Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 n.1 Texcoco Jan./Feb. 2018
https://doi.org/10.29312/remexca.v9i1.849
Articles
Combinatorial aptitude and resistance to leaf damage of Spodoptera rugiperda (J. E. Smith) in maize germplasm native to Tamaulipas
1Posgrado e Investigación de la Facultad de Ingeniería y Ciencias-Universidad Autónoma de Tamaulipas. Cuarto Piso del Centro de Generación de Conocimiento Centro Universitario Victoria, Cd. Victoria, Tamaulipas. CP. 87000, (resendizmorelos.mod4@gmail.com
2Genética-Colegio de Postgraduados, Montecillo, Edo. de México. (jpecina@colpos.mx; carmen@colpos.mx). CP. 56230.
3Campo Experimental Río Bravo-INIFAP. Carretera Matamoros-Reynosa km 6, Tamaulipas. CP. 88900. (reyes.cesar@inifap.gob.mx).
An agricultural pest of economic importance is Spodoptera frugiperda, in Tamaulipas it is common the incidence in corn, causing foliar damage which reduces the yield of grain, a solution to this problem is to use resistant cultivars; the native germplasm of Tamaulipas co-evolved with this insect, causing resistance to it. In order to evaluate the genetic effects and resistance of maize cultivars derived from native germplasm, foliar damage caused by S. frugiperda was determined in six inbred lines and their 30 crosses, under application conditions and non-application of insecticide in the Location of Güemez, Tamaulipas (spring-summer, 2015), a dialelic analysis was carried out with Griffing’s design I. For foliar damage, there were significant effects of general combinatorial aptitude (ACG), specific (ACE) and ACG interaction×insecticide treatment; there were only significant effects of ACG in the non-application treatment. The genetic expression of this germplasm determined by the mean values of the leaf damage showed a wide variation, the line LlHL5S3 had significant and negative effects of ACG and the crosses TGL2S3×LlHL5S3 the greater effects of negative ACE and less leaf damage (1.12), the crosses PWL1S3×TGL2S3, PWL1S3×LlNL4S3, TML3S3×LlNL4S3 and TML3S3×PWL6S3 excelled with leaf damage greater than 1.70; however, they had a reduction of less than 15.5% of grain yield. The variation of leaf damage depended on additive and non-additive effects and there was resistance to S. frugiperda, both due to non-preference and tolerance to leaf damage.
Keywords: Zea mays; armyworm; combinatorial ability; diallelic
Una plaga agrícola de importancia económica es S. frugiperda, en Tamaulipas es común la incidencia en maíz, provocando daño foliar lo que reduce el rendimiento de grano, una solución a esta problemática es utilizar cultivares resistentes; el germoplasma nativo de Tamaulipas a coevolucionado con este insecto, provocando resistencia al mismo. Con el objetivo de evaluar los efectos genéticos y la resistencia de cultivares de maíz derivados de germoplasma nativo, se determinó el daño foliar provocado por S. frugiperda en seis líneas endogámicas y sus 30 cruzas, en condiciones de aplicación y no aplicación de insecticida en la localidad de Güémez (primavera- verano, 2015), se realizó un análisis dialélico con el diseño I de Griffing. Para el daño foliar hubo efectos significativos de aptitud combinatoria general (ACG), específica (ACE) e interacción ACG×tratamiento de insecticida; solo existieron efectos significativos de ACG en el tratamiento de no aplicación. La expresión genética de este germoplasma determinada por los valores medios del daño foliar mostró una amplia variación, la línea LlHL5S3 tuvo efectos significativos y negativos de ACG y la cruza TGL2S3×LlHL5S3 los mayores efectos de ACE negativos y un menor daño foliar (1.12), las cruzas PWL1S3×TGL2S3, PWL1S3×LlNL4S3, TML3S3×LlNL4S3 y TML3S3×PWL6S3 sobresalieron con daño foliar mayor a 1.7; sin embargo, tuvieron una reducción inferior a 15.5% del rendimiento de grano. La variación del daño foliar dependió de efectos aditivos y no aditivos y existió resistencia a S. frugiperda, tanto por no preferencia como por tolerancia al daño foliar.
Palabras clave: Zea mays; aptitud combinatoria; dialélico; gusano cogollero
Introduction
The agricultural regions of the center and south of Tamaulipas, present environmental conditions of dry tropics, humid and High Valleys (Castro et al., 2013) and varied and extreme climate, for which, the maize is established under diverse systems of production (Resendiz et al., 2014), in some of these we use native germplasm with high variability, resistance to environmental stress conditions and grain yield potential, among other desirable characteristics (Pecina et al., 2011).
Most of these production systems are established in favorable environments for the development of S. frugiperda or cogollero worm (Blanco et al., 2014, Loera and Castillo, 2015), therefore, their presence is common in agroecosystems dedicated to the production of corn in these regions, mainly in the spring-summer agricultural cycle, presents favorable conditions of temperature and humidity for the growth and development of this insect (Resendiz et al., 2016), reaching to develop more than four generations during a same agricultural cycle (Blanco et al., 2014).
Feeding mainly of foliar tissues in development, causing decrease of the foliar area of the corn plant, in extreme cases up to 70% of this, which can reduce the yield of grain (Hruska and Gould, 1997); This insect also feeds on other developing tissues such as stems, inflorescences, bracts and grain (Valdez-Torres et al., 2012; Loera and Castillo, 2015), which favors the infestation by microcoleoptera and fungi such as Asperguillus spp. (Rodríguez-del-Bosque et al., 2010) and Fusarium spp, microorganisms that produce mycotoxins (García-Aguirre and Martínez-Flores, 2010, Martínez et al., 2013), this in addition to diminishing grain yield, affects the quality of it (Resendiz et al., 2016), is a plague of economic importance in corn.
Its control is often carried out through the application of chemical insecticides, increasing production costs (Barrientos-Gutiérrez et al., 2013) and the development of resistance of the insect is induced (Ahmad and Arif, 2010), besides, it can be a source of environmental pollution (Devine et al., 2008). On the other hand, many corn production systems in the center and south of Tamaulipas are established on a small scale and for self-consumption, where the cost represented by the use of hybrid cultivars is unviable and the low adaptation of these to these specific environments increases this problematic (Turrent et al., 2012; García-Salazar and Ramírez-Jaspeado, 2014). A viable solution is to form and release cultivars that can be used in the specific environmental conditions of each region and resistant to S. frugiperda and that do not increase production costs.
In this sense, in a program of genetic improvement, the choice of germplasm to use is a decisive decision for the success of it, therefore, the use of native germplasm as a base population for the development of cultivars resistant to S. frugiperda is a viable alternative, since this germplasm has high adaptation to the environmental conditions of these regions (Castro et al., 2013), wide variability (Pecina et al., 2011; Castro et al., 2013), it has also developed under the constant presence of this lepidopteran, which is a source of characteristics that provide resistance to attack (Cantú et al., 2012, Loera and Castillo, 2015). The use of this germplasm in breeding programs allows the conservation, management and exploitation of this phytogenetic resource, contributing to the reduction of genetic loss and erosion (González et al., 2014).
The planning of a plant breeding program is based on knowing the genetic component of the base germplasm used (Gutiérrez et al., 2004); that is, to understand the gene action that controls the characters of interest; In this sense, the combinatorial aptitude of the parents allows selecting those with an outstanding average behavior in a series of crosses (Luna-Ortega et al., 2013) and identifying specific combinations with a behavior higher than expected based on the average of the parents that intervene in the crossing and in this way, define heterotic patterns (Guillén-de la Cruz et al., 2009), which constitute a source of germplasm for the generation of elite lines of great utility in a breeding program dynamic; the above can be achieved through the evaluation of diallel crosses (Antuna et al., 2003).
The evaluation of general combinatorial aptitude (ACG) and specific (ACE) by diallel crosses, allows efficient classification of parents based on the behavior of their progeny (Antuna et al., 2003), is considered effective in identifying useful sources of germplasm for maize breeding programs (Ávila et al., 2009) in this sense, the ACG determines the additive portion of the genetic effects that control the expression of the phenotypic characteristics of interest, while the ACE non-additive effects, this is the gene action of dominance and epistasis (Camposeco et al., 2015).
In this way, when there are greater effects of ACG, it is feasible to take advantage of the additive portion of the available genetic variance, through any recurrent selection methodology; while the existence of a greater ACE favors the exploitation of the non-additive variance, through the implementation of a reciprocal recurrent selection or hybridization program (Preciado et al., 2005). The objective of the present work was to evaluate the genetic effects of leaf damage caused by Spodoptera frugiperda and the resistance to it of lines derived from maize germplasm native to Tamaulipas and its crosses.
Materials and methods
The experiment was carried out during the spring-summer 2015 cycle, in the Experimental Field “Ing. Herminio García González” of the Autonomous University of Tamaulipas located in the municipality of Güemez, Tamaulipas, at 23º 56ʼ 26” north latitude and 99º 05ʼ 59” west longitude, at an altitude of 193 meters above sea level. With a subtropical, semi-dry and warm extreme climate, average annual temperature of 23.8 °C, with an annual rainfall of 721.1 mm.
They were evaluated six maize S3 inbred lines, PWL1S3 and PWL6S3 derived from a native population of Padilla C-3001, TGL2S3 and TML3S3 derived from populations C-3007 and C-3012 from Tula and LlNL4S3 and LlHL5S3 from populations C-3033 and C-3040 of Llera, Tam. and its direct and reciprocal crosses, giving a total of 36 cultivars; were established in two treatments, the first with insecticide for the control of S. frugiperda with three applications of Denim® 19 CE (emamectin benzoate) at a dose of 200 ml ha-1 in each application; the first application was made at the time of the complete expansion of sheet 5, second to sheet 9 and third to leaf flag and the second without insecticide.
The planting was done manually on September 11, 2015, the cultivars were established at a population density of 50 000 plants ha-1 under irrigation conditions, the fertilizer dose of 120N-60P-00K, 50% was applied of N and 100% of P in the sowing and the rest of the N in the first weeding, 32 days after sowing; weed control was performed manually at the time of complete expansion of leaves 8 and 14. The experimental plot was a furrow of 5 m in length and a separation of 0.80 m between rows, the experiment was established under a block design complete at random, with an arrangement of divided plots, where the large plot was the treatments with and without insecticide to control S. frugiperda and the small plot the cultivars, with three repetitions.
Foliar damage was determined by S. frugiperda, using the modified damage scale of Fernandez and Exposito (2000) (Table 1), at the moment of male flowering by individual plant in all the plants of the experimental plot, to later obtain the average per floor.
Table 1 Modified visual scale of leaf damage caused by S. frugiperda in cultivars derived from germplasm native corn of Tamaulipas.
| Grade | Damage characteristics |
| 0 | No visible damage |
| 1 | Perforations in the shape of a window, circular, elongated small smaller than 5 mm in less than 20% of the leaf area of the plant |
| 2 | Circular or elongated perforations between 5 to 10 mm, affecting between 20 and 40% of the foliar area of the plant |
| 3 | Circular or elongated perforations greater than 10 mm, affecting 40 to 60% of the foliar area of the plant with less than 20% destruction of the whorl |
| 4 | Circular or elongated perforations greater than 10 mm, affecting between 60 to 80% of the foliar area of the plant with the verticil destroyed more than 50% |
| 5 | Circular or elongated perforations greater than 10 mm, affecting between 80 to 100% of the leaf area of the plant with the whorl completely destroyed |
The yield of grain per plant (RGP), was calculated as the product of the weight of ear per experimental plot at harvest, by the content of dry matter of the grain, standardized to 14% of humidity of the grain, by the proportion of the weight of grain with respect to the total weight of ear and averaged by the total number of plants per plot. By means of the difference between treatments with and without insecticide the decrease of the RGP was calculated because of the foliar damage of S. frugiperda in grams and percentage.
An analysis of variance and a diallyl analysis were carried out under Griffing method I and model I (fixed effects), to determine general combinatorial aptitude (ACG) and specific (ACE), maternal (EM) and reciprocal effects (ER) using the DIALLEL-SAS05 program proposed by Zhang and Kang (2005). On the other hand, we considered as low preference cultivars for S. frugiperda, those that had a foliar damage inferior to the value of the mean minus the variance (μ - σ) and cultivars of high preference, to those that had a superior foliar damage to the value of the mean plus the variance (μ + σ) (De la Cruz-Lazaro et al., 2010).
Results and discussion
The analysis of variance detected significant statistical differences (p≤ 0.01) between the insecticide application treatments for the variable leaf damage of S. frugiperda (Table 2), with an average foliar damage of 0.14 in the insecticide treatment, lower than the treatment without insecticide with an average leaf damage of 1.64.
Table 2 Statistical significance of genetic effects of 6 lines S3 progenitor of corn and their crosses for foliar damage of S. frugiperda with and without insecticide.
| Source of variation | Leaf damage of S. frugiperda | ||
| Combined | TCI | TSI | |
| Trat | <0.001 | - | - |
| Cruza | 0.018 | 0.903 | 0.044 |
| Cruza×trat | 0.03 | - | - |
| Acg | 0.012 | 0.813 | 0.012 |
| Ace | 0.042 | 0.51 | 0.137 |
| Acg×trat | 0.035 | - | - |
| Ace×trat | 0.45 | - | - |
| Rec | 0.349 | 0.954 | 0.42 |
| Rec×trat | 0.676 | - | - |
| Mat | 0.128 | 0.754 | 0.227 |
| Mat×trat | 0.631 | - | - |
| Nomat | 0.536 | 0.932 | 0.573 |
| Nomat×trat | 0.639 | - | - |
TCI = treatment with insecticide; TSI = treatment without insecticide; ACG = general combinatorial aptitude; ACE = specific combinatorial aptitude; TRAT = insecticide treatment; REC = reciprocal effects; MAT = maternal effects; NoMAT = non-maternal effects.
We also detected significant differences between the crosses evaluated for this same variable, this is indicative of the existence of genetic variability for this variable between the parents; since according to Guillen-de la Cruz et al. (2009) and De la Cruz-Lazaro et al. (2010) as the genetic diversity of the parents increases, the differences between their crosses are increased, both in agronomic and physiological characteristics, it can cause differences in the tolerance to a pest and its preference (Zavala, 2010). S. frugiperda (Camarena, 2009). Statistical significance (p≤ 0.05) was found for the genetic effects of ACG of foliar damage of S. frugiperda, similarly occurred for ACE (Table 2).
This indicates that the variation between the crosses evaluated for foliar damage of S. frugiperda, is due to both additive and non-additive gene action (Camposeco et al., 2015). In this sense, the sum of squares of crosses (data not shown) shows that, the variation corresponding to the effects of ACG for foliar damage of S. frugiperda was 25.9%, while for the effects of ACE the results were 45.7 % and for reciprocal effects of 28.4% which shows that the variation of leaf damage was controlled to a greater extent by non-additive effects (Widstrom et al., 1972).
On the other hand, for the interaction of Cruza×Trat in this variable, significant effects were present (p≤ 0.05), this shows evidence of different foliar damage of S. frugiperda among the crosses within each of the evaluated treatments, which it could be indicative of genetic variability among these cultivars for the preference of this insect, as mentioned by Medina et al. (2001) and González et al. (2008). Similarly, the significance in the ACG×TRAT interaction demonstrates that the additive effects for this variable were expressed according to the environmental condition in which the plant was developed is necessary, that the evaluation and selection of cultivars be carried out in different environmental conditions, in order to know the interactions between these and the various factors involved in their behavior, both biotic and abiotic (Callejas and Ochando, 2005) and in this way, to be able to identify with greater precision the effects of ACG and thus then choose the parents according to the specific needs of the improvement program (Yan and Hunt, 2002).
On the other hand, there was no significance (p> 0.05) for the reciprocal effects, nor for the interaction with insecticide treatment and therefore, neither for maternal, not maternal effects and their respective interactions with the insecticide treatment, so it can be considered that there were no cytoplasmic or extranuclear factors, or interaction of these with nuclear factors involved in the expression of this variable (Ávila et al., 2009).
Taking into account the significant interaction of ACG×TRAT (Table 2), a diallel analysis was performed within each of the treatments, not finding significant effects of ACG in the treatment with insecticide; conversely, significant effects of ACG were observed in the treatment without insecticide (Table 2); in this treatment for the variable foliar damage by S. frugiperda, the PWL1S3 line presented significant effects of ACG with a positive value (Table 3).
Table 3 Estimated effects of general combinatorial aptitude on the diagonal and specific combinatorial ability on the diagonal of 30 crosses and 6 maize lines progenitor for foliar damage of S. frugiperda.
| Progenitor | PWL1S3 | TGL2S3 | TML3S3 | LlNL4S3 | LlHL5S3 | PWL6S3 | ||
| PWL1S3 | 0.146 | * | 0.134 | -0.166 | -0.03 | -0.009 | -0.022 | |
| TGL2S3 | -0.042 | -0.099 | -0.171 | -0.2 | * | 0.022 | ||
| TML3S3 | 0.108 | 0.002 | -0.067 | 0.074 | ||||
| LlNL4S3 | 0.101 | 0.026 | -0.032 | |||||
| LlHL5S3 | -0.177 | * | -0.023 | |||||
| PWL6S3 | -0.137 |
*= significance a p≤ 0.05.
Conversely, the LlHL5S3 line had significant and negative ACG effects; these results indicate that both lines have a high contribution in the expression of the variation of this characteristic, positively and negatively, in their respective progenies and that the additive effects are important, so it is feasible to exploit the additive proportion of the variance genetic available in these lines, by any variant of recurrent selection (Guillén-de la Cruzet al., 2009; Coutiño et al., 2010) to modify the resistance to damage of S. frugiperda (Widstrom et al., 1992) in the rest of the lines evaluated did not show significance for this effect (Table 3).
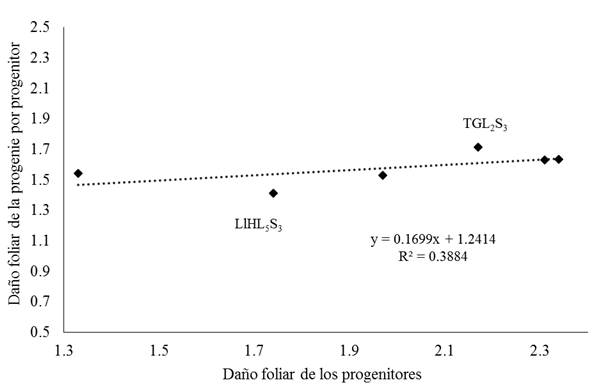
The positive correlation (r= 0.62) of the foliar damage of S. frugiperda of the progenitors with the average damage of the F1 crosses that form their respective progenies, corroborates that the resistance to damage caused by this insect within these inbred lines, is controlled by additive effects (Figure 1). In this sense, the susceptibility of the PWL1S3 line is demonstrated with a high level of damage when evaluated as line per se (2.17) and with the average damage of all its progeny (1.71); conversely, a lower level of susceptibility was observed in the LlHL5S3line, both when evaluated as line per se (1.74), and when its progeny was evaluated (1.41); the above, also demonstrates the amplitude of the phenotypic and genotypic variability of the parents evaluated.
For the same variable there were only significant (p≤ 0.05) and negative effects of ACE in the cross TGL2S3×LlHL5S3 (Cuadro 3), if we consider the absence of reciprocal effects (Table 2) this indicates that the direct and reciprocal crosses of the lines TGL2S3 and LlHL5S3 present a leaf damage less than that of their parents; from the above it is suggested that the non-additive gene action is involved in the level of foliar damage by S. frugiperda in the indicated crosses and that the non-preference of this insect for this germplasm can be increased by hybridization processes, according to a Preciado et al. (2005), who mention that characteristics of corn, controlled by non-additive effects can be modified by hybridization methods.
The LlHL5S3line presented significance for negative ACG effects and is also one of the progenitors of the higher ACE cross (TGL2S3×LlHL5S3) (Table 3). These results corroborate that at higher ACE crosses there is at least one high ACG line (Reyes et al., 2004; Escorcia-Gutiérrez et al., 2010) it can be inferred that the high ACG of at least one parent is an indicator of greater ACE in their progeny, on the other hand, if the low ACG of the progenitor is considered. line TGL2S3 (Table 3), we can deduce the existence of genetic divergence between it and the line LlHL5S3 according to what was mentioned by Romero et al. (2002), who found that differential levels of ACG indicate genetic divergence between parents; which explains the high ACE in the crosses that these two parents participate (Table 3).
The regression analysis between leaf damage values of F1 crosses and the average of their parents (Figure 2), shows relative dominance exhibited by these crosses, as a function of heterosis. All F1 crosses had lower leaf damage of S. frugiperda, compared to the average values of their parents (Figure 2) and except for four crosses (PWL1S3×PWL6S3, TGL2S3×PWL6S3, TML3S3×PWL6S3, LlNL4S3×PWL6S3) they showed less damage than the less preferred parent (Table 4), due to the fact that the PWL6S3 line participates in these crosses, which presented the lowest level of leaf damage (1.33) by S. frugiperda (Table 4).
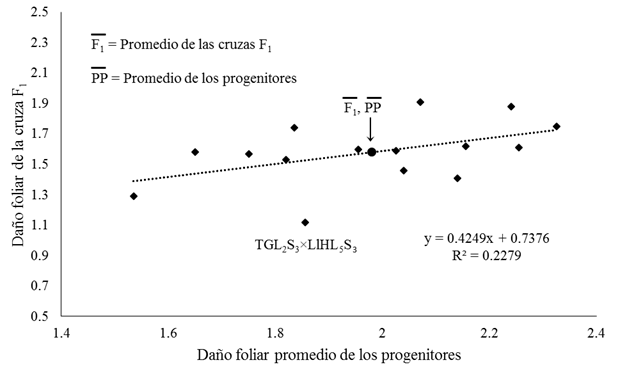
Figure 2 Relation of the foliar damage of S. frugiperda of the F1 crosses of maize and the average progenitor conditions without in insecticidal application.
Table 4 Leaf damage by S. frugiperda and decrease in grain yield per plant, of maize progenitor lines and their crosses.
| Cultivar | DFoSI | Decrease in the RGP | ||
| (g) | (%) | |||
| PWL1S3 | 2.17 | S* | 36.08 | 39.9 |
| TGL2S3 | 1.97 | S* | - | - |
| TML3S3 | 2.34 | S** | 24.39 | 34.2 |
| LlNL4S3 | 2.31 | R** | 20.4 | 24.6 |
| LlHL5S3 | 1.74 | 7.23 | 6.7 | |
| PWL6S3 | 1.33 | R* | 19.09 | 18.5 |
| PWL1S3×TGL2S3 | 1.91 | 11.92 | 6.9 | |
| PWL1S3×TML3S3 | 1.61 | 38.04 | 32.7 | |
| PWL1S3×LlNL4S3 | 1.88 | 18.53 | 15.1 | |
| PWL1S3×LlHL5S3 | 1.6 | 29.8 | 28.2 | |
| PWL1S3×PWL6S3 | 1.57 | - | - | |
| TGL2S3×TML3S3 | 1.62 | 15.29 | 16.7 | |
| TGL2S3×LlNL4S3 | 1.41 | 17.54 | 9.1 | |
| TGL2S3×LlHL5S3 | 1.12 | R* | 19.05 | 15.8 |
| TGL2S3×PWL6S3 | 1.58 | 30.14 | 24.5 | |
| TML3S3×LlNL4S3 | 1.75 | - | - | |
| TML3S3×LlHL5S3 | 1.46 | 25.98 | 24.8 | |
| TML3S3×PWL6S3 | 1.74 | 15.76 | 12.8 | |
| LlNL4S3×LlHL5S3 | 1.59 | 26.12 | 23.2 | |
| LlNL4S3×PWL6S3 | 1.53 | 26.32 | 21.6 | |
| LlHL5S3×PWL6S3 | 1.29 | R* | 40.37 | 32.1 |
DFoSI = foliar damage of S. frugiperda in treatment without insecticide; RGP = grain yield per plant; S** = values greater than μ + 2σ; S* = values greater than μ + σ; R* = values less than μ-σ.
All the points that were distributed uniformly and closely along the trend line, indicate that the variation in leaf damage of the F1 crosses, depended mainly on additive gene action, in a contrary way, the non-additive effects (dominance and epistasis), are identified in points that deviate considerably from this line (Figure 2). The negative non-additive effects are found in points farther below the trend line, for the case of the direct and reciprocal crosses of the lines TGL2S3 and LlHL5S3, this is corroborated in Table 3, where estimated effects of ACE negative in the crossing of these two lines.
In Figure 2, the dispersion of most of the points was established along the regression line, corroborating the results of the diallel analysis, which showed significant effects of ACG that favor less leaf damage in the progeny of these lines, established in conditions without insecticide. Likewise, there are points that deviate considerably from the regression line and lower damage values predominate in the F1 crosses, compared to their parents (negative non-additive effects), such as in the TGL2S3×LlHL5S3, cross, which suggests positive dominance for the resistance to the attack of S. frugiperda.
The genetic expression of the crosses and progenitors, determined by the mean values of the foliar damage by S. frugiperda in the treatment without insecticide, showed a wide variation, the crosses TGL2S3×LlHL5S3 and LlHL5S3×PWL6S3 and the parent PWL6S3 had a foliar damage inferior to 1.35, within which the cross stands out TGL2S3×LlHL5S3, with the lowest level of leaf damage (1.12) (Table 4) and with greater estimated effects of ACE (Table 3), therefore, it can be considered that these cultivars have low preference on the part of S. frugiperda (Casmuz et al., 2010). Therefore, the line LlHL5S3 is a good option to be used in breeding programs, especially for the derivation of lines of higher inbreeding with high ACG for low preference of S. frugiperda or in a hybridization program taking advantage of the high ACE that showed with the TGL2S3 line (Table 3).
On the other hand, for the foliar damage of S. frugiperda in the treatment without insecticide, it was observed that the lines PWL1S3, TML3S3 and LlNL4S3 presented an average superior to 2 with a decrease in the average grain yield of 32.9% (Table 4), these lines can be considered with high preference on the part of S. frugiperda and susceptible to leaf damage caused by this pest, while the TGL2S3 line, despite having a damage greater than 1.9, did not register a decrease in grain yield, can be considered with high preference on the part of S. frugiperda, but with tolerance to leaf damage caused by this insect; finally, the PWL6S3 line presented the least leaf damage (1.33), consequently the decrease in grain yield due to this damage was only 18.5% (Table 4).
Within the crosses evaluated, the PWL1S3×TGL2S3, PWL1S3×LlNL4S3, TML3S3×LlNL4S3 andTML3S3×PWL6S3 showed an average leaf damage greater than 1.7, but a low level of grain yield reduction, lower than 15.5% (Table 4) , so it can be inferred that these crosses have tolerance to foliar damage caused by S. frugiperda (Casmuz et al., 2010) in a contrary manner, crosses PWL1S3×TML3S3, LlHL5S3×PWL6S3 and PWL1S3×LlHL5S3 had lower leaf damage to 1.62, but had a decrease in grain yield of 32.7, 32.1 and 28.2% respectively and can be considered to have a low tolerance to leaf damage; finally, the cross TGL2S3×LlHL5S3 had a low leaf damage (1.29) of S. frugiperda and consequently a reduction in grain yield of only 15.8% (Table 4).
Conclusions
Within the evaluated germplasm there is resistance to S. frugiperda, both due to non-preference and tolerance to leaf damage, which caused a smaller decrease in grain yield due to the incidence of this pest; In general, there was heterosis for the non-preference of this pest, so this germplasm can be considered as a source of characteristics that provide resistance to S. frugiperda.
The variation of the foliar damage of S. frugiperda in the germplasm evaluated, depended as much on additive as non-additive effects, and the additive effects depended on the application condition or without insecticide, considering that it is feasible the inclusion of this germplasm in programs of selection or hybridization for the improvement of resistance to S. frugiperda.
The effects of non-additive type were of greater importance for foliar damage caused by S. frugiperda in the germplasm evaluated, so that processes of improvement through reciprocal recurrent selection or hybridization are viable.
Literatura citada
Ahmad, M. and Arif, M. I. 2010. Resistance of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) to endosulfan, organophosphorus and pyrethroid insecticides in Pakistan. Crop Prot. 29:1428-1433. [ Links ]
Antuna, G. O.; Rincón, S. F.; Gutiérrez, R. E.; Ruiz, T. N. A. y Bustamante, G. L. 2003. Componentes genéticos de caracteres agronómicos y de calidad fisiológica de semillas en líneas de maíz. Rev. Fitotec. Méx. 26:11-17. [ Links ]
Ávila, P. M. A.; Rodríguez, H. S. A.; Vázquez, B. M. E.; Borrego, E. F.; Lozano, R. A. J. y López, B. A. 2009. Aptitud combinatoria y efectos recíprocos en líneas endogámicas de maíz de valles altos del centro de México. Agric. Téc. Méx. 3:285-293. [ Links ]
Barrientos, G. J. E.; Huerta, de la P. A.; Escobedo, G. J. S. y López, O. J. F. 2013. Manejo convencional de Spodoptera exigua en cultivos del municipio de Los Reyes de Juárez, Puebla. Rev. Méx. Cienc. Agríc. 4:1197-1208. [ Links ]
Blanco, J. C. A.; Pellegaud, G.; Nava, C. U.; Lugo, B. D.; Vega, A. P.; Coello, J.; Terán, V. A. P. and Vargas, C. J. 2014. Maize pests in Mexico and challenges for the adoption of integrated pest management programs. J. Integ. Pest Manag. 5:1-9. [ Links ]
Callejas, C. y Ochando, M. D. 2005. Variabilidad genética en Bemisia tabaci (Gennadius) en mecanismos de resistencia inducida en plantas de tomate. Bol. San. Veg. Plagas. 31:71-77. [ Links ]
Camarena, G. G. 2009. Señales en la interacción planta insecto. Rev. Chapingo Ser. Cienc. Forest. Ambiente. 15:81-85. [ Links ]
Camposeco, M. N.; Robledo, T. V.; Valdez, A. L. A.; Ramírez, G. F.; Mendoza, V. R. y Benavides, M. A. 2015. Estimación de la aptitud combinatoria en poblaciones de tomate de cáscara. Rev. Méx. Cienc. Agríc. 6:437-451. [ Links ]
Cantú, A. M. A.; Reyes, M. C. A. y Rodríguez, B. L. A. 2012. La fecha de siembra: una alternativa para incrementar la producción de maíz. Fundación Produce Tamaulipas, AC. 7-15 pp. [ Links ]
Casmuz, A.; Juárez, M. L.; Socías, M. G.; Murúa, M. G.; Prieto, S.; Medina, S.; Willink, E. y Gastaminza, G. 2010. Revisión de los hospederos del gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Argent. 69:209-231. [ Links ]
Castro; N. S.; López, S. J. A.; Pecina, M. J. A.; Mendoza, C. M. C. y Reyes, M. C. A. 2013. Exploración de germoplasma nativo de maíz en el centro y sur de Tamaulipas, México. Rev. Méx. Cienc. Agríc. 4:645-653. [ Links ]
Coutiño, E. B.; Vidal, M. V. A.; Cruz, G. B. y Cruz, V. C. 2010. Aptitud combinatoria general y específica del contenido de azúcares en maíces criollos eloteros. Rev. Fitotec. Méx. 33:57-61. [ Links ]
De la Cruz, L. E.; Castañón, N. G.; Brito, M. N. P.; Gómez, V. A.; Robledo, T. V. y Lozano, R. A. J. 2010. Heterosis y aptitud combinatoria de poblaciones de maíz tropical. Фhyton. 79:11-17. [ Links ]
Devine, G. J.; Eza, D.; Ogusuku, E. y Furlong, M. J. 2008. Uso de insecticidas: contexto y consecuencias ecológicas. Rev. Perú Med. Exp. Salud Pública. 25:74-100. [ Links ]
Escorcia, G. N.; Molina, G. J. D.; Castillo, G. F. y Mejía, C. J. A. 2010. Rendimiento, heterosis y depresión endogámica de cruzas simples de maíz. Rev. Fitotec. Méx. 33:271-279. [ Links ]
Fernández, J. L. y Expósito, I. E. 2000. Nuevo método para el muestreo de Spodoptera frugiperda (J. E. Smith) en el cultivo del maíz en Cuba. Centro Agrícola. 27:32-38. [ Links ]
García, A. G. y Martínez, F. R. 2010. Especies de Fusarium en granos de maíz recién cosechado y desgranado en el campo en la región de Ciudad Serdán, Puebla. Rev. Mex. Biod. 81:15-20. [ Links ]
García, S. J. A. y Ramírez, J. R. 2014. El mercado de la semilla mejorada de maíz (Zea mays L.) en México. Un análisis del saldo comercial por entidad federativa. Rev. Fitotec. Méx. 1:69-77. [ Links ]
González, H. A.; Pérez, L. D. J.; Domínguez, L. A.; Franco, M. O.; Balbuena, M. A.; Ramos, M. A.; Sahagún, C. J. 2008. Variabilidad genética, diversidad fenotípica e identificación de poblaciones sobresalientes de maíz cacahuacintle. Ciencia Ergo. Sum. 3:297-305. [ Links ]
González, M. J.; López, S. J. A.; Briones, E. F.; Varela, F. S. E.; Reyes, M. C. A. y Pecina, M. J. A. 2014. Programa de manejo, conservación y mejoramiento de maíz nativo de la Facultad de Ingeniería y Ciencias de la UAT. Investigación y Ciencia de la Universidad Autónoma de Aguascalientes. 62:77-84. [ Links ]
Guillen, de la C. P.; de la Cruz, L. E.; Castañon, N. G.; Osorio, O. R.; Brito, M. N. P.; Lozano, del R. A. y López, N. U. 2009. Aptitud combinatoria general y específica de germoplasma tropical de maíz. Trop. Subt. Agroecosys. 10:101-107. [ Links ]
Gutiérrez, R. E.; Espinoza, B. A.; Palomo, G. A.; Lozano, G. J. y Antuna, G. O. 2004. Aptitud combinatoria de híbridos de maíz para la Comarca Lagunera. Rev. Fitotec. Méx. 27:7-11. [ Links ]
Hruska, A. J. and Gould, F. 1997. Fall armyworm (Lepidoptera: Noctuidae) and Diatraea lineolata (Lepidoptera: Pyralidae): impact of larval population level and temporal occurrence on maize yield in Nicaragua. J. Econ. Entomol. 90: 611-622. [ Links ]
Loera, G. J. y Castillo, T. H. 2015. Control del gusano cogollero en maíz. INIFAP boletín informativo. Boletín electrónico año 1. núm. 3. 2 p. [ Links ]
Luna, O. J. G.; García, H. J. L.; Valdez, C. R. D.; Gallegos, R. M. Á.; Preciado, R. P.; Guerrero, G. C. y Espinoza, B. A. 2013. Aptitud combinatoria y componentes genéticos en líneas de maíz. Universidad y Ciencia. 29:243-253. [ Links ]
Martínez, P. H. Y.; Hernández, D. S.; Reyes, M. C. A. y Vázquez, C. G. 2013. El género Aspergillus y sus micotoxinas en maíz en México: Problemática y Perspectivas. Revista Mexicana de Fitopatología 31:126-146. [ Links ]
Medina, M.; Abadie, T.; Vilaró, D. y Ceretta, S. 2001. Estudio metodológico de adaptación de cultivares de maíz para silo a las condiciones de Uruguay. Agrociencia Uruguay. 5:23-31. [ Links ]
Pecina, M. J. A.; Mendoza, C. M. C.; López, S. J. A.; Castillo, G. F.; Mendoza, R. M. y Ortiz, C. J. 2011. Rendimiento de grano y sus componentes en maíces nativos de Tamaulipas evaluados en ambientes contrastantes. Rev. Fitotec. Méx. 34:85-92. [ Links ]
Preciado, O. R. E.; Terrón, I. A. D.; Gómez, M. N. O. y Robledo, G. E. I. 2005. Componentes genéticos en poblaciones heteróticamente contrastantes de maíz de origen tropical y subtropical. Agron. Mesoam. 16:145-151. [ Links ]
Reséndiz, R. Z.; López, S. J. A.; Briones, E. F.; Mendoza, C. M. C. y Varela, F. S. E. 2014. Situación actual de los sistemas de producción de grano de maíz en Tamaulipas, México. Investigación y Ciencia de la Universidad Autónoma de Aguascalientes. 62:70-76. [ Links ]
Reséndiz, R. Z.; López, S. J. A.; Osorio, H. E.; Estrada, D. B.; Pecina, M. J. A.; Mendoza, C. M. C. y Reyes, M. C. A. 2016. Importancia de la resistencia del maíz nativo al ataque de larvas de lepidópteros. Temas de Ciencia y Tecnología. 20:3-14. [ Links ]
Reyes, M. C. A. y Cantú, A. M. A. 2004. H-437, H-439 y H-440: nuevos híbridos trilineales de maíz de grano blanco para el noreste de México y regiones similares. In: Castillo, T. H.; González, Q. J. y Garza, G. L. (Eds.). Día del agricultor 2004. INIFAP-CIRNE. México, D. F. 10-12. pp. [ Links ]
Rodríguez, del B. L. A.; Cantú, A. M. A. and Reyes, M. C. A. 2010. Effect of planting date and hybrid selection on Helicoverpa zea and Spodoptera frugiperda (Lepidoptera: Noctuidae) damage on maize ears in Northeastern México. Southwestern Entomologist. 35:157-164. [ Links ]
Romero, P. J.; Castillo, G. F. y Ortega, P. R. 2002. Cruzas de poblaciones nativas de maíz de la raza chalqueño: II. Grupos genéticos, divergencia genética y heterosis. Rev. Fitotec. Méx. 25:107-115. [ Links ]
Turrent, F. A.; Wise, T. A. y Garvey, E. 2012. Factibilidad de alcanzar el potencial productivo de maíz de México. Mexican Rural Development Research Report No. 24, octubre 2012 Woodrow Wilson International Center for Scholars. [ Links ]
Valdez, T. J. B.; Soto, L. F.; Osuna, E. T. y Báez, S. M. A. 2012. Modelos de predicción fenológica para maíz blanco (Zea mays L.) y gusano cogollero (Spodoptera frugiperda J. E. Smith). Agrociencia. 46:399-410. [ Links ]
Widstrom, N. W.; Williams, W. P.; Wiseman, B. R. and Davis, F. M. 1992. Recurrent selection for resistance to leaf feeding by fall armyworm on maize. Crop Sci. 32:1171-1174. [ Links ]
Widstrom, N. W.; Wiseman, B. R. and McMillian, W. W. 1972. Resistance among some maize inbreds and single crosses to fall armyworm injury. Crop Sci. 12:290-292. [ Links ]
Yan, W. and Hunt, L. A. 2002. Biplot analysis of diallel data. Crop Sci. 42:21-30. [ Links ]
Zavala, J. A. 2010. Respuestas inmunológicas de las plantas frente al ataque de insectos. Ciencia Hoy. 20:52-59. [ Links ]
Zhang, Y. and Kang, M. S. 2005. DIALLEL-SAS05: a comprehensive program for Griffing’s and Gardner-Eberhart analyses. Agron. J. 97:1097-1106. [ Links ]
Received: November 00, 2017; Accepted: January 00, 2018











 text in
text in