Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 n.7 Texcoco Sep./Nov. 2021 Epub Mar 22, 2022
https://doi.org/10.29312/remexca.v12i7.2910
Articles
Co-inoculation with rhizobia and arbuscular mycorrhizal fungi in seedlings of Prosopis laevigata
1Universidad Autónoma de San Luis Potosí-Facultad de Agronomía y Veterinaria. Carretera San Luis-Matehuala km 14.5, Ejido Palma de la Cruz, Soledad de Graciano Sánchez, San Luis Potosí, México. CP. 78321. (gcf7631@gmail.com; heriberto.mendez@uaslp.mx).
2Consejo Nacional de Ciencia y Tecnología-Universidad Autónoma de San Luis Potosí-Coordinación para la Innovación y Aplicación de la Ciencia y la Tecnología. Av. Sierra Leona núm. 550, Col. Lomas 2a, San Luis Potosí, México. CP. 78210. (vallejo.pmr@gmail.com).
Inoculation with rhizobia and arbuscular mycorrhizal fungi (HMA), single or combined, may contribute to the growth and establishment of Prosopis laevigata because of a positive interaction of symbionts with the plant. The objective was to evaluate the effect of single and combined inoculation of three local isolates of rhizobia (R1, R2 and R3) and two strains of HMA (HMA1 and HMA2) on mycorrhizal colonization, nodulation, growth and biomass production of P. laevigata in substrate with pH close to neutrality. The experiment was carried out in a greenhouse, at the Faculty of Agronomy and Veterinary of the UASLP in 2020-2021. Thirteen treatments were evaluated in a completely randomized design with five repetitions. The variables studied were number of spores g-1 of substrate, mycorrhizal colonization and visual density, total nodules, effective nodules, number of leaflets, height, stem diameter and biomass yield. Inoculation promoted greater colonization, nodulation and plant growth with respect to the non-inoculated control. With HMA1, HMA2, R1, R3, R1+HMA1, R3+HMA1, R1+HMA2 and R3+HMA2, results superior to those of the rest of the treatments were obtained. It is concluded that inoculation with local isolates of rhizobia and their combination with HMA favors the development of mycorrhizal structures, nodulation, growth and biomass production of P. laevigata grown in substrate with neutral pH. Isolates R1, R3, strains HMA1, HMA2 and combinations R1+HMA1, R1+HMA2, R3+HMA1 and R3+HMA2 were registered as effective inoculants to increase the growth of P. laevigata.
Keywords: Rhizobium; forage legumes; HMA
La inoculación con rizobios y hongos micorrízicos arbusculares (HMA), de manera simple o combinada, puede contribuir con el crecimiento y establecimiento de Prosopis laevigata por una interacción positiva de los simbiontes con la planta. El objetivo fue evaluar el efecto de la inoculación simple y combinada de tres aislados locales de rizobios (R1, R2 y R3) y dos cepas de HMA (HMA1 y HMA2) en la colonización micorrízica, la nodulación, el crecimiento y producción de biomasa de P. laevigata en sustrato con pH cercano a la neutralidad. El experimento se desarrolló en invernadero, en la Facultad de Agronomía y Veterinaria de la UASLP en 2020-2021. Se evaluaron 13 tratamientos en un diseño completamente aleatorizado con cinco repeticiones. Se estudiaron las variables número de esporas g-1 de sustrato, colonización y densidad visual micorrízica, nódulos totales, nódulos efectivos, número de foliolos, altura, diámetro de tallo y rendimiento de biomasa. La inoculación promovió mayor colonización, nodulación y crecimiento vegetal con respecto al testigo sin inocular. Con HMA1, HMA2, R1, R3, R1+HMA1, R3+HMA1, R1+HMA2 y R3+HMA2, se obtuvieron resultados superiores que el resto de los tratamientos. Se concluye que la inoculación con aislados locales de rizobios y su combinación con HMA favorece el desarrollo de estructuras micorrízicas, la nodulación, el crecimiento y la producción de biomasa de P. laevigata crecido en sustrato con pH neutro. Se registró a los aislados R1, R3, las cepas HMA1, HMA2 y las combinaciones R1+HMA1, R1+HMA2, R3+HMA1 y R3+HMA2 como inoculantes efectivos para aumentar el crecimiento de P. laevigata.
Palabras clave: Rhizobium; HMA; leguminosas forrajeras
Introduction
Intensive and extensive livestock farming has been one of the factors that has had a negative impact on soil degradation and on the environment. This is due, among other causes, to the amount of waste that originates in intensive livestock farming (Pinos-Rodríguez et al., 2012) and to the deforestation caused by the felling of forests to establish pastures in large areas of land (Budowski, 1984), as well as the mismanagement of stocking rate or overgrazing in the case of extensive livestock farming (SEMARNAT, 2015). Some measures that support the recovery of degraded soils are to increase and maintain the diversity of plant species and to include herbaceous and shrubby legumes in the system (Crespo, 2009).
Prosopis laevigata (Humb. & Bonpl. ex Willd.) M.C. Johnst, better known in Mexico as mesquite, is a legume that develops in the arid and semi-arid regions of central and northern Mexico. Mesquite has multiple uses, since its wood is used as fuel, for fence construction; its pods are used as fodder and as food even for man; it produces resin that is used in the manufacture of glues, varnishes; while its flowers are important in the production of honey (Rodríguez et al., 2014). From the environmental point of view, it is known that it prevents desertification and erosion processes due to its high soil-retention capacity, improves fertility, among other attributes (Palacios et al., 2016).
Legumes, in addition to presenting great importance as fodder, due to their nutritional qualities, their high protein content and digestibility with respect to other groups of plants, can improve the structure and fertility of the soil through the biological fixation of nitrogen, this ability is carried out through the symbiosis they establish with bacteria known as rhizobia (Bianco and Cenzano, 2018). This quality facilitates adequate nitrogen nutrition to this group of plants, which, in turn, decreases the need to apply nitrogen fertilizers to ensure their productivity (Cantaro-Segura et al., 2019).
Arbuscular mycorrhizal fungi (HMA) are soil microorganisms that form symbiosis with 80% of land plants (Vierheilig, 2004). These fungi colonize the roots of these plants, producing specialized structures in the rhizosphere through which they help the host to increase the acquisition of nutrients and water (Posada et al., 2008; Leigh et al., 2009).
It is also proven that a tripartite symbiosis is established between rhizobia-plant-HMA, which, together with other soil microorganisms, contributes to improving the nutritional status and yields of plants, in addition to preserving the fertility of soils (Toro et al., 2008).
Although the benefit experienced by P. laevigata seedlings with HMA inoculation has been proven (Gardezi et al., 2020), the effectiveness of the symbiosis will depend, to some extent, on the infectivity of the strains (Tapia et al., 2010) and the soil conditions where its establishment is intended (Gryndler et al., 2009). In the case of inoculation with rhizobia in P. laevigata, the information is very scarce to be a legume with the potential to establish symbiosis with these bacteria; for this reason, it is necessary to study the effects of rhizobia-HMA inoculation and co-inoculation in this species.
Through some studies, it has been proven that certain species of HMA have affinity for one or another type of soil, rather than between host and symbiont (González et al., 2016). For example, Glomus cubense is usually more effective in medium to high fertility soils. In contrast, Funneliformis mosseae is more effective in low-fertility soils with a tendency to acidity (Rivera and Fernández, 2003).
In the case of rhizobia, the specificity between the species and host plant is known, although some plant species are considered promiscuous because they show high compatibility with a wide variety of species of these bacteria. But also, the soil can be decisive for the proper functioning of the symbiosis because there are certain factors that inhibit it, such as salinity, pH, deficiency or toxicity of certain chemical elements (P, Ca, Mo and Al), presence of combined nitrogen (NO3 -) and excess or deficit of water, etc. (De Souza et al., 2003). The information generated from these studies will help to ensure that the management of the inoculation of these microorganisms is effective depending on the productivity of the plants in different conditions.
Considering the benefits resulting from the aforementioned tripartite symbiosis, using local isolates from different shrubby legumes, single inoculated or co-inoculated with HMA, could contribute to improving nutrition, as well as increasing the growth and biomass production of P. laevigata seedlings. Based on this hypothesis, this work was carried out to evaluate the effect of the single and combined inoculation of two strains of HMA and three local isolates of nitrogen-fixing bacteria (rhizobia) from the rhizosphere of Leucaena leucocephala and Vachellia schaffneri to determine the behavior of mycorrhizal structures, nodulation and growth of P. laevigata.
Materials and methods
Experiment conditions
The experiment was carried out in a greenhouse at the Faculty of Agronomy and Veterinary of the UASLP. As a substrate for plant growth, a mixture of soil and river sand was prepared in a 1:1 ratio. The soil used comes from an experimental area of the faculty; the sand was extracted from a riverbed. One kilogram of substrate was added to each nursery bag whose dimensions were 20 x 12.5 cm in height and width respectively. The prepared substrate presented 35, 66.3, 3 300 and 540 mg kg-1 of P, K, Ca and Mg respectively and 34.5 mg kg-1 (0.7% of the bases) of Na, 1.92% of organic matter (OM) as well as a pH= 6.9.
The methods used to determine these values were as follows: pH (H2O) by potentiometer (1:2.5, soil:water ratio) (NC ISO 10390, 1999), organic matter by the Walkley and Black method (NC 51, 1999), assimilable phosphorus (P) by the extraction method with H2SO4 0.05 mol L-1, Ca and Mg by complexometry and Na and K by flame photometry (NC 52, 1999).
According to the values of the chemical analysis, the substrate had low concentrations of K and OM, high values of assimilable P, Ca and Mg, an acceptable percentage of Na and pH close to neutrality (Paneque and Calaña, 2011). The substrate had an average HMA spore number of 140 spores 50 g-1.
Experimental design
The experiment was designed with 13 treatments, which consisted of the inoculation of two strains of HMA (HMA1 and HMA2) and three isolates of rhizobia (R1, R2 and R3) inoculated individually and in combinations, plus a non-inoculated control and a fertilization treatment as a reference. The treatments were made up as follows: non-inoculated control; single inoculation treatments (HMA1, HMA2, R1, R2, R3); combined inoculation or co-inoculation treatments (HMA1+R1, HMA1+R2, HMA1+R3, HMA2+R1, HMA2+R2, HMA2+R3), control without inoculation with fertilization (Fert). The treatments were distributed in a completely randomized design with five repetitions. The experimental unit was made up of each bag with a plant of P. laevigata.
Inoculants used
The inoculum HMA1 [IMCAM 4 Glomus cubense (Rodríguez & Dalpé)] is from the collection of arbuscular mycorrhizal fungi of the National Institute of Agricultural Sciences (INCA, for its acronym in Spanish) in San José de las Lajas, Mayabeque, Cuba. The inoculum HMA2 [Claroideoglomus claroideum (Schenck & Sm.) Walker & Schüßler] was isolated from a plot in the peanut-producing area of Ciudad Fernández, SLP, Mexico (22° 00’ 47.36” north latitude 100° 21’ 14.66” west longitude), from a soil whose textural characteristic are sandy and low clay content. Both were prepared from spores extracted from inoculums previously multiplied in a clay substrate and autoclaved at 120 °C for one hour for three consecutive days; Sorghum vulgare L. was used as a trap culture.
The culture of rhizobia cells was prepared in liquid Yeast-Mannitol-Agar (YMA) medium at 28 °C and under agitation conditions for 24-30 h (Vincent, 1970). Rhizobia were isolated from plants of Vachellia schaffneri (R1 and R2) and Leucaena leucocephala (R3). The root samples of these plants were collected in their natural habitats located in sites near the Venustiano Carranza-Pajacuaran road, Michoacan (20º 07’ 09.8” north latitude 102º 36’ 52.5” west longitude) and in the locality Tocoy in the municipality of San Antonio, San Luis Potosí (21º 38’ 19.0” west longitude 98º 52’ 15.0” west longitude), respectively.
Propagation of P. laevigata
The plants of P. laevigata (mesquite) were propagated from seed. The seeds were subjected to scarification to facilitate their germination, which consisted of immersing them in water at 80 °C for two minutes, then they were immersed in a solution of 5% sodium hypochlorite for three minutes, then they were germinated in germination trays of 200 cavities with Peat moss as substrate. When the seedlings reached between 5-6 cm in height, they were transplanted into the bags containing the soil-sand mixture and inoculated. In the fertilization treatment, starting 21 days after transplantation (dat), 0.125 g of water-soluble fertilizer 17-17-17 (vigoroso excelso) was applied to each bag every 15 days, until 210 dat. The mesquite was kept growing in the bags for 12 months, throughout the period, the application of 200 ml of water to each experimental unit on alternate days or according to the needs of the plants was maintained.
Application of inoculants
The inoculation of HMA strains (HMA1 and HMA2) was carried out by applying 0.5 ml of ringer solution (the ringer solution contained NaCl 7.5 g, KCl 0.75 g, CaCl2 0.1 g and NaHCO3 0.1 g in 1 L of H2O) containing 60 spores in each case, directly around the roots of the seedlings. In the case of rhizobia (R1, R2 and R3), 1 ml of the inoculum was applied to the substrate in a region very close to the root crown. Each 1 ml of inoculum contained 108 colony-forming units (CFUs).
Evaluation of growth variables
Plant growth was evaluated based on plant height, stem diameter and number of leaflets per plant. The plant height was measured from the substrate surface to the terminal bud (cm plant) using a graduated ruler of 40 cm in length and 1 mm in precision. The diameter of the stem (mm plant) was measured with a digital king foot at 1 cm high from the surface of the substrate. At the end of the experiment (365 dat), the root was separated from the aerial part of each plant. The samples were then placed in the oven at 65°C for approximately 72 h until constant weight values were reached. The weight of MS was recorded on a Precisa LS320M SCS balance.
Evaluation of nodulation
The number of total nodules and their effectiveness were assessed as indicated in FAO (1985). For this, the roots were extracted from the bag separating them from the substrate and carefully washed with running water. Once the roots were cleaned, the total nodules were quantified and their effectiveness was determined by observing the internal coloration by means of a cross-section of the nodule, those that exhibited red to pink coloration were considered as effective nodules for evidencing the presence of leghemoglobin, while those of white color were considered as young nodule (non-effective nodule).
Evaluation of fungal variables of mycorrhiza
Mycorrhizal colonization was determined in roots previously washed with running water and then air-dried. For the determinations, approximately 200 mg of rootlets were weighed and dried at 70 °C and stained according to the methodology described by Phillips and Hayman in 1970. The frequency of mycorrhizal colonization, which expresses the degree of occupation of the rootlets by HMAs, was evaluated by the intercept method (Giovannetti and Mosse, 1980) and the visual density or intensity of colonization according to Trouvelot et al. (1986). The quantification of the number of spores (spores 50 g) was carried out from samples of 50 g of substrate from the pots, extracting these structures by wet sieving and decanting and their observation under a microscope (Herrera et al., 1995).
Statistical analysis
Data were assessed using simple classification analysis of variance. Duncan’s multiple comparison test was used in cases where there was a significant effect of the treatments, to establish the differences between the means.
To meet the assumptions of normality, the data corresponding to the variable number of nodules were transformed by the square root function and natural logarithm for the visual density. In the case of (%) of colonization and (%) of effective nodules, which did not meet the assumptions of normality even after a transformation of the data, the Kruskal-Wallis test was performed to determine the existence of differences between the means, then the Games-Howell test was performed to establish the differences between the means. The IBM SPSS Statistics statistical program for Windows version 23 (Armonk, New York, United States) was used.
Results and discussion
Fungal and nodulation variables
The treatments studied generated significant effects (p< 0.001) on the variables that characterize the mycorrhizal structures associated with P. laevigata. Treatments inoculated with both HMA and co-inoculated had a greater number of spores than the non-inoculated control and the fertilization treatment, except when combined with the isolate R2 (Figure 1A). Mycorrhizal colonization was favored when the strains of HMA were combined with the isolates of rhizobia R1 and R3 (R1+HMA1, R3+HMA1, R1+HMA2 and R3+HMA2), standing out significantly (p< 0.001) from the rest of the treatments.
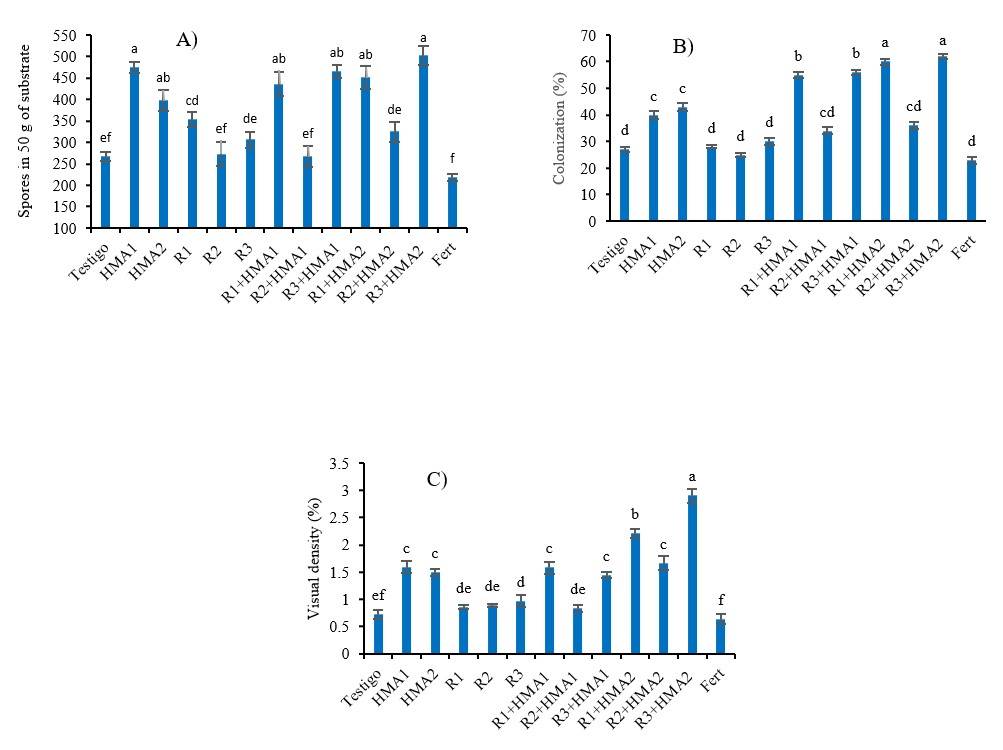
Figure 1 Effect of various inoculation and co-inoculation treatments on spore number (A), mycorrhizal colonization; and (B) and visual density (C) of P. laevigata roots. abcdef= values with different letters in each variable differ significantly according to Duncan’s test (p< 0.05) for the variables of spore number and (%) of visual density, and according to the Games-Howell test (p< 0.05) for the variable (%) of colonization. The bars show the standard error ± of the means. R1, R2 and R3 (Rhizobia isolates). HMA1 (Glomus cubense) and HMA2 (Claroideoglomus claroideum). Fert (0.125 g of water-soluble fertilizer 17-17-17).
Also, both rhizobia, R1 and R3, combined with HMA2 reached higher percentages of colonization than when combined with HMA1 (Figure 1B). In the case of visual density (%), R3+HMA2 followed by R1+HMA2 were the treatments with the highest values in this variable and the other treatments inoculated with HMAs, except R2+HMA1, exceeded those that were not inoculated with HMAs (Figure 1C). The non-inoculated control and the fertilizer treatment had the lowest values of spore number, colonization and visual density (Figure 1).
The nodulation of P. laevigata was higher (p< 0.001) with the inoculation of the isolates R1 and R3 and their combinations with HMAs (R1+HMA1, R3+HMA1, R1+HMA2 and R3+HMA2), with respect to the rest of the treatments and the control. In turn, the nodulation of R3 was greater when combined with both strains of HMA (R3+HMA1 and R3+HMA2) than with its single inoculation (Figure 2A). The percentage of effective nodules behaved similarly to the number of nodules, with the difference that R1, R3 and their combinations with HMA1 and HMA2 did not have differences between them but with the rest of the treatments (Figure 2B).
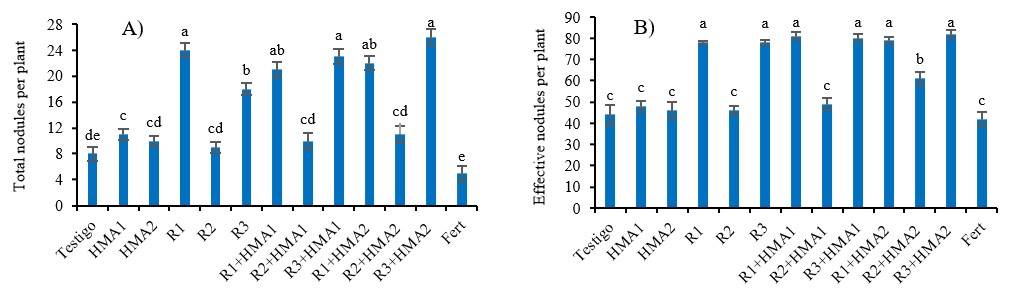
Figure 2 Effect of various inoculation and co-inoculation treatments on the number of total nodules (A) and the number of effective nodules (B) of P. laevigata . abcde= values different letters in each variable differ significantly according to Duncan’s test (p< 0.05) for the variable of number of total nodules per plant, and according to the Games-Howell test (p< 0.05) for the variable of effective nodules per plant (%). The bars show the standard error ± of the means. R1, R2 and R3 (rhizobia isolates). HMA1 (Glomus cubense) and HMA2 (Claroideoglomus claroideum). Fert (0.125 g of water-soluble fertilizer 17-17-17).
In this research, it was found that the inoculation of HMAs, alone or in combination with rhizobia isolates, favors the generation of mycorrhizal structures and greater colonization in P. laevigata (Figure 1). Similarly, the inoculation and co-inoculation of rhizobia, simply and combined with HMAs, produced the highest number of total and effective nodules in the species studied (Figure 2). Similar results, but only with HMA inoculation, were found by Monroy-Ata et al. (2007), when inoculating seedlings of P. laevigata with an inoculum reproduced from HMA of the rhizosphere of Bouteloa gracilis, the percentage of colonization reached values greater than 50%.
In Prosopis juliflora, results have been found that suggest that inoculation combined with isolates of rhizobia and HMA favors both the colonization and formation of mycorrhizal structures and the nodulation of rhizobia with respect to the inoculation of rhizobia individually in soils with sodium chloride (NaCl) amendments (Dixon et al., 1993). Our research is pioneering in demonstrating that the inoculation of HMA and rhizobia in P. laevigata favors the formation of mycorrhizal structures and nodulation.
The control treatment also presented spores at a similar level as other inoculated treatments (Figure 1A), which is due to the presence of HMAs resident in the substrate. Other inoculated treatments had up to twice as many spores as the control. The colonization of roots with HMAs resident in the soil is a process whose magnitude is influenced by the effectiveness of the strain according to soil and climatic conditions and the affinity between symbiont and host (Rivera and Fernández, 2003; João et al., 2016).
It is possible to consider that the spores registered in the inoculated treatments corresponded in a greater proportion to the inoculum than to the residents of the soil. The above, due to the homogeneity observed in the morphological characteristics of the spores when quantifying them. However, in this study the number of spores of each type of present in the sample was not recorded. This suggests that the inoculated HMA strains were more effective in promoting the development of mycorrhizal structures than those originally present in the substrate (Figure 1). However, this assertion requires greater methodological rigor for its confirmation.
It has been shown that, by inoculating the roots of plants with effective strains, both of HMA and rhizobia, the production of fungal structures and nodulation, respectively, increases. However, there are factors that influence the effectiveness of the strains. In the case of HMAs, different species behave differently according to certain ranges of soil fertility and pH, which indicates a certain between species or strain-type of soil (Rivera and Fernández, 2003; João et al., 2016).
On the other hand, although rhizobia are common inhabitants in agricultural soils, their population is usually insufficient to achieve a beneficial relationship with the legume, which makes it necessary to apply inoculants to the seed or young roots to guarantee symbiosis and with it the biological fixation of nitrogen (Das and Varma, 2009).
Mesquite growth variables
Inoculation favored the growth of P. laevigata, however, the number of leaflets was higher (p< 0.001) than the control only when the isolate R3 was co-inoculated with both mycorrhizal strains (R3+HMA1 and R3+HMA2) (Figure 3A). On the other hand, both the strains of HMA and the rhizobia R1 and R3 single-inoculated, as in their combinations, produced plants with greater height, like the fertilized treatment, with significant difference over the treatments with R2 alone and co-inoculated with HMAs (R2+HMA1 and R2+HMA2), which behaved similarly to the control without inoculation (Figure 3B).
Stem diameter was also positively influenced by inoculation, with all treatments being superior to the control, except the isolate R2 inoculated individually. Treatments R1, R3, R1+HMA1, R3+HMA1, R1+HMA2 and R3+HMA2 stood out for generating plants with a larger (p< 0.001) stem diameter than the rest of the treatments (p< 0.001) (Figure 3C).
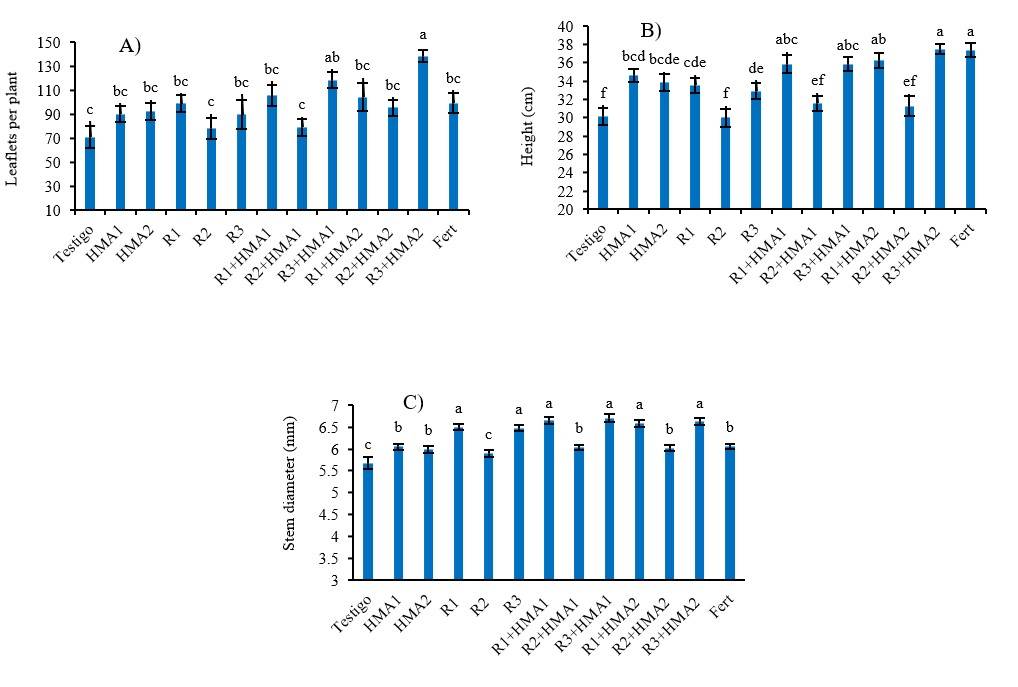
Figure 3 Effect of various inoculation and co-inoculation treatments on the number of leaflets (A) height (B); and stem diameter (C) of P. laevigata. abcdef= values with different letters in each variable differ significantly according to Duncan’s test (p< 0.05). The bars show the standard error ± of the means. R1, R2 and R3 (rhizobia isolates). HMA1(Glomus cubense) and HMA2 (Claroideoglomus claroideum). Fert (0.125 g of water-soluble fertilizer 17-17-17).
Biomass production was also influenced by inoculation, but in the case of the aerial part, it was the isolate R1 and the combination R3+HMA2 those that reached higher MS values. Also, all inoculated treatments and the fertilized treatment produced more biomass than the non-inoculated control (p< 0.001), except when the isolate R2 was present, both single inoculated and combined with HMAs (Figure 4A). The strain HMA1, isolates R1 and R3 and the combinations R1+HMA1, R3+HMA1, R1+HMA2 and R3+HMA2 produced greater root biomass (p< 0.001) than the treatments with the presence of the isolate R2, and the non-inoculated control and the fertilized treatment (Figure 4B).
The increase in growth provided by inoculation that was recorded in this research partially coincides with the results found by Gardezi et al. (2020), who found that, in Xerosol and Litosol-type soils, with alkaline and neutral pH respectively, inoculation with Glomus sp., together with the application of organic matter, favored the growth of shoots and roots in seedlings of P. laevigata under greenhouse conditions. In our study, inoculated HMAs produced greater colonization and density of spores, greater height and biomass production than the control, even when inoculated individually (Figures 1, 3 and 4), which demonstrates the effectiveness of these strains in soils with characteristics similar to those of the substrate used.
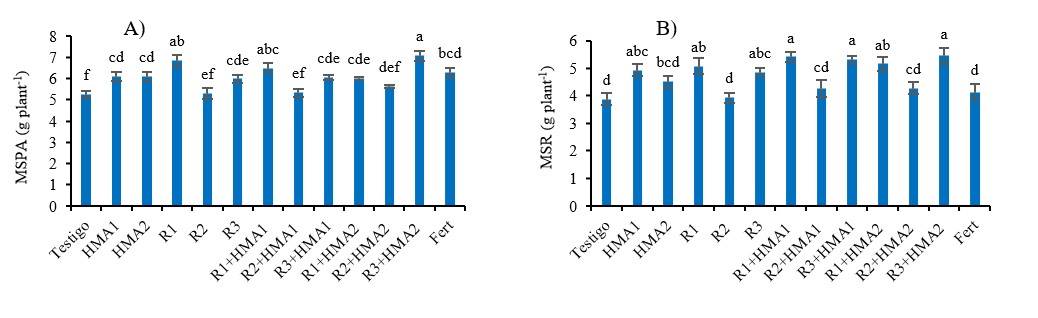
Figure 4 Effect of various inoculation and co-inoculation treatments on the production of dry mass of the aerial part and root of P. laevigata at 180 dat. Values with different letters in each variable differ significantly according to Duncan’s test (p< 0.05). The bars show the standard error ± of the means. MS PA (dry mass of the aerial part). MS R (dry mass of the root). R1, R2 and R3 (rhizobia isolates). HMA1 (Glomus cubense) and HMA2 (Claroideoglomus claroideum). Fert (0.125 g of water-soluble fertilizer 17-17-17).
In another tree legume species such as Leucaena leucocephala, Quintana et al. (2014), when using the inoculation of mycorrhizal fungi and rhizobia in an isolated and combined way, obtained higher values in the growth and biomass variables of the crop in relation to the non-inoculated control, and the combined inoculations were more effective than the single inoculations.
In this research, it can be observed that, mainly for the variables of plant height and MS, the treatments inoculated with both microorganisms in a single and combined way were superior to the control, except when it was inoculated with the isolate R2, in a single way and in its combinations with HMAs (R2+HMA1 and R2+HMA2) (Figures 3 and 4).
In this sense, it is known that most species of rhizobia experience a strain-species of legume specificity (Balatti, 1996). It should be noted that the interaction between the host and symbionts inoculated with the microorganisms present in the substrate, which was not sterilized, must be taken into account. Despite this, the effect on plants is observed with inoculation and co-inoculation treatments, which shows that the strains used are effective, except R2.
The higher height and biomass production experienced by mesquite seedlings inoculated with HMA1, HMA2, R1, R3, R1+HMA1, R1+HMA2, R3+HMA1 and R3+HMA2 coincides with the colonization values, spore number and nodulation of rhizobia. In turn, the highest values in these variables were reached when both symbionts were combined (Figures 1, 2, 3 and 4). This relationship between increased fungal structures, nodulation and growth of P. laevigata plants demonstrates the benefit of tripartite symbiotic interaction (rhizobia-legume-HMA) mentioned above by several authors such as Rabie et al. (2005) and Lara et al. (2019). Tripartite symbiosis favors the development of each organism individually and together, which represents opportunities for better growth of the associated plants, and, consequently, greater productivity (Toro et al., 2008).
The synergy between HMAs and rhizobia in favor of plant productivity has also been corroborated by authors such as González et al. (2012), who, when evaluating the effect of co-osculation of rhizobia isolates and a strain of HMA (G. cubense), in Kudzu plants (Pueraria phaseoloides), obtained increases in yields and nutritional content of grass in the treatments where both microorganisms were combined.
The control treatment established in this investigation resulted in the presence of rhizobia nodules in their roots. Evidently, such nodules were produced by the native rhizobia or residents of the soil present in the substrate. Like any legume, nodulation of P. laevigata occurs naturally in several types of soils.
Although there is no information related to nodulation by native rhizobia in P. laevigata, the isolation of these microorganisms in other species of the genus such as Prosopis juliflora and Prosopis alba has been reported by Kulkarni and Nautiyal (1999) and Velázquez et al. (2001) respectively.
However, this research is the first to document the beneficial effect of dual symbiotic association on P. laevigata in pH-neutral soils. This evidences the effectiveness of the isolates and strains tested in this study.
It is interesting that these isolates presented compatibility with P. laevigata, even in the case of R1 and R3, they proved to be almost as efficient in some of the variables measured as their combinations with HMAs (R1+HMA1, R1+HMA2, R3+HMA1 and R3+HMA2). Our results provide new rhizobia isolates compatible with P. laevigata and that are good growth promoters, which increases the possibility of generating inoculants that can promote good results in other species, even in crops.
Conclusions
The combination of rhizobia isolates and HMA strains in soils with a pH close to neutrality favors the development of mycorrhizal structures, nodulation, growth and biomass production of Prosopis laevigata. The isolates R1 and R3 (from the rhizosphere of Vachellia schaffneri and Leucaena leucocephala respectively), the strains HMA1 and HMA2 (Glomus cubense and Claroideuglomus claroideum) and the combinations R1+HMA1, R3+HMA1, R1+HMA2 and R3+HMA2 are effective inoculants to increase the growth of P. laevigata.
Acknowledgements
To the support received through the project C18-FAI-05-54.54 and to the CONACYT grant (638999) for doctoral studies of the first author. To the Soil and Plant Tissue Analysis Laboratory of the National Institute of Agricultural Sciences (INCA, for its acronym in Spanish) of Cuba, for its support with the analysis of soil samples.
REFERENCES
Balatti, P. A. 1996. Interacciones tempranas Rhizobio-leguminosa. Rev. de la Facultad de Agronomía. 101(1):91-108. http://sedici.unlp.edu.ar/handle/10915/69406. [ Links ]
Bianco, L. y Cenzano, A. M. 2018. Leguminosas nativas: estrategias adaptativas y capacidad para la fijación biológica de nitrógeno. Implicancia ecológica. IDESIA. Chile. Arica. 36(4):71-80. http://dx.doi.org/10.4067/S0718-34292018005002601. [ Links ]
Budowski, G. 1984. Los sistemas agroforestales en América Central. In: agroforestería. Actas del Seminario. (Ed). J. Heuveldop y J. Lagemann. CATIE. Turrialba, Costa Rica. 112 p. [ Links ]
Cantaro-Segura, H.; Huaringa-Joaquín, A. y Zúñiga-Dávil, D. 2019. Efectividad simbiótica de dos cepas de Rhizobium sp. en cuatro variedades de frijol (Phaseolus vulgaris L.) en Perú. Idesia (Arica). 37(4):73-81. Doi: https://dx.doi.org/10.4067/S0718-34292019000400073. [ Links ]
Crespo, G. 2009. Recuperación de la fertilidad del suelo en áreas ganaderas degradadas Rev. Cubana de Ciencia Agrícola. Instituto de Ciencia Animal. 43(4):355-360. https://www.redalyc.org/pdf/1930/193014888005.pdf. [ Links ]
Das, A. and Varma, A. 2009. Symbiosis: ‘the art of living’. In: (Ed.). Varma, A. and Kharkwal, A. C. Symbiotic Fungi,). (Ed.). Springer Berlin Heidelberg. Soil Biol. 18. 1-28 pp. ISBN 978-3-540-95893-2. doi: 10.1007/978-3-540-95894-9-1. [ Links ]
FAO. 1985. Les inoculums de légumineuses et leurs applications. Organisation des Nations Unies pour l’Alimentation et l´Agriculture. Roma. 63 p. [ Links ]
De Souza, D.; Colozzi-Filho, A.; Balota, E. L. and Hungria, M. 2003. Long-term effects of agricultural practices on microbial community. In: García-Torres, L; Benites, J.; Martínez-Vilela, A. and Holgado-Cabrera, A. (Ed.). Conservation Agriculture. Springer, Dordrecht. 301-306 pp. https://doi.org/10.1007/978-94-017-1143-2-36. [ Links ]
Gardezi, A. K.; Márquez-Berber, S. R.; Velarde, E. V.; Escobar, M. O.; Escalona-Maurice, M. J.; Haro-Aguilar, G. y Larqué-Saavedra, M. U. 2020. Inoculación de (Prosopis laevigata) por hongos micorrízicos arbusculares en diferentes dosis de materia orgánica en dos tipos de suelo. Inter. J. Environ. Agric. Res. 5(11):01-08. doi: https://dx.doi.org/10.5281/ zenodo.4297161. [ Links ]
Giovannetti, M. and Mosse, B. 1980. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist. 84(3):489-500. https://www.jstor.org/stable/2432123. [ Links ]
González, P. J.; Ramírez, J. F.; Rivera, R.; Hernández, A. y Crespo, G. 2016. Efectividad de la inoculación de hongos micorrízicos arbusculares en dos leguminosas forrajeras cultivadas en dos tipos de suelos. Tropical Grassland. 4(2):82-90. doi: 10.17138/TGFT(4)82-90. [ Links ]
Gryndler, M.; Hršelová, H.; Cajthaml, T.; Havránková, M.; Rezácová, V.; Gryndlerová, H. and Larsen, J. 2009. Influence of soil organic matter decomposition on arbuscular mycorrhizal fungi in terms of symbiotic hyphal growth and root colonization. Mycorrhiza. 19(4):255-266. https://doi.org/10.1007/s00572-008-0217-y. [ Links ]
Herrera, R. A.; Ferrer, R. L.; Furrazola, E. y Orozco, M. O. 1995. Estrategia de funcionamiento de las micorrizas VA en un bosque tropical. Biodiversidad en Iberoamérica. Ecosistemas, Evolución y Procesos sociales. (Ed.). Monasterio, M. Programa Iberoamericano de Ciencia y Tecnología para el desarrollo. Subprograma XII. Diversidad Biológica. Mérida. [ Links ]
João, J. P.; Espinosa, A.; Ruiz, L.; Simó, J. y Rivera, R. 2016. Efectividad de cepas de HMA en el cultivo de la yuca (Manihot esculenta Crantz) en dos tipos de suelos. Cultivos Tropicales. 37(1):48-56. http://scielo.sld.cu/scielo.php?script=sci-arttext&pid=S0258-59362016000100007 &lng =es&tlng=es . [ Links ]
Kulkarni, S. and Nautiyal, C. S. 1999. Characterization of high-temperature tolerant rhizobia isolated from Prosopis juliflora grown in alkaline soil. J. Gen. Appl. Microbiol. 45(5):213-220. https://doi.org/10.2323/jgam.45.213. [ Links ]
Lara, L.; Hernández, L. G.; Reyes, J. J.; Rangel, P. P. y Zulueta, R. 2019. Respuesta agronómica de Phaseolus vulgaris a la biofertilización en campo. Rev. Mex. Cienc. Agríc. 10(5):1035-1046. https://doi.org/10.29312/remexca.v10i5.936. [ Links ]
Leigh, J.; Hodge, A. and Fitter, A. H. 2009. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 181(1):199-207. https://doi.org/10.1111/j.1469-8137.2008.02630.x. [ Links ]
Monroy-Ata, A.; Estévez-Torres, J.; García-Sánchez, R. y Ríos-Gómez, R. 2007. Establecimiento de plantas mediante el uso de micorrizas y de islas de recursos en un matorral xerófilo deteriorado. Boletín de la Sociedad Botánica de México 80. 49-57 pp. doi: 10.17129/botsci.1756. [ Links ]
NC 51. 1999. Determinación del por ciento de materia orgánica. Manual de procedimientos. Comité Técnico de Normalización Núm. 3. Calidad del suelo. Análisis químico. La Habana. Oficina Nacional de Normalización. 9 p. [ Links ]
NC 52. 1999. Determinación de las formas móviles de fósforo y potasio. Manual de procedimientos. Comité Técnico de Normalización Núm. 3. Calidad del suelo. Análisis químico. La Habana. Oficina Nacional de Normalización. 12 p. [ Links ]
NC ISO 10390. 1999. Determinación de pH. Manual de procedimientos. Comité Técnico de Normalización Núm. 3. Calidad del suelo. Análisis químico. La Habana. Oficina Nacional de Normalización. 10 p. [ Links ]
Paneque, V. M. y Calaña, J. M. 2001. La fertilización de los cultivos. Aspectos teórico-prácticos para su recomendación. Departamento de biofertilizantes y nutrición de las plantas. La Habana. INCA. 29 p. [ Links ]
Palacios, R. A.; Rodríguez, L. R. y Hernández, F. M. 2016. Distribución potencial de Prosopis laevigata (Humb. & Bonpl. ex Willd) MC Johnston basada en un modelo de nicho ecológico. Rev. Mex. Cienc. Bosque. 34(7):35-46. doi:10.29298/rmcf.v7i34.81. [ Links ]
Phillips, J. M. and Hayman, D. S. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycororrhizal fungi for rapid assessment to infection. Trans. Brit. Mycol. Soc. 55(1):158-161. https://www.cabdirect.org/cabdirect/abstract/ 19711101080. [ Links ]
Pinos-Rodríguez, J. M.; García-López, J. C.; Peña-Avelino, J. A.; Rendo-Huerta, J. A.; González-González, C. y Tristán, P. F. 2012. Impactos y regulaciones ambientales del estiércol generado por los sistemas ganaderos de algunos países de América. Agrociencia. 46(4):359-370. http://www.scielo.org.mx/scielo.php?script=sci-arttext&pid=S1405-31952012000400004 . [ Links ]
Posada, R. H.; Franco, L. A.; Ramos, C.; Plazas, L. S.; Suárez, J. C. and Álvarez, F. 2008. Effect of physical, chemical and environmental characteristics on arbuscular mycorrhizal fungi in Brachiaria decumbens (Stapf) pastures. J. Appl Microbiol. 104(1):132-140. https://doi.org/ 10.1111/j.1365-2672.2007.03533.x. [ Links ]
Quintana, L. J.; Herrera, R.; Furrazola, E. y Hernández, C. 2014. Efecto de inoculaciones conjuntas de Rhizobium-Micorrizas Arbusculares en Leucaena leucocephala CV: Perú. Centro Agrícola. 41(3):17-21. https://www.researchgate.net/publication/280577699-Efecto-de-inoculaciones-conjuntas-de-rhizobium-micorrizas-arbusculares-en-eucaena-leucocephala-CV-Peru. [ Links ]
Rabie, G. H.; Aboul-Nasr, M. B. and Al-Humiany, A. 2005. Increased salinity tolerance of cowpea plants by dualinoculation of an arbuscular mycorrhizal fungus Glomus clarum and a nitrogen-fixer Azospirillum brasilense. Mycobiology. 33(1):51-60. doi:10.4489/MYCO. 2005.33.1.051. [ Links ]
Rivera, R. y Fernández, K. 2003. Bases científico-técnicas para el manejo de los sistemas agrícolas micorrizados eficientemente. In: Rivera, R. y Fernández, K. (Ed.). Manejo efectivo de la simbiosis micorrízica, una vía hacia la agricultura sostenible. Estudio de caso: el Caribe. INCA. La Habana. 166-169. Doi:10.13140/2.1.1813.9203. [ Links ]
Rodríguez, E. N.; Rojo, G. E.; Ramírez, B.; Martínez, R.; Cong Hermida, M de la C.; Medina, S. M. y Piña, H. H. 2014. Análisis técnico del árbol del mezquite (Prosopis laevigata Humb. & Bonpl. Ex Willd.) En México. Ra Ximhai. 10(3):173-193. https://www.redalyc.org /articulo.oa?id=46131111013. [ Links ]
SEMARNAT. 2015. Informe de la Situación Medio Ambiente en México. https://apps1.semarnat.gob.mx:8443/dgeia/informe15/tema/pdf/Informe15-completo.pdf. [ Links ]
Tapia, J.; Ferrera, R. J.; Varela, L.; Rodríguez, J. C.; Soria, J. C. y Tiscareño, M. A. 2010. Infectividad y efectividad de hongos micorrízicos arbusculares nativos de suelos salinos en el cultivo de lechuga (Lactuca sativa). Rev. Mex. Micol. 31:69-74. http://www.scielo.org.mx/scielo.php?script=sci-arttext&pid=S0187-31802010000100010. [ Links ]
Toro, M.; Bazó, I. y López, M. 2008. Micorrizas arbusculares y bacterias promotoras de crecimiento vegetal, Biofertilizantes nativos de sistemas agrícolas bajo manejo conservacionista. Agronomía Tropical. 58(3):215-221. http://ve.scielo.org/scielo.php? script=sci-arttext&pid=S0002-192X2008000300002&lng= es&nrm=iso. [ Links ]
Trouvelot, A.; Kough, J. L. and Gianinazzi-Pearson, V. 1986. Mesure du taux de micorrización VA d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V.and Gianinazzi S. (Ed). Physiological and genetical aspects of mycorrhizae. INRA, Paris. 217-221 pp. [ Links ]
Velázquez, E.; Igual, J. M.; Willems, A.; Fernández, M. P.; Muñoz, E.; Mateos, P. F.; Abril, A.; Toro, N.; Normy, P.; Cervantes, M.; Gillis, M. y Martínez-Molina, E. 2001. Mesorhizobium chacoense sp. nov, a novel species that nodulates Prosopis alba in the Chaco Arido region, Argentina. Int. J. Syst. Evol. Microbiol. 51(3):1011-1021. https://doi.org/10.1099/ 00207713-51-3-1011. [ Links ]
Vierheilig, H. 2004. Regulatory mechanisms during the plant - arbuscular mycorrhizal fungus interaction. Canadian J. Bot. 82(8):1166-1176. https://doi.org/10.1139/b04-015. [ Links ]
Vincent, J. M. A. 1970. Manual for the practical study of rootnodule bacteria. In: International Biological Programme Handbook. Blackwele scientific num. 15. 1-13 pp. https://www.scielo.br/scielo.php?script=sci-nlinks&ref=000149&pid=S0100-0683200900050001500031&lng=en . [ Links ]
Received: March 01, 2021; Accepted: June 01, 2021











 text in
text in 


