characteristics that increase the probability of survival in a given environment (Martínez et al., 2016), without underestimating the existence of some type of pressure of different nature at the present time. Larios et al. (2014) established that the critical resources with which competition operates vary between environments and are increased by resources such as soil nutrients and water in dry environments.
Unfortunately, the ultimate effect of intraspecific competition manifests itself in future generations (Valeria et al., 2017); therefore, this type of competition would tend to regulate the size of the populations of Mexican fire barrel cactus. Number and weight of cactus seed also depend on the growing environment, age and adaptation of the reproductive system (Ayala-Cordero et al., 2004) and pollinators (Valiente-Banuet, 2002).
Seed weight
In the seed weight (SW) of Ferocactus pilosus, populations II, III and IV were outstanding, with 0.92 ±0.27, 0.9 ±0.3 and 0.88 ±0.34 mg, each, statistically different according to the Tukey test (α≤ 0.05). Some explanations around this response could be due to a similarity in the best use of low soil moisture, nutritional reserves, solar radiation, temperature, age of the plant, prevailing between flowering and seed formation and maturity (Ayala-Cordero et al., 2004; Flores and Jurado, 2011) in growing environments with some degree of similarity between the three populations of barrel cactus, with repercussions on seed weight.
The weak to moderate positive correlation that existed between SW with PL, ED, RMx, RMn and FV would indicate that the weight gain of the seed does not depend on the size of the fruit, which is highly variable and therefore an undesirable attribute for F. Pilosus. Among these, the low contribution of PL in SW would also have been found in F. robustus and Pterocereus gaumeri (Méndez et al., 2005; González and Navarro, 2011), in contrast to the positive response obtained in F. cylindraceus, F. wizlizenii and Echinocactus platyacanthus (McInthosh, 2002; Díaz et al., 2008).
The seeds of the barrel cacti of populations II, III and IV, due to their greater weight, could have an advantage in terms of survival, viability, germination and establishment, with respect to the seeds of populations I and V of F. pilosus; nevertheless, the weight of the seed decreases as its number in the fruit increases. In the same sense, the seeds of the Mexican fire barrel cactus with variable weight in a population would be expected to exhibit memory of hydration and alternating germination (Contreras-Quiróz et al., 2016a, 2016b).
The variation in weight of seeds of the same species responds to a differential biological capacity (Sánchez-Salas et al., 2006). An ideal advantage of large seeds, such as those of barrel cactus populations II, III and IV, is to have higher nutritional reserves and despite germinating slowly, they have high germination and emergence rates (Brown et al., 2003). In reality, the Mexican fire barrel cactus has a high mortality rate during germination and establishment in natural conditions, which motivates the transplantation of individuals reproduced ex situ.
Conclusions
The proposed hypothesis was partially satisfied, because the physical characteristics of the semiarid zone of the Highlands of Tamaulipas defined three groups of populations of Ferocactus pilosus with a certain degree of similarity and only the number and weight of the seed suffered a variation because of these group differences. The affinity and group responses occurred in the populations of Mexican fire barrel cactus of the Ejidos La Perdida, Estanque de los Walle and Joya de Herrera, as well as for Villa de Miquihuana and Ejido Magdaleno Cedillo, in a different group.
Acknowledgements
The first author expresses his gratitude to CONACYT-Mexico for the granting of the scholarship for the development of postgraduate studies, identified with No. 290817-DBIO-2011-03. In the same way, the participation of the anonymous reviewers designated by the editorial group of the Mexican Journal of Agricultural Sciences of INIFAP is appreciated, who, in anonymity, allowed improving the thematic content.
REFERENCES
Alanís-Flores, G. J. y Velasco-Macías, C. G. 2008. Importancia de las cactáceas como recurso natural en el noreste de México. Ciencia UANL. 11(001):5-11. [ Links ]
Ayala-Cordero, G.; Terrazas, T.; López-Mata, L. y Trejo, C. 2004. Variación en el tamaño y peso de la semilla y su relación con la germinación en una población de Stenocereus beneckei. Interciencia. 29(12):692-697. [ Links ]
Ballesteros-Barrera, C.; Aguilar-Romero, O.; Zarate-Hernández, R. y Ballesteros-Tapia, L. 2017. Distribución geográfica y conservación de nueve especies del género Ferocactus (Cactaceae) en México. Rev. Fitotec. Mex. 40(2):131-140. [ Links ]
Brown, J.; Enrigth, N. J. and Miller, B. P. 2003. Seed production and germination in two rare and three common co-occurring Acacia species from southeast Australia. Australian Ecol. 28(3):271-280. https://doi.org/10.1046/j.1442-9993.2003.t01-4-01287.x. [ Links ]
Canazza, M. C.; Scalon, S. P. Q.; Sari, A. P.; Rosa, Y. B. C. J. and Robaina, A. D. 2009. Biometria de frutos e sementes e germinação de magonia pubescens ST. Hil (Sapindaceae). Rev. Brasileira de Sementes. 31(2):202-211. https://doi.org/10.1590/S0101-31222009000200024. [ Links ]
Contreras-Quiroz, M. R.; Pando-Moreno, M.; Flores, J. and Jurado, E. 2016. Effects of wetting and drying cycles on the germination of nine species of the Chihuahuan desert. Bot. Sci. 94(2):221-228. https://doi.org/10.17129/botsci.457. [ Links ]
Contreras-Quiroz, M. R.; Pando-Moreno, M.; Jurado, E.; Bauk, K.; Gurvich, E. and Flores. J. 2016b. Is seed hydration memory dependent on climate? testing this hypothesis with Mexican and Argentinian cacti species. J. Environ. 130:94-97. https://doi.org/10.1016/j.jaridenv.2016.03.001. [ Links ]
Del-Castillo, R. F. 1986. Semillas, germinación y establecimiento de Ferocactus histrix. Cactáceas y Suculentas Mexicanas. 3(1):5-10. [ Links ]
Díaz, H. H.; Navarro, C. M. C and Rodríguez, M. C. M. 2008. Aspectos de la morfometría y fenología reproductiva de Echinocactus platyacanthus en la barranca Huexotitlanapa de Tecali de Herrera. Cactáceas y Suculentas Mexicanas . 53(4):100-107. [ Links ]
Durant, S. M.; Pettorelli, N.; Bashir, S.; Wooddroffe, R.; Wacher, T.; Ornelas, P.; Ransom, C.; Abáigar, T.; Abdelgadir, M.; El Alqamy, H.; Beddiaf, M.; Belbachir, F.; Belbachir-Bazi, A.; Berbash, A. A.; Beudels-Jamar, R.; Boitani, L.; Breitenmoser, C.; Cano, M.; Chardonnet, P.; Collen, B.; Cornforth, W. A.; Cuzin, F.; Gerngross, P.; Haddane, B.; Hadjeloum, M.; Jacobson, A.; Jebali, A.; Lamarque, F.; Mallon, D.; Minkowsky, K.; Monfort, S.; Ndoassal, B.; Newby, J.; Ngakoutou, B. E.; Niagate, B.; Purchase, G.; Samaïla, S.; Samna, A. K.; Sillero-Zubiri, C.; Soultan, A.E.; Stanley Price, M .R. and Billie, J. E. M. 2012. Forgotten biodiversity in desert ecosystems. Science. 336(6087):1379-1380. https://doi.org/10.1126/science.336.6087.1379.. [ Links ]
Fitz, M. B. and Fitz, M. W. A. 2017. Ferocactus pilosus. The IUCN red list of threatened species 2017: e.T152928A121553200. https://doi.org/10.2305/IUCN.UK.2017-3.RLTS.t152928A121553200.en . [ Links ]
Flores, J. and Jurado, E. 2011. Germinación de especies de cactáceas en categoría de riesgo del Desierto Chihuahuense. Rev. Mex. Cienc. Forest. 2(8):59-70. [ Links ]
Flores-Martínez, A.; Manzanero, G. I.; Golubov, J. y Mandujano, M. C. 2013. Biología floral de Mammillaria huitzilopochtli, una especie rara que habita acantilados. Bot. Sci. 91(3):349-356. [ Links ]
Goettsch, B.; Hilton, T. C.; Cruz, P. G.; Duffy, J. P.; Frances, A. and Hernández, H. M. 2015. High proportion of cactus species threatened with extinction. Natural Plants. 15142:1-7. https://doi.org/10.1038/NPLANTS.2015.142. [ Links ]
González, M. E. M. y Navarro, C. M. C. 2011. Fenología reproductive de Ferocactus robustus en San Mateo Talixpan, Tecamachalco, Puebla, México. Cactáceaes y Suculentas Mexicanas. 56(4):100-111. [ Links ]
Grime, J. P. 1977. Evidence for the existence of three primary strategies in plants its relevance to ecological and evolutionary theory. The American Naturalist. 111(982):1168-1194. http://www.jstor.org/stable/2460262. [ Links ]
Grivet, D.; Robledo, A. J. J., Smouse, P. E. and Sork, V. 2009. Relative contribution of contemporary polen and seed dispersal to the effective parental size of seedling population of California valley oak (Quercus lobata Née). Mol. Ecol. 18(19):30967-3979. https://doi.org10.1111/j.1365-294X.2009.04326.x. [ Links ]
Hernández, B. T. y Treviño, C. J. 1998. Notas referentes al fruto de Ariocarpus agavoides (Castañeda) anderson. Cactáceas y Suculentas Mexicanas . 43(4):80-84. [ Links ]
INEGI. 2005. Instituto Nacional de Estadística, Geografía e Informática. Guía para la interpretación de cartografía: uso de suelo y vegetación. Instituto Nacional de Estadística, Geografía e Informática. Aguascalientes, México. [ Links ]
INEGI. 2006. Instituto Nacional de Estadística, Geografía e Informática. Propuesta de clasificación: Sistema de clasificación de la cubierta de la tierra. Instituto nacional de estadística, geografía e informática, Aguascalientes, México. [ Links ]
INEGI-CONABIO-INE. 2008. Instituto Nacional de Estadística, Geografía e Informática Comisión Nacional para el Conocimiento y Uso de la Biodiversidad-Instituto Nacional de Ecología. Ecorregiones Terrestres de México. http://www.conabio.gob.mx/informacion/ metadata/gis/ecort08gw.xml?-xsl=/db/metadata/xsl/fgdc-html.xsl&-indent=no. [ Links ]
IUCN. 2016. International Union for Conservation of Nature and Natural Resources. Red list of threatened species. Gland, Switzerland, international union for the conservation of nature. http://iucnredlist.org. Version 2016-1. [ Links ]
Jiménez, S. C. L. 2011. Las cactáceas mexicanas y los riesgos que enfrentan. Rev. Digital Universitaria. 12(1):1-23. [ Links ]
Lara, J. E. I.; Treviño, C. J.; Estrada, D. B.; Poot, P. W. A.; Vargas, T. V. y Ballesteros, B. C. 2016. Determinación de las especies nodriza de Ferocactus pilosus (Galeotti) Werderm. (Cactaceae) en Miquihuana, Tamaulipas, México. Rev. Mex. Agroecos. 3(2):184-194. [ Links ]
Larios, E.; Búrquez, A.; Becerra, J. and Venable, L. D. 2014. Natural selection on seed size through the life cycle of a desert annual plant. Ecology. 95(11):321. https://doi.org/10.1890/13-1965. [ Links ]
Linkies, A.; Graeber, K.; Knight, C. and Leubner-Metzger, G. 2010. The evolution of seeds. New Phytologist. 186(4):817-831. https://doi.org/10.1111/j.1469-8137.2010.03249.x. [ Links ]
Loza, C. S.; Terrazas, T. and López-Mata, L. 2012. Fruits, seeds, and germination in five species of globose cacteae (cactaceae). Interciencia . 37(3):197-203. https://www.interciencia.net/ wp-content/uploads/2018/01/197-c-terrazas-7.pdf. [ Links ]
Martínez, I.; García, D. and Obeso, J. R. 2016. Allometric allocation in fruit and seed packaging conditions the conflict among selective pressures on seed size. Ev. Ecol. 21(4):517-533. https://doi.org/10.1007/s10682-006-9132-x. [ Links ]
McIntosh, M. E. 2002. Flowering phenology and reproductive output two sister’s species of Ferocactus (Cactaceae). Plant Ecol. 159:1-13. https://doi.org/10.1023/A:1015589002987. [ Links ]
Méndez, M.; Durán, R.; Dorantes, A.; Dzib, G.; Simá, L.; Simá, P. and Orellana, R. 2005. Floral demography and reproductive systema of Pterocereus gaumeri, a rare columnar cactus endemic to Mexico. J. Arid Environ. 62(3):363-376. https://doi.org/10.1016/j.jaridenv.2004.12.002. [ Links ]
Morláns, M. A. 2004. Introducción a la ecología de poblaciones. cátedra de ecología. FCA-Universidad Nacional de Catamarca. Argentina. 16 p. [ Links ]
Nora, S.; Albaladejo, R. G.; González, M. S. C.; Robledo, A. J. J. y Aparicio, A. 2011. Movimiento de genes polen y semillas en poblaciones fragmentadas de plantas. Ecosistemas. 20(2-3):35-45. http://www.revistaecosistemas.net/articulo.asp?Id=690. [ Links ]
Núñez-Colín, C. A.; Rodríguez-Pérez, J. E.; Nieto-Ángel, R. y Barrientos-Priego, A. F. 2004. Construcción de dendrogramas de taxonomía numérica mediante el coeficiente de distancia X 2: una revisión. Rev. Chapingo Ser. Hortic. 10(2):229-237. [ Links ]
Ordiales-Plaza, R. 2000. MideBMP, versión 4.2. Estación experimental de zonas áridas. Almería, España. 8 p. [ Links ]
Raisman, J. S. y González, A. M. 2013. Hipertextos del área de biología. http://www.biologia.edu.mx. [ Links ]
Rzedowski, J. 2006. Vegetación de México. 1ra . Ed. digital. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. México, DF. 504 p. [ Links ]
Salinas-Rodríguez, M. M. 2018. La sierra madre oriental como reservorio de diversidad vegetal. Ciencia UANL . 21(88):46-51. [ Links ]
Sánchez-Salas, J.; Flores, J. y Martínez-García, E. 2006. Efecto del tamaño de semilla en la germinación de Astrophytum myriostigma Lemaire (Cacataceae), especie amenazada de extinción. Interciencia . 31(5):371-375. [ Links ]
Santos-Díaz, M. S.; Pérez-Molphe, E.; Ramírez-Malagón, R.; Núñez-Palenius, H. G. and Ochoa-Alejo, N. 2010. Mexican threatened cacti: current status and strategies for their conservation. Chapter 1. In: Tepper GH, Ed. Species diversity and extintion. Hauppauge, Nueva York, USA. Nova science publishers, Inc. 1-60 pp. [ Links ]
Schwinning, S. and Kelly, C. K. 2013. Plant competition, temporal niches and implications for productivity and adaptability to climate change in water-limited environments. Functkional ecology. 27:886-897. https://doi.org/10.1111/1365-2435.12115. [ Links ]
SEMARNAT. 2010. Secretaría de Medio Ambiente y Recursos Naturales. Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental especies nativas de México de flora y fauna silvestres-categorías de riesgo y especificaciones para su inclusión, exclusión o cambio lista de especies en riesgo. Diario oficial de la federación. 30 de diciembre de 2010, Segunda Sección. México, DF. [ Links ]
Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembsky, S.; Lindner, A.; Konaté, S. and Linsenmair, E. K. 2017. Bee pollinization increases yield quantity and quality of cash crops in burkina faso, West Africa science report on line. 7(1):17691. https://doi.org/10.1038/s41 598-017-17790-2. [ Links ]
Tardieu, F. 2013. Plant response to environmental conditions: assessing potential production, water demand and negative effects of water deficit. Frontiers in physiology. 4(17):11. https://doi.org/10.3389/fphys.2013.00017. [ Links ]
Tilman, D. 1990. Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos. 58(1):3-15. https://doi.org/10.2307/3565355 [ Links ]
Torroba, P.; Hernández, Á. y Zaldívar, P. 2013. Caracterización morfométrica de las semillas de frutos carnosos de la flora del norte de la península ibérica. Ecología. 25(1):175-196. [ Links ]
Valeria, G.; Bedmar, F.; Diez, U. P. y Hernán, P. 2017. Dinámica de emergencia y competencia intraespecífica en Conyza sumatrensis. Agrociencia Uruguay. 21(1):69-77. http://www.scielo.edu.uy/pdf/agro/v21n1/2301-1548-agro-21-01-00069.pdf. [ Links ]
Valiente-Banuet, A. 2002. Vulnerabilidad de los sistemas de polinización de las cactaceas columnares de México. Rev. Chil. Histor. Natur. 75(1):99-104. [ Links ]
Received: October 01, 2022; Accepted: January 01, 2023











 text in
text in 


