Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 n.7 Texcoco Sep./Nov. 2021 Epub Mar 22, 2022
https://doi.org/10.29312/remexca.v12i7.2632
Articles
Morphological characterization of Moringa oleifera accessions from the South-Southeast of Mexico
1Campus Veracruz-Colegio de Postgraduados. Carretera Xalapa-Veracruz km 88.5, Predio Tepetates, Manlio F. Altamirano, Veracruz. CP. 91700. Tel. 229 2010770. (rafael.ruiz@colpos.mx; geliseo@colpos.mx; clandero@colpos.mx).
2Universidad Autónoma Chapingo. Carretera. México-Texcoco km 38.5, Chapingo, Texcoco, Estado de México. CP. 56230. Tel. 595 9521540. (fredy.morales@colpos.mx).
3Colegio de Postgraduados. Carretera México-Texcoco km 36.5, Montecillo, Texcoco, Estado de México. CP. 56230. Tel. 595 9570887. (msoto@colpos.mx).
Moringa oleifera is a plant with great capacity to adapt to different edaphoclimatic conditions. Environmental factors influence the morphology and phenology of this species. The objective was to characterize the morphology of 20 accessions of Moringa oleifera from the South-Southeast of Mexico. The seeds were collected from commercial cultivations in the states of Veracruz, Oaxaca, Guerrero, Chiapas and Yucatan. The seeds were sown in containers and transplanted two months after germination in the field with a completely randomized block design (CRBD). Every seven days the quantitative variables were recorded, and 301 days after transplantation, the morphological descriptors were evaluated. It was observed that accession C2 presented the highest growth (273 cm). Accession Y2 had a diameter of 43.22 mm and accession Y3 had 54 leaves. A high variation was found in leaf size, flower and stem color and the onset of flowering. Principal component analysis identified three groups. Principal component analysis (PCA) showed that the first five components explain 99.21% of the total variation and that components 1 (52.87%) and 2 (37.54%) contribute 90.41%. From the cluster analysis, three groups with 0.76 similarity resulted, based on Euclidean similarity. The morphological differentiation of the various accessions of moringa allowed corroborating varietal differentiation and the need to implement a genetic program of conservation, selection and breeding of moringa in the South-Southeast of Mexico.
Keywords: accessions; agroecosystems; moringa; phenotypes
La Moringa oleifera es una planta con gran capacidad de adaptación a diferentes condiciones edafoclimáticas. Los factores ambientales influyen en la morfología y fenología de esta especie. El objetivo fue caracterizar la morfología de 20 accesiones de Moringa oleifera provenientes del sur-sureste de México. Las semillas fueron recolectadas en cultivos comerciales de los estados de Veracruz, Oaxaca, Guerrero, Chiapas y Yucatán. Las semillas fueron sembradas en contenedores y se trasplantaron dos meses después de su germinación en campo con un diseño de bloques completamente al azar (DBCA). Cada siete días se registraron las variables cuantitativas y a los 301 días después del trasplante se evaluaron los descriptores morfológicos. Se observó que la accesión C2 presentó el mayor crecimiento (273 cm). La accesión Y2 presentó un diámetro de 43.22 mm y la accesión Y3 presentó 54 hojas. Se encontró una alta variación en el tamaño de la hoja, color de la flor y tallo e inicio de floración. El análisis de componentes principales identificó tres grupos. El análisis de componentes principales (PCA) mostró que los primeros cinco componentes explican 99.21% de la variación total y que los componentes 1 (52.87%) y 2 (37.54%) contribuyen con 90.41%. Del análisis de conglomerados resultaron tres grupos con 0.76 de similitud, basado en la similitud euclidiana. La diferenciación morfológica de los diversos accesos de moringa permitió corroborar diferenciación varietal y la necesidad de instrumentar un programa genético de conservación, selección y fitomejoramiento de moringa en el sur-sureste de México.
Palabras clave: accesiones; agroecosistemas; fenotipos; moringa
Introduction
Plants have, in general, the ability to adapt to the various conditions that exist on the planet. This physiological adjustment allows them to survive in diverse and adverse climates. However, external factors influence growth and production through direct influence on their physiological and biochemical processes (Tesfay et al., 2011; Santiago and Bezerra, 2017). Moringa oleifera Lam., is a perennial, fast-growing plant species that presents great agroecological plasticity (Pérez et al., 2010). The global importance of this plant lies in its use to contribute to the improvement of nutrition and human health, to ensure food security, to promote economic development in rural areas and to mitigate the effects of climate change (NRC, 2006).
All parts of moringa have bioactive principles of nutritional and medicinal importance (Martín et al., 2013). In addition, it has a high storage capacity of active compounds that is determined by the variety or by the modification that the accession has undergone in the collected environment (Baiyeri et al., 2015). In India, the varieties of moringa: PKM-1, PKM-2, Jaffna, Chavakacheri Murungai, Chemmurungai, Kaadumurungai palmurungai, Puna murungai and Kodikkal murungai have been identified, which show phenological and morphological differences.
Morphological characterization is a fundamental tool for the selection, conservation, breeding and creation of new varieties (Popoola et al., 2016; Kumar et al., 2017). The study of accessions under homogeneous environmental conditions allows detecting the variability in the growth, flowering, number and size of leaves and fruits and allows identifying the resistance to various types of environmental stress (Resmi et al., 2005). Despite the great adaptability of the moringa plant, deciduous populations have been found in subtropical climates (Folkard et al., 1999).
Knowledge of morphological diversity in moringa can become a resource for its breeding through the section of elite varieties adapted to local conditions (Leone et al., 2015). The South-Southeast of Mexico has several moringa plantations. However, there is no detailed information on the morphological variation of the various accessions. Morphological variation in any plant can be attributed to edaphoclimatic, genetic, agronomic management factors or their combination (Chaves-Bedoya et al., 2017). The objective was to characterize the morphology of M. oleifera accessions from the Southeast of Mexico.
Materials and methods
Study area
The research focused on the south-southeastern region of Mexico due to the presence of commercial cultivations of moringa and this characteristic favored seed availability. The seeds of the accessions studied are from the states of Veracruz, Oaxaca, Guerrero, Chiapas and Yucatán (Table 1, Figure 1). The research was carried out at the College of Postgraduates, Campus Veracruz, geographically located at 19° 16’ 32” north latitude, 96° 16’ 32” west longitude, at an altitude of 16 m (Valdés et al., 2014). The climate is warm subhumid (AW0), with an accumulated annual rainfall of 1 000 mm and an average annual temperature of 27 °C (Olguín, 1999).
Table 1 Origin of the seeds of M. oleifera from the South-Southeast of Mexico.
| Num. | State | Accession | Municipality | Locality | Longitude | Latitude |
| 1 | Veracruz | V1 | Soledad de Doblado | El Progreso | -96.4022719 | 19.0818742 |
| 2 | Veracruz | V2 | Paso del Macho | Loma Pelada | -96.5398368 | 18.9258796 |
| 3 | Veracruz | V3 | Tierra Blanca | Colonia Pemex | -96.3429545 | 18.435 |
| 4 | Veracruz | V4 | Misantla | Santa Cruz Hidalgo | -96.8628092 | 19.9555656 |
| 5 | Oaxaca | O1 | Santa Cruz Xoxocotlán | San Juan Bautista La Raya | -96.7280556 | 16.9791667 |
| 6 | Oaxaca | O2 | Santa María Huatulco | La Herradura | -96.3658333 | 15.7772222 |
| 7 | Oaxaca | O3 | Mariscala de Juárez | Guadalupe la Huertilla | -98.1088889 | 17.8513889 |
| 8 | Oaxaca | O4 | Tuxtepec | San Juan Bautista | -96.1286697 | 18.087694 |
| 9 | Guerrero | G1 | Acapulco de Juárez | Bejuco | -99.6977778 | 16.8216667 |
| 10 | Guerrero | G2 | Acapulco de Juárez | Parotillas | -99.61558371 | 16.8787834 |
| 11 | Guerrero | G3 | Acapulco de Juárez | Concepción | -99.66028879 | 16.8799601 |
| 12 | Guerrero | G4 | Tecpan de Galeana | Mitla | -99.89343517 | 16.8789425 |
| 13 | Chiapas | C1 | Tuzantán | Villa Hidalgo | -92.374722 | 15.108056 |
| 14 | Chiapas | C2 | Tuxtla Gutiérrez | Colonia La Salle | -93.0868889 | 16.7429444 |
| 15 | Chiapas | C3 | Tuxtla Gutiérrez | Santa Cruz | -93.108986 | 16.783481 |
| 16 | Chiapas | C4 | Tuxtla Gutiérrez | San Juan | -93.103645 | 16.747307 |
| 17 | Yucatán | Y1 | Tzucacah | Tzucacah | -89.0391111 | 20.0720278 |
| 18 | Yucatán | Y2 | Mérida | Frac. el Parque | -89.5872222 | 20.9711111 |
| 19 | Yucatán | Y3 | Peto | Teshan | -88.62125 | 20.1486389 |
| 20 | Yucatán | Y4 | Baca | Felipe Carrillo Puerto | -89.6070099 | 20.9954688 |
Biological material
Moringa seeds were collected from different commercial cultivations in the South-Southeast of Mexico during the months from February to May 2018. Thirty healthy seeds were selected from each collection point and the variables of length, thickness and weight of the seed were measured. Twenty accessions were evaluated, which are shown in (Table 2).
Table 2 Morphological variables of moringa accessions from the South-Southeast of Mexico.
| Num. | Accession | Average weight (mg) ± standard error | Average length (mm) ± standard error | Average thickness (diameter) (mm) ± standard error |
| 1 | V1 | 364.33** ±8.19 | 11.93 ±0.26 | 8.33 ±0.14 |
| 2 | V2 | 296.67 ±11.31 | 9.50 ±0.24 | 7.33 ±0.18 |
| 3 | V3 | 325.33 ±10.5 | 8.93 ±0.18 | 8.5 ±0.18 |
| 4 | V4 | 331.43 ±4.59 | 9.57 ±0.2 | 10.43 ±0.3 |
| 5 | O1 | 332.33 ±6.91 | 11.67 ±0.23 | 9.5 ±0.13 |
| 6 | O2 | 379.67 ±8.2 | 11.33 ±0.19 | 10.63 ±0.15 |
| 7 | O3 | 324.67 ±6.19 | 13.53 ±0.25 | 10.77 ±0.09 |
| 8 | O4 | 364.67 ±8.63 | 11.87 ±0.19 | 11.37 ±0.15 |
| 9 | G1 | 309.67 ±9.83 | 12.43 ±0.23 | 9.53 ±0.13 |
| 10 | G2 | 356 ±7.99 | 13.97 ±0.33 | 10 ±0.16 |
| 11 | G3 | 408.67 ±10.42 | 12.97 ±0.28 | 11.07 ±0.13 |
| 12 | G4 | 580.67 ±12.05 | 14.2 ±0.18 | 12.3 ±0.12 |
| 13 | C1 | 485.33 ±10.36 | 14.9 ±0.35 | 10.93 ±0.21 |
| 14 | C2 | 461 ±10.3 | 13.8 ±0.24 | 11.83 ±0.14 |
| 15 | C3 | 480 ±8.04 | 13.87 ±0.25 | 11.97 ±0.14 |
| 16 | C4 | 308.33 ±7.08 | 12.13 ±0.24 | 9.53 ±0.22 |
| 17 | Y1 | 337.67 ±9.38 | 13.03 ±0.23 | 11.2 ±0.18 |
| 18 | Y2 | 460 ±11.25 | 14.3 ±0.35 | 10.6 ±0.19 |
| 19 | Y3 | 349.67 ±8.7 | 12.47 ±0.22 | 10.37 ±0.21 |
| 20 | Y4 | 428.67 ±12.76 | 13.6 ±0.27 | 11.27 ±0.23 |
V= Veracruz; O= Oaxaca; G= Guerrero; C= Chiapas; Y= Yucatán; **= average value of 30 seeds.
Sowing and transplanting
The seeds were sown in black nursery bags of 27 x 27 cm. The substrate used was composed of soil, manure-vermicompost and sand (5:4:1). After 15 days of germination, the seedlings of greater vigor and of a similar size were selected and two months later, they were transplanted in the field. The type of soil in the field was clay-loam.
Treatments and experimental design
The experimental design was completely randomized blocks with five repetitions. The size of the area used was 900 m2. The distance between individuals and furrows was 3 m. Every seven days, the variables of height, basal diameter (10 cm from the ground), number of branches and leaves were recorded. Two liters of water was applied daily to each plant through drip irrigation.
Morphological characterization
For morphological characterization, quantitative and qualitative descriptors were used for each organ of the plant. The quantitative ones recorded were: height, stem diameter, number of leaves, number of branches, number of flowers, leaf length, leaf width, petiole length, leaflet length, leaflet width and days to first flowering. For qualitative descriptors, those published by Mgendi et al. (2011); Zhigila et al. (2015); Popoola et al. (2016) were used. The qualitative descriptors were: leaf petiole color (1: light green, 2: green, 3: light violet, 4: medium violet, 5: intense violet), leaf shape (1: oval, 2: oblong, 3: oblong oval and 4: elliptical), leaf apex (1: obtuse and 2: acute), leaf pubescence (0: absent and 2: present), flower color (1: white, 2: white-cream, 3: white-pink, 4: white-cream-pink and 5: pinkish), purple spots on the flowers (0: absent and 1: present) and anther color (1: yellow and 2: orange).
Statistical analysis
The average values of the descriptors were evaluated by descriptive statistics. The correlation coefficient, principal component analyses and cluster analysis were performed through a hierarchical grouping with coefficient of variation in the unweighted pair group method (Euclidean distance). The two-dimensional scatter plot was performed through the percentage variation of the first two principal component analyses using the PAST program Version 3.0.
Results and discussion
Germination
The highest percentage of germination was obtained in accession G3 (100%) and the lowest percentage was 13% for accessions C4, O1, G4, C1, C4 and Y1. At the time of transplantation, accession V3 measured 108 cm, being the greatest height. Significant statistical differences (p˂ 0.05) were observed in the height and diameter of the stem of the 20 accessions evaluated. Accession C3 had the largest diameter and the largest number of leaves corresponded to accession O4 (Table 3).
Table 3 Variables recorded of Moringa oleifera at the time of transplantation.
| Num. | Accession | Germination (%) | Height (cm) (mean ± standard error) | Diameter (mm) (mean ± standard error) | Number of leaves (mean ± standard error) |
| 1 | V1 | 60* | 101.8 ±5.12 de | 9 ±0.55 cdefgh | 8.6 ±0.24 ab |
| 2 | V2 | 13 | 98.6 ±5.82 de | 8.2 ±0.37 bcdefg | 7 ±0.32 a |
| 3 | V3 | 50 | 108 ±7.35 e | 10.25 ±0.48 gh | 7.25 ±0.48 a |
| 4 | V4 | 63 | 59.4 ±2.86 a | 5.8 ±0.37 a | 6.6 ±0.24 a |
| 5 | O1 | 13 | 98.6 ±3.52 de | 8.6 ±0.4 cdefgh | 7.4 ±0.24 ab |
| 6 | O2 | 10 | 85.8 ±2.29 cde | 8 ±0.55 abcdef | 7.8 ±0.2 ab |
| 7 | O3 | 43 | 84 ± 4.89 bcd | 9.4 ±0.68 defgh | 6.6 ±0.24 a |
| 8 | O4 | 47 | 103.4 ±1.83 de | 10 ±0.32 fgh | 12.4 ±3.71 b |
| 9 | G1 | 90 | 62 ±2.21 ab | 6.2 ±0.37 ab | 6.6 ±0.24 a |
| 10 | G2 | 33 | 84.4 ±3.26 bcd | 7.2 ±0.2 abcd | 7.2 ±0.2 a |
| 11 | G3 | 100 | 72.4 ±3.3 abc | 7 ±0.32 abc | 6 ±0.63 a |
| 12 | G4 | 13 | 95.8 ±2.94 de | 7.6 ±0.24 abcde | 7.8 ±0.37 ab |
| 13 | C1 | 13 | 85.8 ±3.07 cde | 8.6 ±0.4 cdefgh | 6.6 ±0.75 a |
| 14 | C2 | 50 | 82 ±1.64 abcd | 7 ±0.55 abc | 7.4 ±0.68 ab |
| 15 | C3 | 63 | 102.5 ±10.31 de | 10.5 ±0.5 h | 9.75 ±0.25 ab |
| 16 | C4 | 13 | 86.75 ±3.35 cde | 10 ±0.41 fgh | 8.5 ±0.29 ab |
| 17 | Y1 | 13 | 93 ±2.94 cde | 8.6 ±0.4 cdefgh | 6.6 ±0.75 a |
| 18 | Y2 | 77 | 90 ±2.76 cde | 10.2 ±0.37 fgh | 7.2 ±0.37 a |
| 19 | Y3 | 60 | 96.6 ±4.35 de | 9.6 ±0.4 efgh | 8 ±0.32 ab |
| 20 | Y4 | 23 | 99.8 ±7.7 de | 8.4 ±0.4 bcdefgh | 8.2 ±0.2 ab |
*= average value of 10 seedlings. Means with similar letters do not present significant statistical differences (p> 0.05).
Great variation was found in the percentage of germination of the accessions sown. This variation is attributed to seed quality and storage time before sowing (Du Toit et al., 2017). In addition to this, genetic potential and environmental factors such as temperature, precipitation and altitude influence the development of the seed before collection and determine its size and weight (Baiyeri et al., 2015; Ledea-Rodríguez et al., 2018).
This process affects the viability of seeds of more than one year of storage in temperature ranges of 23 to 25 ºC. Therefore, it is recommended that the seeds be preserved in the pods (Fotouo et al., 2015). The average number of germination days of the accessions sown was 11. The earliest gemination occurred at 8 days and the latest at 14 days. This interval is like the 6 and 13 days reported by Popoola et al. (2016); Zaku et al. (2015); while Kumar et al. (2014) mentioned that germination occurs between 10 and 12 days after sowing. Ramos et al. (2010) reported that germination begins from 8 days after sowing and at 25 days, the primary leaves appear.
Height
Accessions C2 and G2 were the largest and reached an average height of 273 and 271 cm, respectively. Accession Y4 had an average height of 164 cm, being the lowest growth recorded during the 301 days after transplantation (DAT) (Figure 2).
At six months after transplantation, the minimum and maximum height were 1.45 and 2.25 m, respectively. These values are lower than the 5.17 and 10.27 m reported by Popoola et al. (2016). This smaller size can be attributed to the lack of precipitation, since water has a direct effect on plant growth because in drought conditions, cell division and expansion decreases (Taiz and Zeiger, 2009).
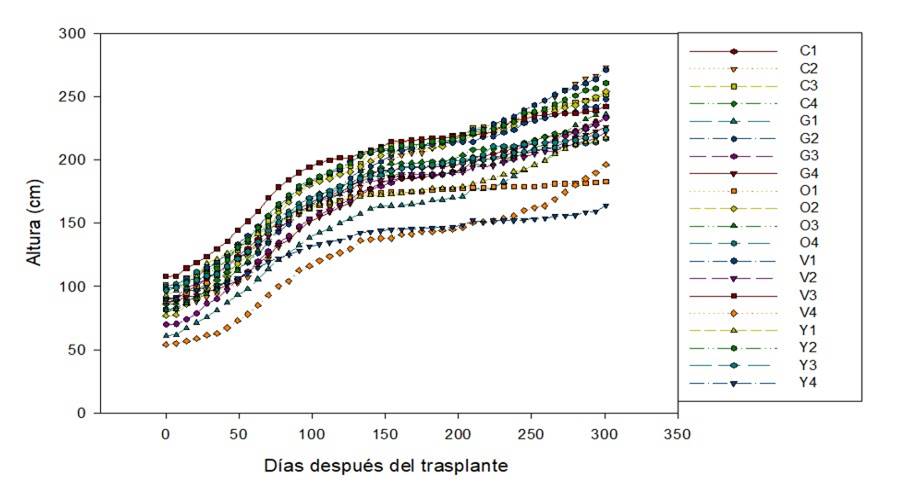
Figure 2 Plant height of the 20 accessions of Moringa oleifera collected in the South-Southeast of Mexico. Period: 0 to 301 DAT.
In relation to the number of branches, there were few and these emerged after 2 m in height. Popoola et al. (2016) mentions that the branching is moderate. Dao and Kabore (2015) recorded in moringa, at two months, from 8 to 15 branches per tree. In this work, there was a monopodial growth in the accessions evaluated.
Stem diameter
The largest diameter at a height of 10 cm from the ground was observed in accession Y2 with an average value of 43.22 mm, and the lowest value corresponded to accession Y4 with 25.61 mm at 301 days after transplantation. The most frequent stem colors were gray and whitish gray. Panshin and Zeeuw (1970) mention that the thickening of the stem is due to related processes in xylem and phloem. Therefore, they may differ between accessions. The thickening of the stem and its morphology among moringa ecotypes is diverse due to the great plasticity it presents (Förster et al., 2015).
Leaves
After transplantation, defoliation was observed in all accessions. At 301 DAT, accession Y3 had 54 leaves, the highest value being. Accession O1 presented 9 leaves, being the accession with the lowest average number of leaves. The shortest leaf length was 28.8 cm (Y3) and the longest was 47 cm (C2). The leaf width fluctuated between 16.8 (O1) and 38.33 cm (Y1). The presence of pubescence was recorded in accessions G4, C3 and G2. These values are higher than the range of 21.4 to 54.2 cm long and 10.1 to 41.6 cm wide reported by Zhigila et al. (2015). Dao and Kabore (2015) reported average leaf lengths from 16 to 44 cm and values from 10.5 to 34 cm wide, with a number of pinnas of 5 to 12 per leaf.
Leaf length is influenced by relative humidity and average annual precipitation. Phenotypic characteristics are affected by edaphic factors and result in epigenetic changes (Shahzad et al., 2013). Moringa leaves are consumed for their high nutritional value (Förster et al., 2015). Therefore, the size, color and number of leaves represent an important characteristic to produce biomass, nutritional content and genetic improvement.
The presence of purple pigmentation in the petiole and rachis of accessions C1, O2 and Y2 was also identified. The dark color on the leaf represents a greater amount of chlorophyll and the existence of this photosynthate promotes greater growth (Opare-Obuobi, 2012).
The violet pigmentation in the leaf petiole is determined by the anthocyanin content. The presence of anthocyanins is determined by environmental conditions and can be purple or pink in color. Usually, crops in areas under drought have higher anthocyanin production as a mechanism to avoid stress (Shahzad et al., 2013). The presence of pubescence on the leaves, tender shoots and filaments of the anther was noted. There was defoliation caused by high temperatures and little precipitation. Vasconcelos et al. (2019) mentioned that water stress caused by lack of precipitation influences leaf morphology and plant physiology. In this work, the effect of stress on the defoliation and yellowing of the leaves was observed.
Flowers
The beginning of flowering was recorded by counting the days since the sowing of the accessions. Accession O4 was the first to start the flowering process at 129 days after sowing (das). The last accession to flower was G3 at 240 das. Accessions Y2 and Y5 had pinkish-white flowers, C2 and V3 creamy white and O1 white flowers. Purple spots were identified in accessions G2 and V1 (Table 4). Flowering in cultivated accessions began at 129 days and continued during the months of October-May. However, the fall of flowers prevented many from reaching anthesis, limiting the pollination process.
Table 4 Central tendency values for quantitative descriptors of Moringa oleifera Lam.
| Num. | Descriptor | Mean | Standard deviation | Maximum | Minimal | Variance |
| 1 | Height (cm) | 231.45* | 27.37 | 273 (C2) | 164 (Y4) | 749.32 |
| 2 | Diameter (mm) | 32.58 | 4.75 | 44 (Y2) | 23.6 (O1) | 22.59 |
| 3 | No. leaves | 20.72 | 9.85 | 54 (Y3) | 9.8 (O1) | 97.09 |
| 4 | No. branches | 2.64 | 2.94 | 12.6 (C2) | 0.4 (O1) | 8.64 |
| 5 | No. flowers | 0.52 | 0.75 | 2.67 (Y1) | 0 (V4, O1 y O3) | 0.56 |
| 6 | No. fruits | 0.35 | 0.43 | 1.6 (O2) | 0 (V4, O1, O3 y G1) | 0.19 |
| 7 | Leaf length (cm) | 37.72 | 4.51 | 47 (G1) | 28.8 (Y3) | 20.33 |
| 8 | Leaf width (cm) | 26.71 | 5.72 | 38.33 (Y1) | 16.8 (O1) | 32.72 |
| 9 | Petiole length (cm) | 10.46 | 1.51 | 13 (C1) | 7.6 (Y3) | 2.27 |
| 10 | Leaflet length (mm) | 15.9 | 2.22 | 21.75 (Y2) | 12 (Y3) | 4.92 |
| 11 | Leaflet width (last) (mm) | 7.44 | 1.18 | 9.2 (V2) | 5 (O3) | 1.4 |
| 12 | Days to first flowering | 180.53 | 28.89 | 240 (G3) | 129.6 (O4) | 834.51 |
*= average value of 5 plants. Values obtained at 301 DAT and beginning of flowering obtained at DAS.
Flowering in moringa can occur once or twice a year, depending on the environmental conditions (temperature and precipitation). Price (2000) mentioned that flowering can occur four times during the year. The pigmentation of the flowers varied from white, creamy white and pinkish white. Some accessions had purple pigmentation on the petals. Popoola et al. (2016) recorded white flowers with purple pigmentation, white or creamy white without pigmentation and 50% of flowering occurred in the 161 and 167 days. Moringa is a species that presents great variability in the color of its flowers and in some varieties of India, flowers with pink and dark pink base have been recorded (PPV and FR, 2001).
Several studies have stated that pink and dark pink flowers receive more visits from bumblebees than yellow ones. This factor represents, apparently, a strategy of adaptation and reproduction (Bradshaw and Schemske, 2003; Reverté et al., 2016). Therefore, cross-pollination between moringa accessions is promoted and facilitates the creation of new varieties. Factors such as temperature and soil moisture influence the increase of flowers, pollen viability and decrease the number of fruits (Muhl et al., 2013).
Pearson’s correlation coefficient (r) of morphological characters
The Pearson correlation matrix among the quantitative descriptors of moringa is shown in Table 5. A positive correlation was found between the length and width of the leaf (r= 0.857), and leaf length and petiole length (r= 0.851). A correlation was also found between diameter and height (r= 0.675). A negative correlation was identified between the days to first flowering and the number of flowers (r= - 0.599) and with the number of fruits (r= - 0.361).
Table 5 Correlation matrix of quantitative descriptors of Moringa oleifera at 301 DAT.
| Height | Diameter | Num. leaves | Num. branches | Num. flowers | Num. fruits | L. leaf | W. leaf | L. petiole | L. leaflet | L. leaflet | DF. flowering | |
| Height (cm) | 1 | |||||||||||
| Diameter (mm) | 0.675* | 1 | ||||||||||
| Num. leaves | 0.042 | 0.103 | 1 | |||||||||
| Num. branches | 0.333* | 0.18 | 0.318* | 1 | ||||||||
| Num. flowers | 0.134 | 0.088 | 0.166 | -0.104 | 1 | |||||||
| Num. fruits | 0.284 | 0.344* | -0.226 | 0.025 | 0.364* | 1 | ||||||
| L. leaf (cm) | 0.368* | 0.382* | -0.422* | -0.363* | 0.073 | 0.106 | 1 | |||||
| W. leaf (cm) | 0.327* | 0.454** | -0.285 | -0.22 | 0.324* | 0.175 | 0.857* | 1 | ||||
| L. petiole (cm) | 0.187 | 0.24 | -0.472* | -0.484* | 0.207 | 0.109 | 0.851* | 0.686* | 1 | |||
| L. leaflet (mm) | 0.249 | 0.386* | -0.47* | -0.048 | -0.088 | 0.24 | 0.288 | 0.219 | 0.304* | 1 | ||
| W. leaflet (mm) | -0.085 | 0.045 | -0.187 | -0.117 | 0.084 | 0.269 | 0.068 | 0.017 | 0.126 | 0.636* | 1 | |
| DF. flowering | -0.161 | -0.134 | -0.127 | -0.113 | -0.599** | -0.361* | -0.1 | -0.19 | -0.219 | 0.078 | -0.34* | 1 |
**= significant correlation at 0.01; *= significant correlation at 0.05.
Principal component analysis
The principal component analysis demonstrated variability in morphological characters in the 20 moringa accessions (Table 6). The percentage of variation was 52.87, 37.54, 6.16, 1.98 and 0.63 for components 1, 2, 3, 4 and 5, respectively.
Table 6 Principal component analysis for morphological descriptors of Moringa oleifera at 301 DAT.
| Num. | Descriptor | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 |
| 1 | Height (cm) | 0.6 | 0.785 | 0.029 | -0.11 | -0.094 |
| 2 | Diameter (mm) | 0.075 | 0.091 | 0.006 | 0.291 | 0.856 |
| 3 | Number leaves | 0.04 | -0.025 | 0.906 | 0.378 | -0.09 |
| 4 | Branches | 0.026 | 0.023 | 0.113 | -0.157 | 0.179 |
| 5 | Number flowers | 0.013 | -0.009 | 0.003 | 0.025 | -0.03 |
| 6 | Number fruits | 0.006 | 0 | -0.01 | -0.009 | 0.038 |
| 7 | Leaf length (cm) | 0.042 | 0.046 | -0.264 | 0.458 | -0.238 |
| 8 | Leaf width (cm) | 0.064 | 0.036 | -0.276 | 0.713 | -0.102 |
| 9 | Petiole length (cm) | 0.013 | 0.002 | -0.093 | 0.114 | -0.062 |
| 10 | Leaflet length (mm) | 0.006 | 0.024 | -0.099 | -0.023 | 0.339 |
| 11 | Leaflet width (last) (mm) | 0.008 | -0.014 | -0.026 | -0.019 | 0.115 |
| 12 | Leaves | 0 | 0 | 0 | 0 | 0 |
| 13 | Leaf petiole color | 0.005 | 0.009 | -0.001 | 0.014 | 0.067 |
| 14 | Leaf shape | 0 | 0 | 0 | 0 | 0 |
| 15 | Leaf apex (in mature leaf) | 0 | 0 | 0 | 0 | 0 |
| 16 | Pubescence on the leaf | 0.004 | -0.003 | 0.001 | -0.011 | -0.029 |
| 17 | Days to first flowering | -0.791 | 0.609 | 0.034 | 0.043 | -0.006 |
| 18 | Flower color | 0.008 | 0.001 | -0.004 | 0.015 | 0.103 |
| 19 | Purple spots on flowers | -0.003 | 0.008 | 0.003 | 0.007 | 0.016 |
| 20 | Anther color | 0.002 | -0.002 | -0.003 | -0.007 | 0.027 |
| Eigenvalue | 938.953 | 666.755 | 109.553 | 35.323 | 11.289 | |
| (%) variance | 52.874 | 37.546 | 6.169 | 1.989 | 0.636 | |
PC= principal component.
The two-dimensional Figure 3 of the analyses of principal components 1 and 2 shows 3 groups. Group A comprises accessions O2, Y2, C2, G2, C3, V2, G4, C1, V3, Y1, O4 and V1. Group B comprises accessions G3, O3, G1, Y3 and C4. In group C, populations V4, O1 and Y4 were identified (Figure 3). The grouping was the result of the PCA based on the analysis of morphological descriptors.
Cluster
Two subgroups were formed in group A: I (O2, Y2, C2, G2) and II (O4, G4, C1, Y1, V2, V3, C3 and V1). In group B, the subgroups: I (O3, G1, C4, G3) and II (Y3) were formed. In group C, accessions V4, O1 and Y4 were identified (Figure 4).
Conclusions
There is a morphological diversity in the moringa accessions from the South-Southeast of Mexico. This diversity can serve to reinforce the knowledge of moringa and expand information regarding its physiology, phenology and production. The morphological knowledge of the accessions will allow the creation of programs of conservation, selection and generation of elite materials with greater adaptive potential, resistance to pests and diseases and with greater productive capacity and nutritional content.
Literatura citada
Baiyeri, K. P.; Apeh, P.; Stevens, G. C.; Ndukwe, O.; Aba, S. C. and Otitoju, G. T. 2015. Growth performance and nutrient quality of three Moringa oleifera accessions grown as potplant under varied manure rates and watering intervals. Afr. J. Biotechnol. 14(24):1996-2004. doi: 10.5897/AJB2014.14359. [ Links ]
Bradshaw, J. H. D. and Schemske, D. W. 2003. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 426(6963):176-178. doi: 10.1038/nature02106. [ Links ]
Chaves-Bedoya, G.; Galvis-Pérez, Z. L. y Ortiz-Rojas, L. Y. 2017. Diversidad genética de Moringa oleifera Lam. En el nororiente colombiano utilizando marcadores RAMs. Rev. Colomb. Cienc. Hortíc. 11(2):408-415. doi: http://dx.doi.org/10.17584/rcch.2017v11i2.7343. [ Links ]
Dao, M. C. E. and Kabore, K. H. 2015. Morphological characteristic variation of eleven provenances of Moringa oleifera seedlings grown in the northern sudanese area of burkina faso. Afr. J. Plant Sci. 9(10):401-411. doi: 10.5897/AJPS2015.1334. [ Links ]
Du-Toit, E. S.; Fotouo, H. and Robbertse, P. J. 2017. Seed storage conditions influence germination of Moringa oleifera Lam. seed. Acta Hortic. 1158:441-446. https://www.cabdirect.org/ cabdirect/abstract/20173287864. [ Links ]
Folkard, G.; Sutherland, J. and Shaw, R. 1999. Water clarification using Moringa oleifera coagulant. ‘Water and environmental health at london and loughborough’ (well), Loughborough University, Loughborough. 109-112 pp. [ Links ]
Förster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R. and Mewis, I. 2015. Ecotype variability in growth and secondary metabolite profile in Moringa oleifera: impact of sulfur and water availability. J. Agric. Food Chem. 63(11):2852-2861. doi: 10.1021/jf506174v. [ Links ]
Fotouo-M, H.; Du-Toit, E. S. and Robbertse, P. J. 2015. Germination and ultrastructural studies of seeds produced by a fast-growing, drought-resistant tree: implications for its domestication and seed storage. AoB Plants. 1-12. doi: 10.1093/aobpla/plv016. [ Links ]
Kumar, A. P.; Sarawgi, A. K.; Bhandarkar, S. and Ojha, G. C. 2017. Agro-morphological characterization and morphological based genetic diversity analysis of Rice (Oryza sativa L.) germplasm. J. Pharmacognosy Phytochem. 6(6):75-80. http://www.phytojournal.com/ archives/?year=2017&vol=6&issue=6&ArticleId=2062. [ Links ]
Ledea-Rodríguez, J. L. G.; Rosell-Alonso, D. G.; Benítez-Jiménez, R. C.; Arias-Pérez, J. V.; Ray-Ramírez, and J. J. y Reyes-Pérez. 2018. Producción de semillas de variedades de Moringa oleifera Lam. En el valle del cauto. Agron. Mesoam. 29(2):415-423. http://dx.doi.org/10.15517/ma.v29i2.29545 . [ Links ]
Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J. and Bertoli, S. 2015. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Inter. J. Mol. Sci. 16(6):12791-12835. Doi: 10.3390/ijms160612791. [ Links ]
Martín, C.; Martín, G.; García, A.; Fernández, T.; Hernández, E. and Puls, J. 2013. Potential applications of Moringa oleifera. A critical review. Pastos y Forrajes. 36(2):137-149. https://www.researchgate.net/publication/303986314. [ Links ]
Mgendi, M. G.; Nyomora, A. M. and Manoko, M. K. 2011. Using morphological markers to assess variations between and within cultivated and non-cultivated provenances of Moringa oleifera Lam. in Tanzania. J. Life Sci. 5:387-392. [ Links ]
NRC. 2006. National Research Council. ‘moringa’ -lost crops of Africa National Academic Press. ISBN: 978-0-309-10333-6. 247-267 pp. [ Links ]
Olguín, C. P. 1999. Fertiirrigación orgánica: investigación y transferencia. Terra Latinoam. 17(3):175-178. https://www.redalyc.org/pdf/573/57317301.pdf. [ Links ]
Opare-Obuobi, K. 2012. Characterisation of local and exotic accessions of moringa (Moringa oleifera Lamarck). Department of crop science. College of Agriculture and Consumer Sciences University of Ghana 132 p. [ Links ]
Panshin, A. J. and Zeeuw, C. D. 1970. Textbook of wood technology. Volume I. 3ra (Ed.). Structure, identification, uses, and properties of the commercial woods of the United States and Canada. Structure, identification, uses, and properties of the commercial woods of the United States and Canada. 63-64 pp. [ Links ]
Pérez, A.; Sánchez, T.; Armengol, N. y Reyes, F. 2010. Características y potencialidades de Moringa oleifera, Lamark: una alternativa para la alimentación animal. Pastos y Forrajes. 33(4):1-16. [ Links ]
Popoola, J. O.; Bello, O. A. and Obembe, O. O. 2016. Phenotypic intraspecific variability among some accessions of Drumstick (Moringa oleifera Lam.). Can. J. Pure Appl. Sci. 10(1):3681-3693. https://www.researchgate.net/publication/289521612. [ Links ]
PPV and FR. 2001. Guidelines for the conduct of test for distinctiveness, uniformity and stability on drumstick (Moringa oleifera Lam). Protection of plant varieties and farmers’ rights authority. government of India. New Delhi, India. 1-29 pp. [ Links ]
Price, L. M. 2000. The Moringa tree: revised in 2000 by Kristin Davis. 1-14 pp. [ Links ]
Ramos, L. M.; Costa, R. S.; Môro, F. V. and Silva, R. C. 2010. Morfología de frutos e sementes e morfofunção de plântulas de Moringa (Moringa oleifera Lam.). Comunicata Scientiae. 1(2):156-160. https://dialnet.unirioja.es/servlet/articulo?codigo=6294707. [ Links ]
Resmi, D. S.; Celine, V. A. and Rajamon, L. 2005. Variability among drumstick (Moringa oleifera Lam.) accessions from Central and Southern Kerala. J. Tropical Agric. 43(1-2):83-85. http://jtropag.kau.in/index.php/ojs2/article/view/141. [ Links ]
Reverté, S.; Retana, J.; Gómez, J. M. and Bosch, J. 2016. Pollinators show flower colour preferences but flowers with similar colours do not attract similar pollinators. Ann. Bot. 118(2):249-257. Doi: 10.1093/aob/mcw103. [ Links ]
Santiago, M. T. B. and Bezerra, N. E. 2017. Ecophysiology of Moringa oleifera Lam. in function of different rainfall conditions. Rev. Geama. 3(4):236-241. [ Links ]
Taiz, L. y Zeiger, E. 2009. Fisiologia vegetal. 4ta . (Ed.). Porto Alegre. Universidad Jaume. Artmed. 656 p. [ Links ]
Tesfay, S. Z.; Bertling, I.; Odindo, A. O.; Workneh, T. S. and Mathaba, N. 2011. Levels of anti-oxidants in different parts of moringa (Moringa oleifera) seedling. Afr. J. Agric. Res. 6(22):5123-5132. Doi: https://doi.org/10.5897/AJAR11.1101. [ Links ]
Valdés, R. O. A.; Wassenaar, O. M. P.; Ruiz R. y Pérez, A. V. 2014. Potencial de la asociación moringa y ricinus en el subtrópico veracruzano. Rev. Mex. Cienc. Agríc. 9(2014):1673-1686. http://cienciasagricolas.inifap.gob.mx/index.php/agricolas/article/view/1056. [ Links ]
Vasconcelos, M. C.; Costa, J. C.; Sousa, J. P. S.; Santana, F. V.; Soares, T. F. S. N.; Oliveira L. F. G. D. and Silva-Mann, R. 2019. Biometric and physiological responses to water restriction in Moringa oleifera seedlings. Floresta e Ambiente. 26(1):1-8. Doi: https://doi.org/ 10.1590/2179-8087.016515. [ Links ]
Zaku, S. G.; Emmanuel, S.; Tukur, A. A. and Kabir, A. 2015. Moringa oleifera: an underutilized tree in Nigeria with amazing versatility: A review. Afr. J. Food Sci. 9(9):456-461. https://doi.org/10.5897/AJFS2015.1346. [ Links ]
Received: August 01, 2021; Accepted: October 01, 2021











 text in
text in 





