Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 n.8 Texcoco Nov./Dec. 2019 Epub Feb 05, 2021
https://doi.org/10.29312/remexca.v10i8.1831
Articles
Abundance and genetic diversity of Fusarium oxysporumand Trichoderma sp. in muse AAB
1Benemérita Universidad Autónoma de Puebla-Facultad de Ingeniería Agrohidráulica. Av. Universidad s/n, San Juan Acateno, Teziutlán, Puebla, México. CP. 73965. Tel. 595 9528969. (Delfino-reyes2001@yahoo.com.mx; moralesuno1@hotmail.com; raulberdeja@yahoo.com.mx; titanmj96@hotmail.com; fabianvm24@hotmail.com).
The occurrence and diversity of Fusarium and Trichoderma species was studied in 29 points of a banana plot (Musa AAB) in Veracruz, Mexico, during November 2017 and April 2018. The plot was divided into two parts; in the first one, fallow and weed removal were carried out (Procedure in culture. 1) while in the second one these activities were not carried out (Procedure in culture; and 2). Fungi were isolated from the soil collected at these sites using potato dextrose and agar (PDA) and K2. At the time of collecting the samples, temperature, precipitation and solar radiation were recorded. The results of the analysis showed 68% more presence of colony forming units (CFU) in soils where no cultivation work was carried out using K2 unlike soils where they were performed using PDA (32%). A small amount of CFU was also observed when the temperature, precipitation and radiation values were high. Molecular analyzes showed 65% more abundance of species in soils without labor compared to cultivated soils (35%), six of 16 Fusarium isolates were Fusarium oxysporum f. sp. melonis and six of 13 Trichoderma isolates were Trichoderma longibrachiatum in the first sampling while nine of 14 Trichoderma isolates were Trichoderma spirale in the second sampling. The analysis of the intrapopulation diversity of seven Trichoderma spirale isolates showed bands of 75 to 500 bp in three loci of the genome in the VB28IT1 isolate with five ISSR.
Keywords: banana; populations; CFU
Se estudió la ocurrencia y diversidad de especies de Fusarium y Trichoderma en 29 puntos de una parcela de plátano (Musa AAB) en Veracruz, México, durante noviembre de 2017 y abril 2018. La parcela se dividió en dos partes; en la primera se realizó barbecho y eliminación de maleza (procedimiento en cultivo 1) mientras que en la segunda no se realizaron estas actividades (procedimiento en cultivo 2). Se aislaron hongos del suelo colectado en estos sitios usando papa dextrosa y agar (PDA) y K2. Al momento de colectar las muestras se registró la temperatura, precipitación y radiación solar. Los resultados del análisis mostraron 68% más presencia de unidades formadoras de colonias (UFC) en suelos donde no se realizaron labores de cultivo usando K2 a diferencia de suelos donde sí se realizaron éstas usando PDA (32%). También se observó poca cantidad de UFC cuando los valores de temperatura, precipitación y radiación fueron altos. Los análisis moleculares mostraron 65% más abundancia de especies en suelos sin labor comparado con suelos cultivados (35%), seis de 16 aislados de Fusarium fueron Fusarium oxysporum f. sp. melonis y seis de 13 aislados de Trichoderma fueron Trichoderma longibrachiatum en el primer muestreo mientras que nueve de 14 aislados de Trichoderma fueron Trichoderma spirale en el segundo muestreo. El análisis de la diversidad intrapoblacional de siete aislados de Trichoderma spirale, mostraron bandas de 75 a 500 bp en tres locis del genoma en el aislado VB28IT1 con cinco ISSR.
Palabras clave: plátano; poblaciones; UFC
Introduction
Fusarium is a genus of fungi that includes a large number of plant pathogenic species. Its high coevolutive capacity with cultivated plants has given it greater genetic variability and increased species diversity. Only the Fusarium oxysporum group includes 120 special forms that cause disease in specific host plants (Bentley et al., 1998; Leong, et al., 2010; Benaouali et al., 2014). Of these special forms F. oxysporum Schlechtend f. sp cubense (E.F. Smith) Snyd and Hans, was the cause of one of the most catastrophic diseases worldwide in bananas (Ploetz, 2015).
It is estimated that between 1960 and 1970 in Mexico, about 40 000 ha of banana of the manzano or Silk variety (Musa AAB) were destroyed by this disease (Orozco et al., 2009) and most of the soils of the producing areas of plantain were infested with F. oxysporum f. sp cubense (Orozco et al., 2009) which made the production of susceptible varieties such as Silk or manzano tree difficult.
Fusarium is a pathogen that forms resistance structures called clamidospores that give it the ability to survive for long periods in the soil, and its main forms of dispersion are by movement of infested soil and runoff water (Retana et al., 2018); however, its abundance and diversity in the soil also depends on other factors such as crop variety, physicochemical characteristics of the soil, climatic conditions and human activity (Bateman and Murray 2001; Bernhoft et al., 2012).
All this interaction of factors, make necessary the study of the behavior, abundance and diversity of the different races and special forms Fusarium oxysporum with the crop and Trichoderma species, which have shown properties in their control (Maina et al., 2016), as well such as the impact of cultural practices that can alter this behavior.
At present, most of the studies carried out directly with diversity of species show the history and evolutionary potential of a population or infer in practical aspects such as the virulence gene flow to other geographical areas of both fungi (McDonald and Linde, 2002); however, few studies have studied the impact of cultural practices at different times of the year and climatic conditions on the abundance and genetic diversity of Fusarium species in the soil (Bernhoft et al., 2012) and their interaction with Trichoderma species, therefore, there is little understanding in this aspect (Maina et al., 2016).
Due to the above, the present study was proposed with the objective of knowing the effect of cultural work (fallow and weed control) and climatic conditions (temperature, relative humidity and precipitation) on the abundance and genetic diversity of populations of Fusarium oxysporum and Trichoderma sp., in the AAB banana crop, using the PDA and K2 culture media for their isolation and the polymerase chain reaction (PCR) technique for identification.
Materials and methods
Site location and sampling
A total of 58 soil samples weighing 500 g were collected on a plot of manzano or Silk banana (Musa AAB) in Platanozapan, Municipality of Tlapacoyan Veracruz, Mexico, 29 were collected in the month of November 2017 and the rest in April 2018. For the collection of samples, sampling sites were georeferenced in the plot, which was previously divided into two parts, in one fallow and weed control was performed, while in the other no work was performed. Each site was marked with wooden stakes every five meters, and in each of them at 0.2 m depth a soil sample was taken which was analyzed in the laboratory of the Faculty of Agro-Hydraulic Engineering of the Benemérita Autonomous University of Puebla, Mex.
Isolation and morphological characterization of the Fusarium and Trichoderma genus
10 g of soil were taken from each sample which were diluted in 90 mL of 0.05% Tween 80, of the dilution obtained, 1 mL was deposited in 9 mL of 0.05% Tween 80 to obtain a second dilution. Of both dilutions 100 μL were extended with a glass triangle on Komana culture medium (K2) and potato dextrose and agar (PDA). From each dilution and culture medium, three repetitions were performed, which were incubated at 30 °C for 48 and 72 hours in the dark. After this time, each and every one of the colonies with morphological characteristics of trichoderma and Fusarium were counted and isolated in a new medium (Chaverri et al., 2003; García-Núñez et al., 2016; Sánchez-López et al., 2012).
Quantification of Fusarium and Trichoderma
Amount of CFU g-1 of soil in PDA and K2 medium: before isolation in new medium and to facilitate counting Trichoderma sp. and Fusarium oxysporum of each repetition made and for each culture medium used (PDA and K2), each of the Petri dishes was divided into 4 equal parts and in each quadrant the existing colonies were counted, then the CFU calculation was performed by box and evaluated the effectiveness of each of the media.
Amount of CFU g-1 of soil with respect to cultural work: the amount of isolated colonies was also analyzed with respect to cultural work done (fallow and weed control) against any work performed.
Amount of CFU g-1 with respect to the seasons of sample collection and climatic conditions: the month of December was chosen as the first season of sample collection and the month of April as the second season in order to compare the effect of the climatic conditions, precipitation, temperature, relative humidity and solar radiation present in these two seasons on the amount of CFU existing in soil in each of the sampled parts of the plot. For the analysis of results obtained from CFU g-1 in each case, a comparison test of means with a variance analysis (Anova) was performed using the statistical package SAS 9.0 for Windows.
Determination of the presence of Fusarium oxysporum f. sp. cubense race 1
To corroborate the presence of race 1 of Fusarium oxysporum, manzano banana plants (AAB) were inoculated for each isolate with 1X 106 CFU. The plants were protected in the greenhouse, 7 days after infection they were evaluated qualitatively on a scale of 1-5 (1= no damage; 2= isolated points in the vascular tissue; 3= discoloration of up to one third of the vascular tissue; 4= discoloration greater than one third of the tissue; and 5= total discoloration of the tissue (Dita et al., 2011), for which the base of the corm was cut transversely and observed in the discoloration of vascular tissues.
Molecular identification and determination of Trichoderma and Fusarium diversity between species and within the most abundant species
For identification, determining the abundance and diversity of the genus studied began with the extraction of DNA from the 14-day-old isolates, seeded in cellophane disk on ADS medium, and incubated at 30 °C, the mycelium obtained was stored in 2 mL eppendorf tubes and lyophilized in a Labconco® lyophilizer..
Portions of 0.05 g of lyophilized dry mycelium were macerated with a pistil in tubes cooled with liquid nitrogen. Subsequently, we proceeded according to the instructions of the Dneasy plant mini kit of the Qiagen brand. The DNA quality obtained from each isolate was determined with a Thermoscientific nanodrop, Nanodrop 2000C model, visualized by an agarose gel in a UVP transilluminator, model: 3UV-LMS26 and was stored in a freezer at -7 °C, for amplification by PCR.
The DNA obtained from each isolate of Fusarium oxysporum and Trichoderma sp. was amplified by polymerase chain reaction (PCR) for the elongation factor gene EF1-(. In the case of Fusarium oxysporum, the primers: TEF1 and TEF2 were used, with a kit for PCR amplification of the GoTaq® brand, Promega Corporation, Madison, WI, USA, for which 9.5 μL of 10% trehalose was used, 5 μL of 5x Buffer, 10 mM of dNTPs, 2.5 mM μL-1 of MgCl2, 1 μM of each first, 5 U μL-1 of Taq DNA polymerase and 15 to 20 ng mL-1 of DNA, for a final volume of 25 μL mixture, which was amplified at 1 cycle of 95 °C for 2 min, 10 cycles of 94 °C for 30 s, 66 °C (Touchdown -1 °C → 56 °C) 30 s and 72 °C per 1 min, 36 cycles of 94 °C for 30 s, 56 °C for 36 s and 72 °C for 1 min and a cycle of 72 °C for 10 min.
In the case of Trichoderma sp., the initiators EF1-728F and EF1-986R described by Carbone and Kohn (1999) were used using a mixture similar to that described for Fusarium oxysporum, with the 94 °C 1 cycle amplification program for 5 min, 35 cycles of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 1 min and a cycle of 72 °C for 10 min (Sanchez Lopez et al., 2012) All samples were amplified in a personal Mastercycler thermocycler (Eppedor®) and its obtained PCR products were visualized in 2% agarose gel, TAE buffer (1X) plus 10 μL of ethidium bromide which was exposed to 80 V in a Thermocientific electrophoresis chamber .
The amplified products were monitored with a 100 bp DNA Ladder® molecular weight marker, Promega Corporation, Madison, WI, USA, purified and sequenced by Macrogen Inc. laboratories (Geumchen-gu, Seoul, Korea). The sequences were edited with the BioEdit v 7.1.9 program (Hall, 1999). The phylogenetic trees were generated by a maximum likelihood analysis (Maximum Likelihood, ML) and nearest neighbor (Neighbor Joining) using Mega v.5 (Tamura et al., 2011), followed by a bootstrap analysis with 1 000 repetitions.
The calculation of the means of interpopulation as well as intrapopulation diversity were obtained with a bootstrap of 1 000 replicas, including in the analysis 21 nucleotide sequences for Trichoderma sp. and 20 for Fusarium oxysporum with the MEGA v.5 program. To have a molecular approximation of the genetic variability within the most abundant species (Trichoderma spirale) the polymorphism of eight of its isolates was measured: VB7IT1, VB28IT1, VB2IT2, VB8IT2, VB11IT2, VB24IT2, VB25IT2, VB29IT2, by genotyping the ISSR markers: (GA)9C, (GA)9T, (CA)8RG, (ACC)9 and (GTG)5, which were previously selected for their polymorphism (66 to 100%) according to Thangavelu et al. (2011); Nirmaladevi et al. (2016). ISSR markers were synthesized by Integrated ADN Technologies.
The amplifications were performed in a final volume of 25 (L of reaction, containing 1X buffer for PCR, 0.2 mM dNTPs, 1.5-2.5 mM MgCl, 0.5 (M of each first, 1 unit of Taq DNA Polymerase (GoTaq®, Promise Corporation, Madison, WI, USA) with 20 ng of genomic DNA. The thermal conditions of the reactions were 94 °C for 5 min followed by 40 cycles of 30 s of denaturation at 94 °C and 48 to 64 °C for 45 s of alignment, 1.3 min of extension at 72 °C and a final extension at 72 ºC for 7 min. The PCR products were separated by electrophoresis on a high resolution molecular grade agarose gel (Sigma-Aldrich®, Sigma-Aldrich Corporation, Inc.) at 3% plus 10 (L of ethidium bromide at 45 V for 4 h, using a 50 bp molecular marker (HyperLadder®, BioLine USA Inc. Tauton, Ma, USA).
Results and discussion
Isolation and morphological characterization of the Fusarium and Trichoderma genus
They were able to obtain 29 isolates in the first season, 16 belonging to Fusarium sp. and 13 belonging to Trichoderma sp., while in the second sampling season a total of 20 isolates were obtained, six belonging to Fusarium sp., and 14 belonging to Trichoderma sp. and one to Hypocrea lixii (Table 3 and 4).
In the Fusarium oxysporum isolates, isolated clamidospores, short conidiophores in monophialides, macroconidia with three or four fusiform or canoe-shaped transverse septa, microconidia abundant in aerial mycelium were observed, while for the Trichoderma genus, subglobital conidiophores, attenuated at the tip , the unicellular clamidospores of globose form found in intercalar and terminal form, conidia of ellipsoidal form and green tonality. Characteristic that coincide with those described by Chaverri et al. (2003).
Quantification of Fusarium and Trichoderma
Amount of CFU g-1 soil in PDA and K2 medium: the presence of Trichoderma sp. in the samples taken in both seasons and both media; however, it was difficult to count due to its rapid growth, which caused the loss of the shape of the colony, generating doubt if it came from one or more spores. In this regard, Al-Sadi et al. (2015) state that Trichoderma sp. species are generally characterized by their rapid growth and ability to survive in variable environmental conditions, in addition to varying their diversity of species from one substrate to another.
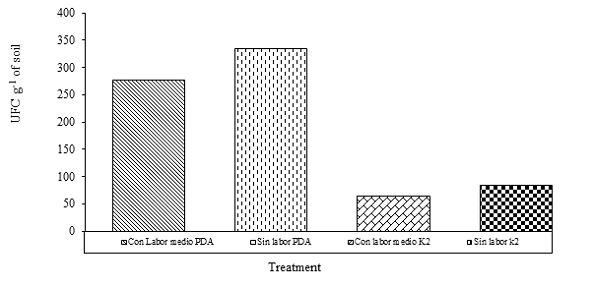
On the other hand, the analysis of variance showed significant differences (p< 0.001) between the averages obtained from the treatments. According to the minimum significant difference test (LSD), the PDA was identified as the medium that showed the best result in isolation in both samples (with and without labor) compared to the K2 medium in terms of growth and development of Fusarium oxysporum; that is, there was an average value greater than 200 CFU g-1 of soil, while treatments with K2 medium the average was between 50 and 100 CFU g-1 of soil (65% higher in PDA than the amount found in K2 medium) (Figure 1).
This suggests that the PDA medium provides the appropriate levels of nutrients, as well as the appropriate conditions during the development of both fungi (López et al., 2004), while the K2 although it has a large number of nutritional elements It is affected by other factors such as pH, fungicides and antibiotics as mentioned by Komada (1975) limiting its development.
However, although authors such as Bragulat et al. (2004) mention that Fusarium sp., does not have large limitations in growth in artificial media there are components that affect its characteristic sporulation (López et al., 2004). In this case, the tone of the Fusarium colonies obtained in PDA was very similar between genders in the first 48 hours of emergency, which made the UFC count difficult.
Similar studies were obtained by Benaouali et al. (2014) when working with Fusarium oxysporum f. sp. radicis lycopersici, who observed better spore growth and pigmentation in the PDA medium compared to other media evaluated. Hence the importance of rapid growth and sporulation in the culture media used because they favor the early identification of fungi preventing CFU from joining.
Although in this study there was fluctuation in the number of isolated CFU per sampled site, it was presented in both media used during the isolation process. These results suggest that there are other factors not analyzed in the study that affect the amounts of CFU specifically per soil site that have no relation to the culture medium. Similar results were obtained by Jiménez-Fernández et al. (2010) who when doing studies of identification and quantification of Fusarium oxysporum in soil finding that, in samples obtained directly from field soils, populations varied in some of their replicas of isolation compared to artificially infested soils.
Amount of CFU g-1 of soil with respect to cultural work: the statistical analysis showed significant differences (p= 0.0352) between treatments, and at least two of them were different (Table 1). The treatment without labor and with PDA medium obtained the highest concentration of CFU g-1 of soil with a difference of 68% greater than treatment four.
Table 1 Analysis of the effect of cultural work on the amount of CFU g-1 of soil.
| Treatment | (CFU g-1 of soil) |
| 1. Without labor +PDA | 420 a |
| 2. Without labor +K2 | 270 ab |
| 3. With labor +PDA | 270 ab |
| 4. With labor +K2 | 135 b |
| LSD | 176.28 |
Means with equal letters are not statistically different, according to the LSD test.
However, although the concentration obtained in the treatments varied among them, statistically the treatments one, two, three and two, three, four were similar indicating the lack of values that show the effectiveness of the cultural work. The effect of agricultural management practices was observed, specifically re-incorporation of senescent plant residues and fungicide application, changing the structure and genetic diversity of the Fusarium communities.
Decomposing plants as a source of nutrients for Fusarium, which can grow saprophytically generating greater abundance and distribution of species in the soil. However, it is necessary to mention that in their study they sampled these soils for five years, unlike the present study where only two seasons were sampled in one year, resulting in a difference in the data obtained.
Amount of CFU g-1 with respect to the seasons of sample collection and climatic conditions: the greatest amount of CFU in the soil was detected in the sampling carried out in the month of December (first season) compared to the month of April where there is a drastic decrease in the amount of CFU, and even in this last season no spores were found in some sampled points (Table 2).
Table 2 Quantity CFU g-1 present in PDA medium and climatic variables of the municipality.
| Características | December (2017) | April (2018) |
| CFU g-1 of soil (medium PDA) | 1098.62 | 297.93 |
| CFU g-1 of soil (medium K2) | 180 | 74.48 |
| Precipitation (mm monthly-1) | 116.2 | 276.8 |
| Average temperature (°C) | 19.38 | 24.34 |
| Relative humidity (%) | 86.09 | 86.83 |
| Radiation (W m-²) | 323.14 | 386.14 |
These results coincide with the increase in temperature, precipitation and solar radiation in the second season of sample collection. The temperature and precipitation present in the sampling seasons can have a significant influence on the diversity of fungal species in a given area.
In this regard, Benaouali et al. (2014), also emphasizes the importance of temperature in the optimal growth of F. oxysporum f. sp. radicis lycopersici on the soil. However, although in both seasons of study the changes in relative humidity were almost nil, there was more solar radiation in the second season which also coincided with a decrease in the amount of CFU, in this context Pinkerton, (2000) mentioned that the amount of absorbed solar radiation reduces the population density of fungi found over 30 cm of the soil surface.
Determination of the presence of Fusarium oxysporum f. sp. cubense race 1
Three plants were observed with slightly necrosed corms, one of these was inoculated by F. oxisporum f. sp. cubensis, while the remaining two were inoculated with F. oxisporum f. sp. melonis according to the identification result of the PCR tests of these isolates. According to Dita et al. (2018) susceptible cultivars such as the AAB group show more severe symptoms or damage than varieties with intermediate resistance when grown under similar environmental conditions and depending on the inoculum pressure and environmental conditions the disease in the plant can be observed early.
Molecular identification and determination of genetic diversity of Trichoderma and Fusarium between species and within the most abundant species
The amplification of the PCR products allowed to visualize in the agarose gel 700 bp for the genus Fusarium sp. and 350 bp for Trichoderma sp. The sequences of identified species were recorded in the National Center for Biotechnology Information (BCNI) (Table 3 and 4). Greater abundance of species was observed in the first season, unlike the second.
Table 3 Isolates obtained in the first sampling season.
| Sample | Isolated | Species | NCBI |
| 1 | VB1IF1 | Fusarium oxysporumvoucher | MK087011 |
| 2 | VB2IF1 | Fusarium oxysporumf. sp.radicis-cucumerinum | MK087012 |
| VB2IIF1 | Fusarium oxysporumf. sp.pisi | MK087013 | |
| 6 | VB6IT1 | Trichoderma spirale | MK086990 |
| 7 | VB7IF1 | Fusarium oxysporumf. sp. | MK087014 |
| VB7IT1 | melonis Trichoderma spirale | MK086984 | |
| 8 | VB8IT1 | Trichoderma longibrachiatum | MK086985 |
| 9 | VB9IT1 | Trichoderma harzianum | MK086986 |
| 10 | VB10IF1 | Fusarium oxysporumf. sp.melonis | MK087015 |
| VB10IT1 | Trichoderma parareesei | MK086987 | |
| 13 | VB13IF1 | Fusarium oxysporumf. sp.melonis | MK087031 |
| 14 | VB14IF1 | Fusarium oxysporumf. sp.radicis-cucumerinum | MK087016 |
| 15 | VB15IF1 | Fusarium oxysporumf. sp.melonis | MK087017 |
| VB15IIF1 | Fusarium oxysporumf. sp.pisi | MK087018 | |
| VB15IT1 | Trichoderma longibrachiatum | MK086988 | |
| 16 | VB16IT1 | Trichoderma longibrachiatum | MK086989 |
| 19 | VB19IF1 | Fusariumsp. | MK087019 |
| 20 | VB20IF1 | Fusariumsp. | MK087020 |
| 21 | VB21IF1 | Fusarium oxysporumf. sp.melonis | MK087021 |
| VB21IT1 | Trichoderma orientale | MK086997 | |
| 22 | VB22IF1 | Fusariumsp | MK087022 |
| VB22IIF1 | Fusarium oxysporumf. sp.melonis | MK087023 | |
| 25 | VB25IT1 | Trichoderma longibrachiatum | MK086998 |
| 26 | VB26IF1 | Fusarium oxysporum | MK087024 |
| VB26IT1 | Fusarium oxysporumf. sp.melonis | MK086991 | |
| 27 | VB27IF1 | Fusarium oxysporumf. sp.radicis-cucumerinum | MK087025 |
| VB27IT1 | Trichoderma longibrachiatum | MK086992 | |
| 28 | VB28IT1 | Trichoderma spirale | MK086999 |
| 29 | VB29IT1 | Trichoderma longibrachiatum | MK086993 |
National Center for Biotechnology Information (BCNI).
Table 4 Isolates obtained in the second sampling season.
| Sample | Isolated | Species | NCBI |
| 2 | VB2IT2 | Trichoderma spirale | MK087001 |
| VB2IIT2 | Trichoderma spirale | MK087002 | |
| 3 | VB3IF2 | Fusarium oxysporumf. sp.cubense | MK087032 |
| 6 | VB6IF2 | Fusariumsp. | MK087026 |
| VB6IT2 | Hypocrea lixii | MK087033 | |
| 7 | VB7IF2 | Fusarium oxysporumf. sp.vasinfectum | MK087027 |
| 8 | VB8IF2 | Fusariumsp. | MK087028 |
| VB8IT2 | Trichoderma spirale | MK087003 | |
| 9 | VB9IF2 | Fusarium oxysporum | MK087029 |
| 10 | VB10IT2 | Trichoderma parareesei | MK087004 |
| VB10IIT2 | Trichodermasp. | MK087005 | |
| 11 | VB11IT2 | Trichoderma spirale | MK087006 |
| 15 | VB15IT2 | Trichoderma spirale | MK086994 |
| 18 | VB18IT2 | Trichoderma koningiopsis | MK086995 |
| 21 | VB21IT2 | Trichoderma hamatum | MK086996 |
| 22 | VB22IF2 | Fusarium oxysporum | MK087030 |
| VB22IT2 | Trichoderma koningiopsis | MK087007 | |
| 24 | VB24IT2 | Trichoderma spirale | MK087008 |
| 25 | VB25IT2 | Trichoderma spirale | MK087000 |
| 27 | VB27IT2 | Trichoderma spirale | MK087009 |
| 29 | VB29IT2 | Trichoderma spirale | MK087010 |
National Center for Biotechnology Information (BCNI).
The species that was found in greater abundance in the first season was Fusarium oxysporum f. sp. melonis with 20%, while in the second one this special form was not found and only four samples were obtained with the Fusarium genus, being isolated from one of them Fusarium oxysporum. F. sp. cubense (Figure 2 and 3). According to Suárez-Estrella (2004), this fungus is able to survive in crop residues, in this case it was found in May, and in soil where no cultivation work was performed.
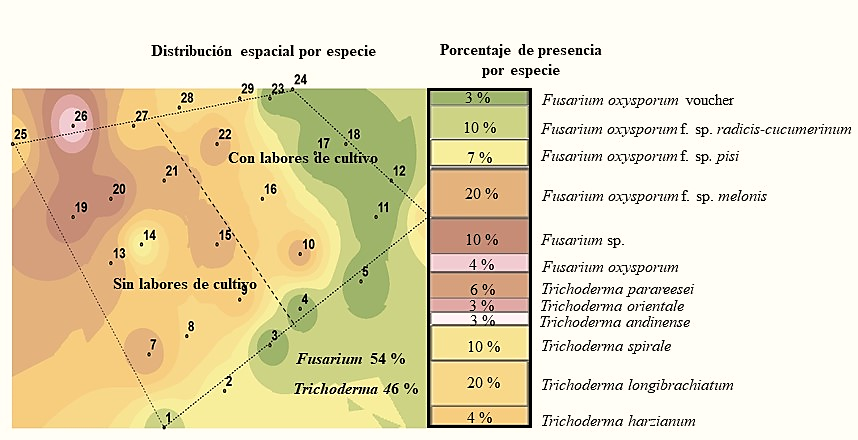
Figure 2 Species of the Trichoderma and Fusarium genus detected in the soil in the first sampling season.
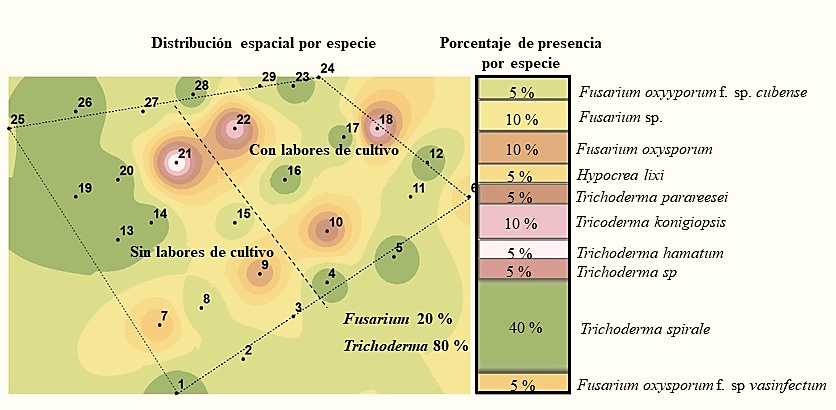
Figure 3 Species of the Trichoderma and Fusarium genus detected in the soil in the second sampling season.
However, in the second sampling season there was a reduction of both the Fusarium genus and the aforementioned species (Figure 2 and 3) and the maintenance of the population density of the Trichoderma genus was observed with 20% presence of Trichoderma longibrachiatum in the first season and 40% of Trichoderma spirale in the second; that is, twice the presence of the latter species. In this context, the species of the Trichoderma genus have a high saprophytic capacity while the majority of Fusarium species are phytopathogenic, so their degree of adaptation is different (Maina et al., 2016).
The DNA sequences analyzed for a Bootstrap above 80% inferred from maximum parsimony (MP), maximum Likelihood (ML) and Neighborhood Joining (NJ) showed a phylogenetic arrangement for both genus (Figure 4 and 5) which grouped isolated sequences of both seasons with 99% similarity; however, it did not show the relationship between kinship and its presence by season or with the cultivation work.
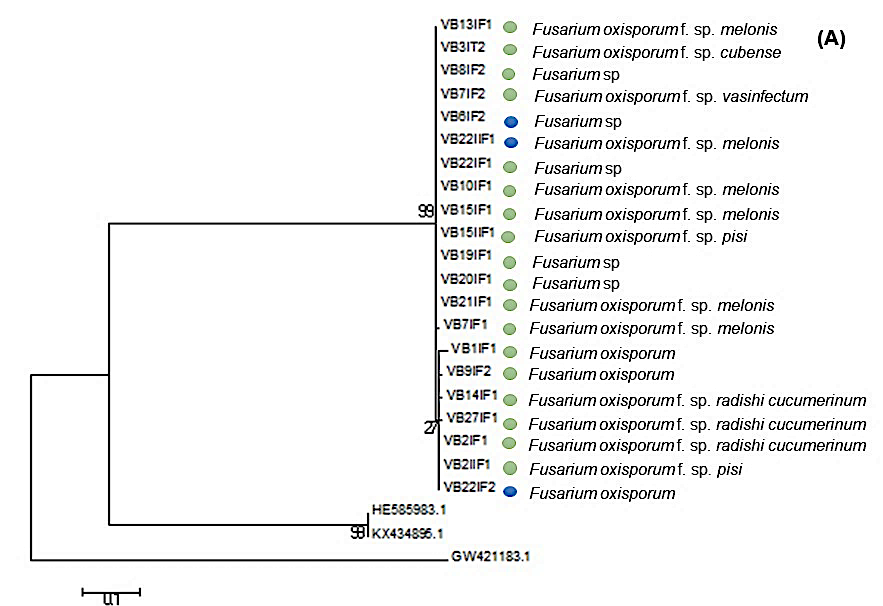
Figure 4 Phylogeny of Fusarium oxysporum species as a result of data analysis of EF1-∝. Bostrap values above 80% inference of maximum parsimony (MP), maximum likelihood (ML) and Neighborhood Joining (NJ). The blue circle indicates that the isolate was obtained from the area where cultivation work was carried out, while the green circle indicates that the isolate was obtained from soil without cultivation work.

Figure 5 Phylogeny of species of Trichoderma sp. as a result of the data analysis of EF1-∝. Bostrap values above 80% inference of maximum parsimony (MP), maximum likelihood (ML) and Neighborhood Joining (NJ). The blue circle indicates that the isolate was obtained from the area where cultivation work was carried out, while the green circle indicates that the isolate was obtained from soil without cultivation work.
These results also showed a greater genetic diversity of species of the Trichoderma genus with an average of the interpopulation diversity of 5.75 obtained from 20 sequences of seven populations of the Trichoderma genus and a differentiation coefficient of 4.27 obtained from a bostrap of 1 000 replicas. While for Fusarium, interpopulation diversity averaged 2.14 with a differentiation coefficient of 4.66 obtained from 21 sequences from eight populations belonging to the genus.
According to studies conducted by Maina et al. (2016) found a negative correlation between the occurrence and diversity of Trichoderma sp and Fusarium sp., in undisturbed areas they observed a high abundance of Trichoderma sp. and low occurrence of Fusarium and conversely in disturbed areas where there was greater occurrence of Fusarium than Trichoderma sp. In this sense we observe a greater number of species from both Fusarium and Trichoderma sp. in soils without cultivation, there is a reduction for the Fusarium genus when doing cultivation work and little influence of these tasks on the amount of Trichoderma species (Figure 4 and 5).
The diversity results of the eight Trichoderma spirale isolates, which was the species that presented the greatest abundance, showed a low intrapopulation diversity observed in the expression of the locis, because only in the VB28IT1 isolate obtained from site 33 bands were amplified which they showed a size between 75 to 500 bp.
In this case, a total of seven bands were amplified from five ISSR; one of the GA9T fragment, five of the ACC6 fragment and one of the GA9C fragment while the CA8RG and GTG5 fragments did not show amplification in any of the isolates. Two populations or more that descend from a common ancestor, have allelic frequencies identical to their ancestor. This is possible when analyzing populations living in moderately small spaces in which there is little incidence of factors or barriers that influence the pressure of genetic selection within the species.
Conclusions
This study reiterates the importance of the proper use of the culture medium for the isolation of species of the Trichoderma and Fusarium genus in soil, because the truthfulness of the results when doing population density studies, as well as population density of abundance and diversity of species. However, in open-field studies the effect of climatic factors, as well as that of cultural activities and times of sample extraction, also influence this type of study.
Finding in these results a greater diversity and abundance of species of both genus in soils where no cultivation work is carried out, in addition to the scarce influence of such work on the amount of Trichoderma species, which showed a succession of T. longibrachiatum by T. spirale from the first to the second season. This sequence and domain expose the biocontrol activity, which could maintain the stability of the populations of the Fusarium genus, especially F. oxysporum f. sp. cubense in banana.
Acknowledgments
To the General Directorate of Higher Education SEP, who through the Department of Academic Improvement (DSA) and program for the professional development of teachers for the higher type (PRODEP) funded this work.
REFERENCES
Bateman, G. L. and Murray, G. 2001. Seasonal variation in population of Fusarium species in wheat-field soil. Appl. Soil Ecol. 18(2):117-128. [ Links ]
Benaouali, H.; Hamini, N.; Bouras, A.; Benichou, S. L.; Mebrouk, K. and Henni, J. E. 2014. Isolation, pathogenicity test and physicochemical studies of Fusarium oxysporum f. sp radicis lycopersici. Adv. Environ. Biol. 8(10):36-49. [ Links ]
Bentley, S.; Pegg, K. G.; Moree, N. Y.; Davis, R. D. and Buddenagen, I. W. 1998. Genetic variation among vegetative compatibility groups of Fusarium oxysporum f. sp. cubense Analyzed by DNA. Fingerprintin. 88(12):1283-1293. [ Links ]
Bernhoft, A.; Torp, M.; Clasen, P. E.; Loes, A. K. and Kristoffersen, A. B. 2012. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Additives y Contaminants. 29(7):1129-1140. [ Links ]
Bragulat, M.; Martínez, E.; Castellá, G. and Cabañes, F. 2004. Selective efficacy of culture media recommended for isolation and enumeration of Fusarium spp. J. Food Protec. 67(1):207-11. [ Links ]
Carbone, I. and Kohn, L. M. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 91(3):553-556. [ Links ]
Chaverri, P.; Castlebury, L. A.; Overton, B. E. and Samuels, G. J. 2003. Hypocrea/Trichoderma: species with conidiophore elongations and green conidia. Mycologia. 95(6):1100-1140. [ Links ]
Dita, M. A.; Waalwijk, C.; Paiva, L. V.; Souza, Jr. M. T. and Kema, G. H. J. 2011. A greenhouse bioassay for the Fusarium oxysporum f. sp. cubense x ‘Grand Naine’ (Musa, AAA, Cavendish subgroup) interaction. V International Bananas Symposium: ISHS-ProMusa Symposium on Global Perspectives on Asian Challenges, Guangzhou, China. Acta Hortic. 897:377-380. [ Links ]
Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E. S and Staver, C. P. (2018) Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Frontiers Plant Sci. 9:1-21. doi: 10.3389/fpls.2018.01468. [ Links ]
García-Núñez, H. G.; Martínez-Campos, A. R.; Hermosa-Prieto, M. R.; Monte-Vázquez, E.; Aguilar-Ortigoza, C. J. and González-Esquivel, C. E. 2016. Morphological and molecular characterization of native isolates of Trichoderma and its potential biocontrol against Phytophthora infestans. Rev. Mex. Fitopatol. 35(1):58-79. [ Links ]
Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 41(1):95-98. [ Links ]
Jiménez-Fernandez, D.; Montez-Borrego, M.; Navas-Cortés Juan, A.; Jiménez-Díaz, R. M.; Landa, B.; B. 2010. Identification and quantification of Fusarium oxysporum in planta and soil by means of an improved specific and quantitative PCR assay. Appl. Soil Ecol. 46(3):372-382. [ Links ]
Komada, H. 1975. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Plant Prot. Res. 8(3):114-125. [ Links ]
Leong, S. K.; Latiffah, Z. and Baharuddin, S. 2010. Genetic diversity of Fusarium oxysporum f. sp. cubense isolates from Malaysia. Afr. J. Microbiol. Res. 4(11):1026-1037. [ Links ]
López, D. and López, M. O. 2004. Influencia de los medios de cultivo en la expresión de los caracteres de valor diagnóstico de las especies del género Fusarium en Cuba. Fitosanidad. 8(3):7-11. [ Links ]
Maina, P. K.; Wachira, P. M.; Okoth, S. A.; Kimenju, J. W. and Mwangi, J. M. 2016. Co-occurrence and Diversity of Soil Trichoderma and Fusarium species from different land use intensities in Machakos County, Kenya. Arch. Current Res. Inter. 4(1):1-13. [ Links ]
McDonald, B. A. and Linde, C. 2002. Pathogen population genetics, evolutionary potential, and durable resistance. Annual Rev. Phytopathol. 40(1):349-379. [ Links ]
Nirmaladevi, D.; Venkataramana, M.; Srivastava, R. K.; Uppalapati, S. R.; Gupta, V. K.; Yli-Mattila, T. and Chandra, N. S. 2016. Molecular phylogeny, pathogenicity and toxigenicity of Fusarium oxysporum f. sp. lycopersici. Nature. 6:1-14. https://www.nature.com/articles/ srep21367.pdf. [ Links ]
Orozco Santos, M.; García Mariscal, K. y Vázquez Jiménez, J. L. 2009. Estado actual del mal de panamá en musáceas en México. In: Reunión de grupos de interés sobre los riesgos de la raza tropical 4 de Fusarium, BBTV y otras plagas de musáceas para la región del OIRSA, América Latina y El Caribe. San Salvador, El Salvador. Resúmenes. Bioversity. 71 p. [ Links ]
Pinkerton, J. N. 2000. Effect of soil solarization and cover crops on populations of selected soilborne plant pathogens in Western Oregon. Plant Dis. 84(9):952-96. [ Links ]
Ploetz, R. C. 2015. Fusarium wilt of banana. Phytopathology. 105(12):1512-1521 [ Links ]
Retana, K.; Ramírez-Coché, J. A.; Castro, O. y Blanco-Meneses, M. 2018. Caracterización morfológica y molecular de Fusarium oxysporum f. sp. apii asociado a la marchitez del apio en costa rica. Agron. Costarric. 42(1):115-126. [ Links ]
Sánchez-López, V.; Martínez-Volañoz, L.; Zavala-González, E. A. y Ramírez-López, M. 2012. Nuevos registros de Trichoderma crassum para México y su variación morfológica en diferentes ecosistemas. Rev. Mex. Micol. 36(2):17-26. [ Links ]
Suárez-Estrella, F.; Vargas García, M.; López, M. and Moreno, J. 2004. Survival of Fusarium oxysporum f. sp. melonis on plant waste. Crop Protection. 23(2):127-133. 10.1016/j.cropro.2003.07.006. [ Links ]
Tamura, K.; Paterson, D.; Peterson, N.; Stecher, G.; Nei, M. and Kumar, S. 2011. MEGA5: Molecular genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28(10):2731-2739. [ Links ]
Thangavelu, R.; Muthu, M. and Mustaffa, M. 2011. Genetic Diversity of Fusarium oxysporum f. sp. cubense Isolates (Foc) of India by Inter Simple Sequence Repeats (ISSR) Analysis. Mol. Biotechnol. 51:203-11. [ Links ]
Received: June 01, 2019; Accepted: October 01, 2019











 text in
text in