Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 spe 20 Texcoco Apr./May. 2018
https://doi.org/10.29312/remexca.v0i20.984
Articles
Application of a film HPMC-paraffin on melon: effect on aromatics and activity of pectinmethylesterase
1Faculty of Chemical Sciences Gómez Palacio-Universidad Juárez of the state of Durango. Avenida article 123 S/N, subdivision Philadelphia CP. 35010, Gómez Palacio, Durango, Mexico. (jresparza02001@yahoo.com; ericksier@gmail.com; luna-141110@hotmail.com; conyavalos@yahoo.com.mx; jorgemezav68@ gmail.com).
2Department of Chemistry-University of the Balearic Islands. Road Valldemossa km 7.5, Palma de Mallorca, Spain. CP. 07122. (rafaelminjares@gmail.com).
The melon is a fruit appreciated for its aroma and flavor, but it has a short storage period. An alternative to extend this period is the use of edible films. In the present study, the effect of the application of an edible film of hydroxypropylmethylcellulose-paraffin (HPMC-PAR) on aromatic components and the activity of melon pectinmethylteterase stored in refrigeration was evaluated. Cantaloupe melon fruits were covered with an edible film of HPMC-PAR and non-covered melons were taken as control. The melons were stored for 20 days at 8 ºC and 80% relative humidity. Every four days, the fruits were subjected to analysis of the concentration of ethylbutyrate, ethylcaproate, butylacetate, benzyl alcohol, methylbutanol and pectylmethylteterase activity (PME). The results showed that melons with film had a higher concentration of the analyzed esters, as well as a lower activity of PME compared to samples without film. However, the alcoholic compounds were not affected by the treatments. Therefore, the application of the film based on HPMC-paraffin promotes the increase of ester compounds during the first days of storage in refrigeration. In addition, the activity of PME can influence the amount of these aromatic compounds of the Cantaloupe melon pulp.
Keywords: aromas; hydroxypropylmethylcellulose; melon; pectinmetillesterase
El melón es un fruto apreciado por su aroma y sabor, pero tiene un corto periodo de almacenamiento. Una alternativa para extender este periodo es el uso de películas comestibles. En el presente estudio se evaluó el efecto de la aplicación de una película comestible de hidroxipropilmetilcelulosa-parafina (HPMC-PAR) sobre componentes aromáticos y la actividad de la pectinmetilesterasa de melón almacenado en refrigeración. Frutos de melón Cantaloupe se cubrieron con una película comestible de HPMC-PAR y melones no cubiertos se tomaron como control. Los melones se almacenaron por 20 días a 8 ºC y 80% de humedad relativa. Cada cuatro días, los frutos se sometieron a análisis de concentración de etilbutirato, etilcaproato, butilacetato, benzilalcohol, metilbutanol y actividad de pectilmetilesterasa (PME). Los resultados mostraron que los melones con película tuvieron una mayor concentración de los ésteres analizados, así como una menor actividad de PME en comparación a muestras sin película. Sin embargo, los compuestos alcohólicos no fueron afectados por los tratamientos. Por lo que, la aplicación de la película a base de HPMC-parafina promueve el aumento de compuestos ésteres durante los primeros días de almacenamiento en refrigeración. Además, la actividad de la PME puede influir en la cantidad de estos compuestos aromáticos de la pulpa del melón Cantaloupe.
Palabras claves: aromas; hidroxipropilmentilcelulosa; melón; pectinmetilesterasa.
Introduction
The Cantaloupe melon is a fruit appreciated for its characteristic aroma and flavor which are decisive for the sensory and commercial quality of the product (Kourkoutas et al., 2006). The aroma and flavor of melon are attributed to its content of volatile aromatic compounds in the pulp, which include esters and alcohols (Aubert et al., 2005), although in some melon varieties up to 240 compounds have been determined by means of gas chromatography (Kourkoutas et al., 2006).
Among the aromatic compounds most abundant in this fruit are esters such as ethylbutanoate, ethylcaproate, and 3-hexyl-2-butanoate, and to a lesser extent sulfur derivatives, aldehydes and alcohols (Obando-Ulloa et al., 2010). On the other hand, the Cantaloupe melon has a short shelf life of 10-15 days (Suslow et al., 2002), therefore, it is necessary to evaluate conservation methods that contribute to maintaining the sensory quality of this fruit during longer storage periods, such as the application of covers or films.
The covers (solutions and emulsions) based on edible polymers such as polysaccharides, proteins or lipids have been applied to various plant products (Bonilla et al., 2013). The application of films on melon has the potential to reduce moisture loss (Reyes et al., 2016), firmness (Reyes et al., 2017), and product respiration rate (Reyes et al., 2016). Likewise, edible covers can protect the product against mechanical, oxidative and microbiological damages, in addition to improving the appearance and preventing the loss of fruit and vegetable aromas (Genskowsky et al., 2015).
The greatest benefit of the use of films and covers is that they can be consumed together with the food product in which they are applied, said films being able to be enriched with compounds that contribute beneficial to health or that improve the sensory properties of the covered food (Perdones et al., 2012). Some polysaccharides used for preparing edible covers are sodium alginate, carrageenan and carboxymethylcellulose (Hamzah et al., 2013; Tavassoli-Kafrani et al., 2016).
Hydroxypropylmethylcellulose (HPMC) is a polymer derived from cellulose used in the formulation of films, which has been tested on various vegetables, resulting in less damage by cold, preservation of firmness and less weight loss in these products (Reyes et al., 2017). In addition, edible films delay the ripening process of fruits (Reyes et al., 2016) so, if the covers can diminish the activity of the pectic enzymes, such as pectinmetillesterase, the fruit will maintain the firmness of the fruit for a longer time your tissues.
The objective of this work was to evaluate the effect of the application of an HPMC-Paraffin film on the concentration of aromatic compounds and the PME activity of the Cantaloupe melon.
Materials and methods
Experimental samples
Cantaloupe melon fruits (Cucumis melo L. var. reticulates) collected in Ceballos, Durango (geographical coordinates latitude 26.526 and length -104.129) in pre-climacteric stage (25 to 27 days after pollination) were used according to the method published by Nishiyama et al. (2007). The fruits were selected in the mature three-quarter maturity state, of similar size and dimensions (fruits of 1.2 to 1.5 kg free of physical damage). The production process was drip irrigation.
Materials and reagents
The HPMC (C56H108O30) was donated by Colorcon (Mexico). The following reagents were purchased from Sigma-Aldrich (St. Louis MI, USA) Sodium metabisulphite, potassium sorbate, propylene glycol monostearate, polygalacturonic acid D-methyl, Tris, ammonium sulfate, sodium dodecyl sulfate, Triton X-100, bromothymol blue and ethylbutyrate, ethylcaproate, butylacetate, benzyl alcohol and methylbutanol standards. Analytical grade paraffin was used (Monterrey, Nuevo León, Mexico).
Treatments
The collected melons were selected free from physical damage and apparent microbial contamination. They were washed with chlorinated water at 200 ppm of sodium hypochlorite, and randomly distributed in two batches: control treatment (without film application), and film (fruits covered with the HPMC-paraffin film). The preparation and application of the HPMC-paraffin film was previously described in Meza et al. (2013). All fruits were then stored in a refrigerator at 8 ±2 °C with relative humidity of 80 ±4% for 20 days. Subsequently, the melons were analyzed every four days throughout the study period (0, 4, 8, 12, 16 and 20 days). The analyzes performed were concentrations of ethylbutyrate, ethylcaproate, butylacetate, benzyl alcohol, methylbutanol and the activity of pectinmethylterasesterase (PME) carried out in triplicate. The treatments (control and film) were repeated eight times and each repetition contained 24 melons.
Analysis of volatile compounds
The analysis was developed with the method proposed by Aubert et al. (2005) with some modifications. A sample composed of 125 g of melon from each of the replicates of the treatments was mixed with 125 ml of n-propyl gallate (10 mM) and ground in a mixer for 2 min, then homogenized for one min with the ultraturrax. The mixture was centrifuged (4 500 rpm, 20 min, 4 °C) and the supernatant was collected.
To carry out the recovery percentage tests, 5 standard solutions of volatile compounds present in the melon were prepared; these solutions were prepared at 40 ppm in dichloromethane. Subsequently, 150 ml of the standard solution (containing ethylbutyrate, ethylcaproate, butylacetate, benzylalcohol and methylbutanol) or the extract obtained from the melon pulp were extracted three times with 50 ml of dichloromethane (3 x 15 min) under constant stirring a temperature of 4 ºC. Subsequently, the mixture was concentrated to 8 ml using a kuderna-danish equipment at 70 °C. The 8 mL were placed in a microkuderna and concentrated up to 1 mL at 45 ºC. The concentrate was injected into an HP 6820 gas chromatograph with a flame ionization detector and a DB-5 capillary column of 30 x 0.25 x 0.25 (Supelco, PA, USA).
The conditions of the chromatograph were 250 °C in the injector, 250 °C in the detector; the column was subjected to a program of 35 ºC of initial temperature with ramp of 5 ºC per min until reaching 150 ºC, the final temperature was maintained for 10 min. Chromatograph readings were recorded and analyzed with the Agilente Cerity NDS software and compared to a standard calibration curve for the compounds mentioned. The results were reported as mg kg-1 of fresh fruit.
Preparation of the sample for the activity of pectinmethylesterase
The analysis was carried out with the method described by Lamikanra and Watson (2004) with some modifications. Slices from the center of the fruit (equator) of approximately 80 x 30 x 2 mm of each treatment were used. To 40 g of slices of melon were added 80 mL of Tris buffer (pH 7.8, 0.05 M) and homogenized in a mixer for 2 min to be centrifuged at 4 ºC and 4 800 G for 30 min. The supernatant was mixed with ammonium sulfate so as to obtain a concentration thereof of 60% and placed in a freezer at -18 °C for 1 h. The mixture was then centrifuged at 4 °C and 4 800 G for 1 h. The supernatant was discarded and the residues were homogenized in 4 ml of Tris for 1 min. The mixture was centrifuged at 4 °C and 4 800 G for 1.5 h. The supernatant was the sample for assay of the enzymatic activity.
Activity of pectinmethylesterase
The test was carried out with a modification of the method proposed by Lamikanra and Watson (2003). A solution of D-methyl ester of polygalacturonic acid (0.1%) was prepared in a 0.4 M NaCl solution. The 0.01% bromothymol blue indicator was used in a potassium phosphate buffer. Before each reaction, the pectic solution (2.5 mL) was adjusted to a pH of 7.5 with 2 M NaOH. To the pectic solution was added the bromothymol blue (0.2 mL), 0.1 mL of the enzyme extract and vortex. The sample was read at an absorbance at 620 nm at 20 and 80 s to determine the rate of the reaction. The results were reported in relative enzymatic activity where 100% activity was from melon samples before treatments.
Experimental design and statistical analysis
A factorial design was used with two factors: storage time (0, 4, 8, 12, 16 and 20 days) and film application (without and with film), and eight repetitions per treatment were carried out. The results of the variables evaluated were analyzed by Analysis of Variance. The difference between treatment means was performed by the multiple comparison test of Fisher’s least significant difference (LSD Fisher) with a significance level of 0.05, using SAS statistical software version 8 (SAS Institute Inc., 2005).
Results and discussion
Volatile compounds
There were significant differences in the concentrations of ethylbutyrate, ethylcaproate and butylacetate between melons with and without film (p≤ 0.05). It was observed that in melons with film, the concentrations of ethylbutyrate and ethylcaproate showed a sudden increase after 4 days of storage (Figure 1 and 2), meanwhile the amount of butylacetate had a sudden increase until 8 days (Figure 3).
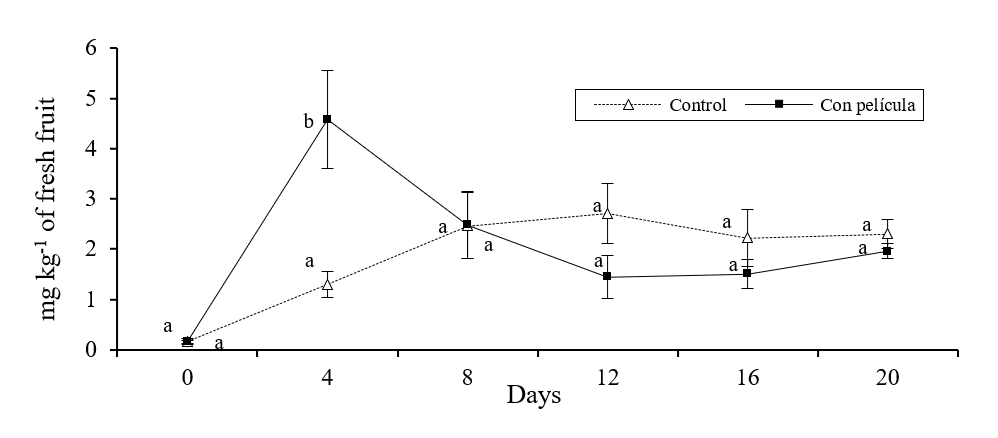
Figure 1 Concentration of ethylbutyrate (mg kg-1 of fresh solid) in melons with and without film stored for 20 days in refrigeration. Bars on the average of the results represents ± standard deviation (n= 4). Different literals indicate significant difference, by Fisher’s LSD (p≤ 0.05), between melons with and without film during the storage time.
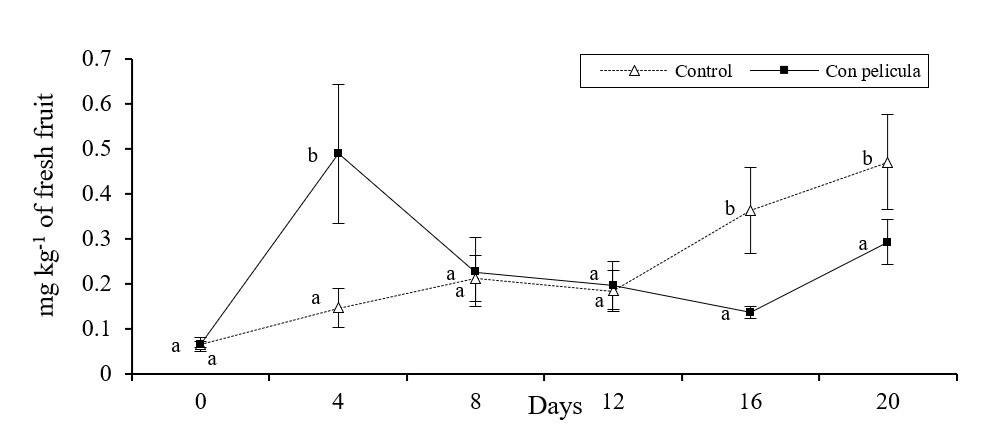
Figure 2 Concentration of ethylcaproate (mg kg-1 of fresh solid) in melons with and without film stored for 20 days in refrigeration. Bars on the average of the results represents ± standard deviation (n= 8). Different literals indicate significant difference, by Fisher’s LSD (p≤ 0.05), between melons with and without film during the storage time.
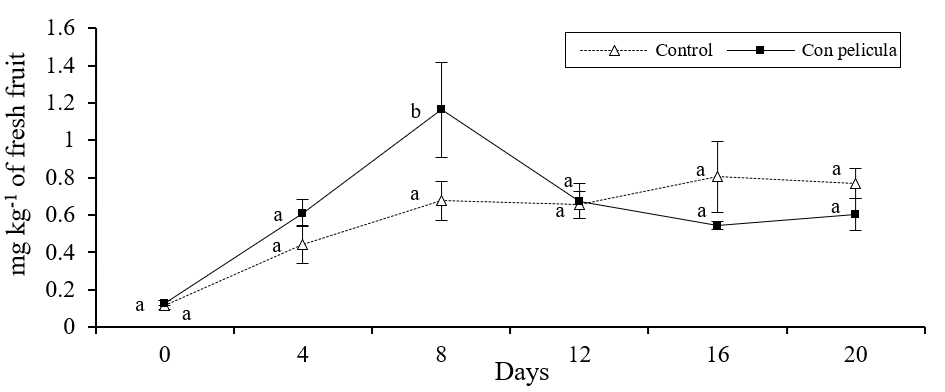
Figure 3 Concentration of butylacetate (mg kg-1 of fresh solid) in melons with and without film stored for 20 days in refrigeration. Bars on the average of the results represents ± standard deviation (n= 8). Different literals indicate significant difference, by Fisher's LSD (p≤ 0.05), between melons with and without film during the storage time.
The concentration of these compounds was significantly lower (p≤ 0.05) in the control fruits during the aforementioned periods of time. Likewise, the melons without cover showed a gradual increase in the concentration of the esters analyzed throughout the study period. In addition, the results obtained in the study showed that the concentration of the monitored alcoholic compounds did not change (p≤ 0.05) between the different treatments during storage. Similar results have been found when covering strawberries with a film of chitosan (Almenar et al., 2009) and mango with carnauba (Dang et al., 2008), the aforementioned authors managed to increase the concentration of aromas with the use of these covers.
Several studies have shown that the production or synthesis of aromatic compounds in fruits and vegetables (especially ester type) are directly related to the maturity and presence and concentration of ethylene (Günther et al., 2015; Li et al., 2016), so that the rapid increase observed in the amount of ester compounds may be related to the relatively high concentration of ethylene found inside the melons covered with the HPMC-paraffin film, previously reported (Meza et al., 2013). The presence of this hormone activates enzymes such as lipases, alcohol acyltransferase and alcohol acetyltransferase, which promote a greater synthesis of ester compounds responsible for aromas and flavors in fruits and vegetables (Hui et al., 2010. In contrast, it was observed that the concentrations of alcohols, present in apple, were not affected by the inhibition of ethylene synthesis (Dandekar et al., 2004).
Activity of pectinmethylesterase (PME)
The PME activity was lower in covered melons during the period of 8 to 16 days of storage (p≤ 0.05) (Figure 4). The fruits with film showed a relative activity of 25% (where 100% is the relative activity of the fruits without treatment at the initial time) after 8 days of storage; while samples without film had relative activity greater than the initial one of up to ~122% in the period of 12-16 days. It has been reported that the use of edible covers can decrease the enzymatic activity of vegetables by promoting the formation of internal modified atmospheres (Meza et al., 2013), which can slow down their maturation process (Reyes et al., 2016).
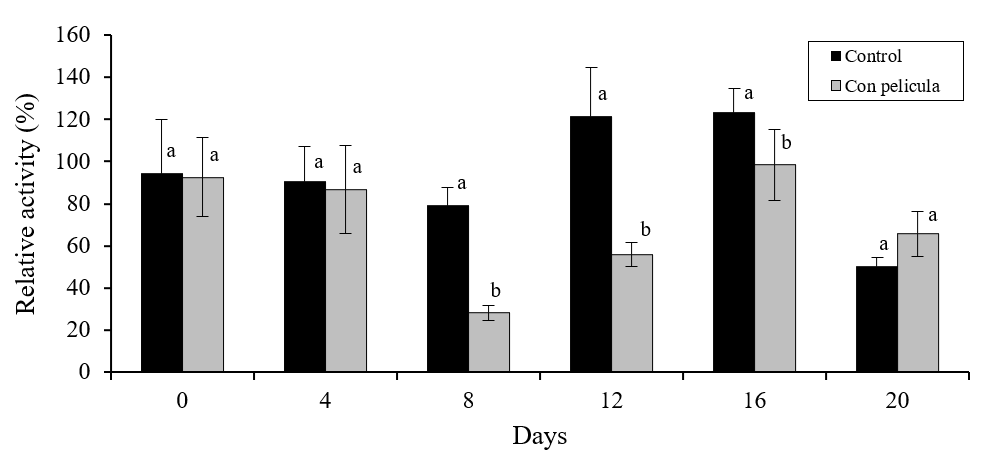
Figure 4 Relative activity of pectinmetillesterase in melon pulp treated with a polymeric coating and stored for 20 days in refrigeration. Bars on the average of the results represents ± standard deviation (n= 8). Different literals indicate significant difference, by Fisher’s LSD (p≤ 0.05), between melons with and without film during the storage time.
The function of the PME is to initiate the change and structural degradation of the pectins, which will directly affect the decrease in the firmness of the plant (Giovane et al., 2004). The synthesis of aromas and changes in texture during the maturation and senescence of fruits has a certain correlation due to changes in cell structure (Dos-Santos et al., 2013).
Some investigations agree that the aromas may be trapped or bound in the cellular structure formed by polysaccharides, mainly pectins (Bezman et al., 2003; Savary et al., 2006; Harker and Johnston, 2008), and probably structural changes may affect the release or accumulation of aromas in the tissue of the fruit, so that fruits with less structural changes, changes promoted by enzymes such as PME, may contain more aromatics.
Conclusions
The application of the HPMC-paraffin-based film accelerated the synthesis of ester compounds in melon during early stages of refrigerated storage. Also, this film decreased the activity of the PME, which can decrease structural changes in the tissues of the fruit and consequently improve the retention of volatile compounds.
Literatura citada
Almenar, E.; Hernández, M. P. and Gavara, P. 2009. Evolution of selected volatiles in chitosan-coated strawberries (Fragaria ananassa) during refrigerated storage. J. Agric. Food Chem. 57(3):974-980. [ Links ]
Bonilla, J.; Atarés, L.; Vargas, M. and Chiralt, A. 2012. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: possibilities and limitations. J. Food Eng. 110(2):208-213. [ Links ]
Dandekar, A. M.; Teo, G.; Defilippi, B. G.; Uratsu, S. L.; Passey, A. J.; Kader, A. A.; Stow, J. R.; Colgan, R. J. and James D. J. 2004. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 13(4):373-384. [ Links ]
Dang, K. H.; Singh, Z. and Swinny, E. E. 2008. Edible coatings influence fruit ripening, quality, and aroma biosynthesis in mango fruit. J. Agric. Food Chem . 56(4):1361-1370. [ Links ]
Dos-Santos, N.; Carmen, B. M. and Fernández, T. J. P. 2013. Aroma volatiles as biomarkers of textural differences at harvest in non-climacteric near-isogenic lines of melon. Food Res. Inter. 54(2):1801-1812. [ Links ]
Genskowsky, E.; Puente, L. A.; Pérez, A. J.A.; Fernández, L. J.; Muñoz, L. A. and Viuda, M. M. 2015. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristoteliachilensis). Food Sci. Technol. 64(2):1057-1062. [ Links ]
Giovane, A.; Servillo, L.; Balestrieri, C.; Raiola, A.; D’avino, R.; Tamburrini M. and Ciardiello L. 2004. Pectin methylesterase inhibitor. Biochimica et Biophysica Acta (BBA). Proteins and Proteomics. 1696(2):245-252. [ Links ]
Günther, C. S.; Marsh, K. B.; Winz, R. A.; Harker, R. F.; Wohlers, M. W.; White, A. and Goddard, M. R. 2015. The impact of cold storage and ethylene on volatile ester production and aroma perception in ‘Hort16A’ kiwifruit. Food Chem. 169(7):5-12. [ Links ]
Hamzah, H. M.; Osman, A.; Tan, Ch. P. and Mohamad- Ghazali, F. 2013. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika). Postharvest Biol. Technol. 75(2):142-146. [ Links ]
Harker, F. R. and Johnston, J. 2008. Importance of texture in fruit and its interaction with flavor. In: Brückner, B. and Wyllie, S. G. (Eds.). Fruit and vegetable flavour: recent advances and future prospects. Abington, Cambridge, UK: Woodhead Pub. Ltd. Chapter. 7. 254-271 pp. [ Links ]
Hui, G. T.; Bin, Y.; Curran, P. and Liu, Sh. Q. 2011. Lipase-catalysed synthesis of natural aroma-active 2-phenylethyl esters in coconut cream. Food Chem . 124(1):80-84. [ Links ]
Kourkoutas, D.; Elmore, J. S. and Mottram, D. S. 2006. Comparison of volatile compositions and flavour properties of cantaloupe Galia and honeydew musk melons. Food Chemistry. 97(1):95-102. [ Links ]
Lamikanra, O. and Watson, M. A. 2003. Temperature and storage duration effect on esterase activity in fresh-cut cantaloupe melon. J. Food Sci. 68(3):790-793. [ Links ]
Lamikanra, O. and Watson, M. A. 2004. Storage effect on lipase activity in fresh-cut cantaloupe melon. J. Food Sci . 69(2):126-130. [ Links ]
Li, Y.; Qi, H.; Jin, Y.; Tian, X.; Sui, L. and Qiu, Y. 2016. Role of ethylene in biosynthetic pathway of related-aroma volatiles derived from amino acids in oriental sweet melons (Cucumis melo var. makuwa Makino). Sci. Hortic. 201(1):24-35. [ Links ]
Meza, V. J. A.; Alanís, G. G.; García, D. C. L.; Fortis, H. M.; Preciado, R. P. and Esparza, R. J. R. 2013. Effect of a film of hydroxypropyl methylcellulose-paraffin in Cantaloupe melón (Cucumis melo) stored in cold. Rev. Mex. Cienc. Agríc. 4(2):259-271. [ Links ]
Nishiyama, K.; Guis, M.; Rose, J. K. C.; Kubo, Y.; Bennett, K. A.; Wangjin, L.; Kato, K.; Ushijima, K.; Nakano, R.; Inaba, A.; Bouyazen, M.; Latche, A.; Pech, J. C. and Benett, A. 2007. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. J. Exp. Bot. 58(6):1281-1290. [ Links ]
Obando, U. J. M.; Ruiz, J.; Monforte, A. J. and Fernández, T. P. 2010. Aroma profile of a collection of near-isogenic lines of melon (Cucumis melo L.). Food Chem . 118(3):815-822. [ Links ]
Perdones, A.; Sánchez, G. L.; Chiralt, A. and Vargas, M. 2012. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 70(3):32-41. [ Links ]
Reyes, A. M. C.; Femenia, A.; Minjares, F. R.; Contreras, E. J. C.; Aguilar, G. C. N.; Esparza, R. J. R. and Meza, V. J. A. 2016. Improvement of the quality and the shelf life of figs (Ficus carica) using an alginate-chitosan edible film. Food Bio. Technol. 9(12):2114-2124. [ Links ]
Reyes, A. M. C.; Minjares, F. J. R.; Esparza, R. J. R.; Contreras, E. J. C.; Montañez, S. J. C. y Meza, V. J. A. 2017. Calidad de melón cantaloupe (Cucumis melo) cubierto con una película comestible de alginato-hpmc-parafina. Nova Scientia. 18(9):222- 238. [ Links ]
SAS. 2005. Institute Inc. SAS/STAT User’s guide, version 8. Fourth Ed. Vol. 1 and 2. SAS Institute Inc., Cary, N.C. USA. [ Links ]
Savary, G.; Guichard, E.; Doublier, J. L. and Cayot, N. 2006. Mixture of aroma compounds: Determination of partition coefficients in complex semi-solid matrices. Food Res. Inter . 39(3):372-379. [ Links ]
Shalit, M.; Katzir, N; Tadmor, Y.; Larkov, O.; Burger, Y.; Shalekhet, F.; Lastochkin, E.; Ravid, U.; Amar, O.; Edelstein, M.; Karchi, Z. and Lewinsohn, E. 2001. Acetyl-CoA: alcohol acetyltransferase activity and aroma formation in ripening melon fruit. J. Agric. Food Chem . 49(2):794-799. [ Links ]
Suslow, T. V; Cantwell, M. and Mitchell, J. 2002. Melón Cantaloupe: (chino o de red) recomendaciones para mantener la calidad postcosecha. http://postharvest.ucdavis.edu/commodity-resources/fact-sheets/datastores/fruit-spanish/? uid=38&ds=802. [ Links ]
Tavassoli, K. E.; Shekarchizadeh, H. and Masoudpour, B. M. 2016. Development of edible films and coatings from alginates and Carrageenans. Carbohydrate Polymers. 137(4):360-374. [ Links ]
Received: January 00, 2018; Accepted: March 00, 2018











 text in
text in 


