Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 n.6 Texcoco Aug./Sep. 2017
Articles
Orthoptera (Caelifera) and its entomopathogens fungi in maize agroecosystems in Erongarícuaro, Michoacán
1Campus de Ciencias Biológicas y Agropecuarias-Universidad Autónoma de Yucatán. Carretera Mérida-Xmatkuil km 15.5, Mérida, Yucatán, México. CP. 97100. Tel. 52(443)3223878. (eslabonmichoacan@hotmail.com).
2Campo Experimental Uruapan-INIFAP. Av. Latinoamericana núm. 110, Col. Revolución, Uruapan, Michoacán, México. CP. 97100. Tel. 52(452)5237392. (minaj47@hotmail.com).
3Instituto de Investigaciones en Ecosistemas y Sustentabilidad-Universidad Nacional Autónoma de México (IIES-UNAM). Antigua Carretera a Pátzcuaro núm. 8701, Col. Ex Hacienda de San José de La Huerta, Morelia, Michoacán, México. CP. 58190 .Tel. 52(443)3223878.
4Centro de Investigaciones Regionales “Dr. Hideyo Noguchi”, Universidad Autónoma de Yucatán. Av. Itzaes por 49 núm. 490, Centro, Mérida, Yucatán, México. CP. 97000. Tel: 52 (999)9255755. (enrique.reyes@correo.uady.mx).
Grasshoppers are a major pest of maize, one of its natural enemies are entomopathogenic fungi (HEP). Proper management of this crop, involving changes in the microhabitat, can contribute to the control of grasshopper populations, as well as the presence of its natural enemies. The effect of two types of agricultural management was evaluated: monoculture (MC-I) of maize and polyculture of maize, pumpkin and bean (PC-I), as well as the vegetation of the monoculture margins (VMM) and the vegetation of the polyculture margins (VMP) on the richness and abundance of grasshoppers and its associated entomopathogenic fungi (HEP) in Erongarícuaro, Michoacán. Grasshoppers were collected monthly with a striking net from July to December 2015 on two plots of each management type and its marginal vegetation at three sites. The grasshopper community (CS) consisted of 17 species, where Sphenarium purpurascens purpurascens Charpentier, was the dominant species (97.8% abundance). The type of agricultural management had no effect on species richness. Richness in the marginal vegetation of the crops did not explain the richness in MC-I and PC-I; however, abundance of S. p. purpurascens in marginal vegetation explained the abundance found in the MC-I and PC-I sites. From the 2920 grasshoppers collected in, 0.41% were infected by Metarhizium Sorokin or Beauveria (Balsamo) Vuillemin. The most frequently infected HEP was Metarhizium. The proportion of infected individuals and PC-I conditions were positively related, compared to VMP. The sites with greater vegetal diversity had greater abundance of grasshoppers and greater infection with HEP.
Keywords: Sphenarium purpurascens purpurascens; Metarhizium; Beauveria; Grasshopper
Los saltamontes son una plaga importante del maíz, uno de sus enemigos naturales son los hongos entomopatógenos (HEP). El manejo adecuado de este cultivo, que involucre cambios en el microhábitat puede contribuir al control de las poblaciones de saltamontes, así como la presencia de sus enemigos naturales. Se evaluó el efecto de dos tipos de manejo agrícola: monocultivo (MC-I) de maíz y policultivo de maíz, calabaza y frijol (PC-I), así como de la vegetación de los márgenes del monocultivo (VMM) y la vegetación de los márgenes del policultivo (VMP), sobre la riqueza y abundancia de saltamontes y sus hongos entomopatógenos (HEP) asociados en Erongarícuaro, Michoacán. Se recolectaron saltamontes mensualmente con una red de golpeo de julio a diciembre de 2015 en dos parcelas de cada tipo de manejo y su vegetación marginal en tres sitios. La comunidad de saltamontes (CS) estuvo conformada por 17 especies, en donde Sphenarium purpurascens purpurascens Charpentier, fue la especie dominante (97.8% de la abundancia). El tipo de manejo agrícola no tuvo efecto sobre la riqueza de especies. La riqueza en la vegetación marginal de los cultivos no explicó la riqueza en el MC-I y PC-I; sin embargo, la abundancia de S. p. purpurascens en la vegetación marginal en los sitios explicó la abundancia encontrada en el MC-I y el PC-I. De 2 920 saltamontes colectados, 0.41% estuvieron infectados por Metarhizium Sorokin o Beauveria (Balsamo) Vuillemin. El HEP con mayor frecuencia de infección fue Metarhizium. La proporción de individuos infectados y las condiciones del PC-I se relacionaron positivamente, en comparación con la VMP. Los sitios con mayor diversidad vegetal tuvieron una mayor abundancia de saltamontes y mayor infección con HEP.
Palabras clave: Sphenarium purpurascens purpurascens; Metarhizium; Beauveria; Saltamontes
Introduction
In México, maize (Zea mays L.) is the main crop due to the area occupied, as it is planted in more than eight million hectares, representing 39% of agricultural land area (González-Estrada et al., 2008) 72.1% of the maize producing units in México have from 0-5 ha, made up of small producers that operate with very little mechanization (SAGARPA, 2010).
In order to produce maize, rural communities use agricultural management systems that are the result of their knowledge and means for the appropriation of natural resources (Toledo, 2010). For example, in Napízaro, in the municipality of Erongarícuaro, Michoacán, two of the agricultural management systems that producers use are monoculture (MC) and milpa or polyculture (PC), consisting of maize, beans (Phaseolus vulgaris L.) and pumpkin (Cucurbita máxima L.) (Del Val et al., 2013). The MC system is characterized by the application of synthetic fertilizers and insecticides and the minimization of plant species diversity (Nicholls and Altieri, 2006). This results in simplification of the system and reduction of the diversity of beneficial organisms (Andow, 1991).
It has been documented that given the mentioned characteristics, the MC favors the proliferation of herbivores (Nicholls, 2008) that increase its density until causing economic damages in the crops, reason why they are considered a plague. This proliferation grows as the area under MC cultivation increases (Brechelt, 2004; Altieri and Nicholls, 2005). In contrast, there are studies showing how pest species, particularly insects, have a smaller impact on agricultural production when PC systems are used compared to MC systems (Altieri and Nicholls, 2013). The main reasons for this difference are the habitat heterogeneity, which results in a lower colonization of herbivores; a lower rate of reproduction as a result of chemical substances produced by the different plants that make up the system; camouflage; feeding inhibition by non-host plants and by a greater diversity of species that act as natural enemies of herbivores (Andow, 1991).
However this is not always true. Altieri (1992) considered that the resulting effect of agricultural management strategies depends to a great extent on the biology of the pest species, the abundance of its natural enemies, the duration of the crop in the agroecosystem and the vegetation around the crop. Entomopathogenic fungi (HEP) are natural enemies of the majority of insects considered pests of economic importance (Dent, 1999; Ames, 2004). There are few studies that have evaluated the distribution of pests insect and its associated HEP in crops. It is essential to study the components that involve the three trophic levels (plant, herbivore and HEP), since these components have implications for the design of integrated pest management strategies (Altieri, 1995). In addition, HEPs can be used as biological control agents as part of integrated pest management.
Representatives of the Acridoidea MacLeay (Orthoptera) superfamily, commonly known as grasshoppers or chapulines, are an important group among insects that affect Mexican crops, as more than 12 species have occasional population outbreaks causing severe damage (Kevan, 1977; Barrientos-Lozano, 2001). HEP, as Beauveria bassiana. (Balsamo), Metarhizium anisopliae (Metchnikoff) Sorokin and Entomophaga grylli Fresen, are natural enemies of grasshoppers and in some cases can regulate its populations (Uribe-González and Santiago-Basilio, 2012) when used as biological control agents in agro-ecosystems (Díaz et al., 2006).
The state of Michoacán is considered one of the most affected by grasshoppers plagues in México (Fontana et al., 2008). Its outbreaks occur cyclically during the rainy season, causing significant damage to crops, which demands the implementation of phytosanitary campaigns, in which the main control strategy is the massive application of synthetic insecticides (Bahena-Juárez and Velázquez-García, 2012), despite the negative consequences it entails for the agroecosystems sustainability and the risk to the producers health (Barrientos-Lozano and Almaguer-Sierra, 2009). In order to develop integrated management strategies for the control of these insects, it is necessary to know the pest response to the variation in the cultivation practices of the region, so that the objective of this paper was to evaluate the effect of the management system (MC or PC) and crop margin vegetation (VMM and VMP) on the richness and abundance of grasshoppers (CS) and its associated HEPs in the municipality of Erongarícuaro, Michoacán.
Materials and methods
Study area and sampling design
The study was conducted in the municipality of Erongarícuaro, which is part of the Basin of Patzcuaro Lake in Michoacan state. The region has a subhumid temperate climate with summer rains (Cw). The temperature range is between 5 and 26°C with an average annual rainfall of 1 040.8 mm (INEGI, 2009). It has a vegetation type of pine and oak forest (Díaz-Barriga et al., 1988).
Three sites with plots for maize production were selected. The first site is located in the town of Ex Hacienda Charahuén (19° 31’ 23”-19° 31’ 13” north latitude and 101° 42’ 32”-101° 42’ 19” west longitude), while the remaining two are in the locality of Napízaro (19° 35’ 94”-19° 35’ 54” N and 101° 41’ 85”-101° 41’ 51” west longitude and 19° 36’ 10”-19° 36’ 00” north latitude and 101° 41’ 93”-101° 41’ 56” west longitude). In all three sites the presence of grasshoppers was observed in the fallow period (May 2015), when most of its species are in the egg phase. The observations were confirmed through interviews with the producers.
In each site two plots with an area of 9×200 m were arbitrarily selected, one with agricultural management of MC of maize and its monoculture marginal vegetation (VMM) and another of PC composed of maize, beans, zucchini and its polyculture marginal vegetation (VMP). Three replicates of each type of agricultural management were carried out, with their respective marginal vegetation, giving a total of six plots, none of which were applied with insecticide.
Grasshoppers were collected once per month during the growing season (July to December, 2015). The collections were made in transects covering the plot longitudinally, one for the interior of the MC (MC-I), another for the PC (PC-I) and another for the marginal vegetation of each one (VMM and VMP). The collections were carried out the third week of each month, with a striking network of 33 cm in diameter, between 10 and 13 h, by 200 strokes of net per transect to a height of 1.50 m. The collected grasshoppers were introduced alive into one-liter plastic containers and transferred to the laboratory. One specimen of each morphospecies was fixed in 70% alcohol and identified to species level based on criteria of Mariño et al. (2011). Some specimens were identified to subfamily level, due to the absence of adult male specimens. The findings were corroborated by the Colección Nacional de Insectos del Instituto de Biología of the UNAM.
Isolation and identification of HEP
Grasshoppers collected in the field were confined in one liter containers at a density of ten specimens per container at room temperature. They were feed with commercial oat flakes and lettuce disinfected by immersion in 5% sodium hypochlorite solution for 20 min and then rinsed with distilled water. This procedure was performed for up to two weeks. The dead individuals were placed in individual wet chambers, consisting of a 3.5×4.5 cm plastic container with a lid and a filter paper in the bottom, moistened every 2 d with 100 μL of distilled water for 10 d, in order to induce sporulation of HEP.
The isolation of HEP was carried out in a laminar flow hood and consisted of the scraping of conidia present on the cadaver and its seeding in potato dextrose agar medium (PDA) in Petri dishes, which were incubated at 27 °C for four to six days. During this period, mycelial growth was observed every 48 h. The pure cultures were obtained by performing re-insultation of the fungus from the original culture (Monzón, 2001). The isolates obtained will be integrated into the HEP collection of the Centro Nacional de Referencia de Control Biológico (CNRCB).
The identification of grasshopper HEP was performed by macro and microscopic observation of the growth morphology and fungus structures (mycelium, conidia and conidiophores). Dichotomous keys (Samson et al., 1988) were used to identify HEP genres. HEP richness and the number of infected grasshoppers were recorded.
A Bray-Curtis matrix was constructed with data similarity measures of the cumulative abundance of the six samples of each species using the Biodiversity Pro version 2 package (McAleece, 1997), in order to obtain the ecological distances expressing the differences in the structure and composition of the grasshopper community among plots (Bray and Curtis, 1957) as well as an array of geographical distances between plots transformed log+1 to homogenize the extreme distances (Su et al., 2004; Manly, 2006). With these two matrices, the Mantel (1967) test was applied with 10 000 permutations, using the XLSTAT statistical software (Addinsoft, 2009) for Excel, to explore the spatial independence of the samples and use them as replicas in the statistical analyzes.
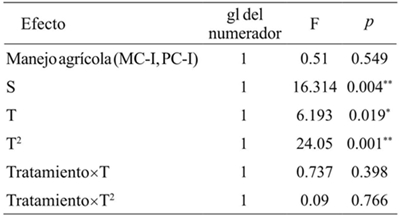
To test the effect of treatments on the abundance of grasshopper species and on abundance of the dominant species, a mixed linear model was used using the statistical software R version 3.2.3. (Pinheiro and Bates, 2000). The model was adjusted using the “lme” function of the “nlme” library for R (Pinheiro et al., 2007). As predictors there were included the agricultural management, time, both linear term (T) as square (T2) and their interaction. The observed species richness and abundance of the dominant species in the marginal vegetation were included as covariates (S) in wealth and abundance models, respectively. The site and plot were included as random factors, in order to model the spatial association of treatments and the different collections over time, respectively. The model’s equations for richness and abundance were:
Where: S= covariables: grasshopper species richness and abundance of the dominant species in the marginal vegetation of monoculture and polyculture; T= time.
In both cases the response variable was transformed with log (response+1) in order to meet the assumptions of variance homogeneity and residues normality. Finally, the association of the frequency of HEP infection isolated in grasshoppers with the treatments in which they were collected was reviewed, using a Chi square test.
Results and discussion
CS composition
2920 grasshoppers belonging to two families of four subfamilies, ten genera and 17 species were collected. 97.8% of all individuals collected belonged to a single species, Sphenarium purpurascens purpurascens Charpentier (Table 1).

Tratamientos: MC-I= interior del monocultivo, PC-I= interior del policultivo. Covariables: VMM= vegetación marginal del monocultivo y VMP= vegetación marginal del policultivo. Datos acumulados de muestreos con redes de golpeo realizados entre julio y diciembre de 2015.
Table 1 Grasshoppers species and their abundance in two types of agricultural management and vegetation of the plots margins in Erongarícuaro, Michoacán.
The apparent dominance of S. p. purpurascens in the study sites implies that this is the most economically important species for mazie producers in the region. Mantel’s test showed that the structure and composition of the CS in the plots were not correlated with geographical distances between them (R= -0.178, p= 0.348) so they were considered as independent samples for comparative analysis (Table 2).
Evaluation of the management system on the richness of grasshopper species
Species richness changed significantly in the T being the quadratic term that best described the change (p< 0.05). The agricultural management had no significant effect on overall species richness (p> 0.05) nor modified the temporal trend of richness (p> 0.05). Species richness in the marginal vegetation did not explain species richness in plots (p> 0.05, Table 3). The variation explained by the fixed effects of the model was 29%. The random effects (site/plot) did not have an additional explanatory capacity.

Modelo lineal mixto tratamientos: MC-I= interior del monocultivo; PC-I= interior del policultivo. S: covariables T= tiempo; T2= tiempo cuadrático; gl= grados de libertad; F= de distribución F de Fisher. *= p< 0.05; **= p< 0.01.
Table 3 Grasshoppers richness in two types of agricultural management and vegetation of the plots margins in Erongarícuaro, Michoacán.
Abundance of S. p. purpurascens
Abundance of S. purpurascens purpurascens changed significantly in the T. The quadratic term was the one that best described this change (p< 0.05). The agricultural management had no significant effect on the abundance of S. p. purpurascens, or alter the temporal trend of abundance (p> 0.05, Table 4). The abundance of this species in the VMM and VMP sites and the abundance in the MC-I and PC-I (p< 0.05, Figure 1 and Table 4). The variation explained by the fixed effects of the model was 57%. The random effects did not have an additional explanatory capacity.
Tratamiento: MC-I0= interior del monocultivo; PC-I= interior del policultivo S= covariables (vegetación marginal del monocultivo y vegetación marginal del policultivo). T= tiempo; T2= tiempo cuadrático. gl= grados de libertad; F=distribución de Fisher; *= p< 0.05; **= p< 0.01.
Table 4 Comparison of the abundance of Sphenarium pupurascens purpurascens two types of agricultural management and the vegetation of plots margins in Erongarícuaro, Michoacán.

Figure 1 Average monthly abundance of S. p. purpurascens in two types of agricultural management and margins plots vegetation in Erongarícuaro, Michoacán,2015. N= 72. Treatments: MC-I= monocultures interior; PC-I= polyculture interior. Covariables: VMM= marginal vegetation of monoculture; VMP=marginal vegetation of polyculture
The PC-I did not hinder the attack of S. p. purpurascens, so no low abundances were reported. In fact, in September, the higher abundance was recorded in this type of agricultural management than in the MC-I. This insect is considered polyphagous (Cano-Santana and Oyama, 1994; Tamayo-Mejía, 2009), and according to the results of this study, plant diversity increased its abundance, since VMM and PMV showed higher values than those recorded in MC-I and PC-I. S. p. purpuascens abundance in margins vegetation influenced the abundance found in the MC-I and PC-I (Table 4).
Cerritos and Cano-Santana (2008) reported that the density of S. purpurascens eggs was 14.9 times higher at the edges of the alfalfa plots than within the plots. This contrasts with Andow (1991) that a particular pest will find less possible hosts in a more diverse habitat and, therefore, pest insects are less harmful in polycultures compared to monocultures. However, insects with a broad spectrum of hosts usually do not reduce their population by crop diversity (Andow, 1991). Therefore, other strategies should be used for their control.
The maximum abundance of S. p. purpurascens of PC-1 the VMM and VMP was recorded in August except the MC-I which increased in September and subsequently decreased. Cano-Santana (1994) reported that the highest densities of S. purpurascens were recorded in early July on abrupt and flat sites s of the Reserve Pedregal de San Angel in México City in 1990 and 1991. In 1991 it was observed that after achieving the máximum density, the population declined drastically in subsequent months.
High humidity combined with low temperatures could have caused high mortality of first instars of S. purpurascens.Tamayo-Mejía (2009) reported that the 1 and 2 nymph stages of grasshopper Melanoplus differentialis (Thomas) and S. purpurascens showed a 50% mortality in field, caused by biotic and abiotic factors. Based on the results of this paper and those mentioned above it is inferred that the dynamics of populations of S. p. purpurascens is affected by environmental conditions and herbaceous vegetation of the study sites (Cano-Santana, 1994; Cerritos and Cano-Santana, 2008; Tamayo-Mejía, 2009).
It is necessary a precise study of the interaction between S. p. purpurascens and weeds found in the plot margins, since it may be useful for manipulation of the composition of weeds species in crop margins to function as trap vegetation. It is important to carry out assessments in plants that have demonstrated repellent properties and manipulate its presence within the crops, in order to push away S. p. purpurascens from the main crop. Among the species that can be evaluated are include castorbean (Ricinus communis L.), garlic (Allium sativum L.) and marigolds (Tagetes erecta L.) (Grainge and Ahmed, 1988; Rodríguez and Nieto, 1997; Vidal et al., 2008). This would allow having areas that concentrate a greater diversity of plants with a specific purpose within the agroecosystem, in order to minimize the damage that the grasshopper causes to the main crop.
In this paper the abundance of S. p. purpurascens in the VMM and the VMP determined the abundance of the species within the plots regardless of the type of agricultural management used (MC-I or PC-I). Besides the integrated pest management, there is another alternative to regulate S. purpurascens populations, consisting of harvesting to use it as a food source. At present this insect is sold in markets in the State of Mexico and the Central Valleys of Oaxaca, where it forms part of the regional cuisine (Marcos et al., 2015).
Cerritos and Cano-Santana (2008) compared the impact of malathion insecticide application with the capture of S. purpurascens for human consumption on the density of ootheques m-2 within the alfalfa plot and its margins in two years. The ootheques density was lower in plots where malathion was applied with respect to those of mechanical control through harvesting. However, the density of ootheques in the mechanical control was lower compared to the control. The eggs density at the plots margins was 14.9 times higher than those found within the plots. The oviposition areas of female S. p. purpurascens were found most frequently in the edges of the plots, because in these areas the soil is less compact (Cerritos and Cano-Santana, 2008). The mechanical control method is a viable alternative to regulate populations of S. p. purpurascens, plus it is a profitable product for the above regions (Cerritos and Cano-Santana, 2008).
HEP isolated in S. p. purpurascens
Of 2920 individuals collected, 12 species of S. p. purpurascens were found infected by HEP, which corresponds to 0.41% of the total (Table 5). All HEP were isolated from adult individuals, but two nymphs on the site of the VMM infected with Metarhizium sp. Sorokin. 75% of infected grasshoppers had Metarhizium sp. and the remaining 25% were found infected by Beauveria sp. (Balsamo) Vuillemin. 16.7% of the grasshoppers infected with HEP were collected in the MC-I, 41.7% in the PC-I, 16.6% in the VMM and finally 25% in the VMP. Generally speaking, the fungus with the highest infection frequency of grasshoppers in the region was Metarhizium sp., Finding an association between the proportion of infected grasshoppers with this HEP on the PC-I, compared to those who were found infected in the VMP (x 2 = 3.84, p= 0.04). In the case of the other sites no significant association (p> 0.05) was found (Table 5).
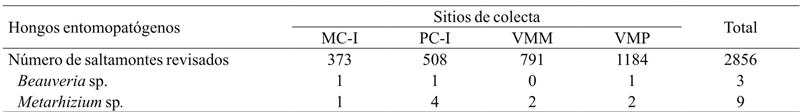
MC-I= interior del monocultivo; PC-I= interior del policultivo; VMM= vegetación de los márgenes del monocultivo; VMP= vegetación de los márgenes del policultivo.
Table 5 Sphenarium purpurascens purpurascens infected with entomopathogenic fungi in maize agroecosystems of the Lake of Patzcuaro Basin region.
HEP found infecting S. p. purpurascens, had minimal presence in maize agroecosystems. It is known that Metarhizium and Beauveria show low levels of natural infection in CS compared to E. grylli, which it is a species that was expected to find in this research, as is has been documented infecting different populations of grasshoppers in México and in other countries in the Americas (Sánchez et al., 2011; Uribe-González y Santiago-Basilio, 2012). However, in this paper there was an association between Metarhizium sp. and PC-I compared to the VMP, so that the infection of Metarhizium seems to be favored by plant diversity, since in these systems, the microclimate changes mainly by increasing relative humidity, favoring the presence of HEP (Van Huis, 1997).
Conclusions
17 species were found, of which the most abundant was S. p. Purpurascens. The abundance of this species in the vegetation of the crops margins influenced the abundance of the interior of the crops. The frequency of HEP infection was low. Metarhizium sp. had a higher infection frequency of in PC-I compared to VMP. Of the HEP isolates three were Beauveria sp. and nine were Metarhizium sp.
Literatura citada
Addinsoft. 2009. XLSTAT versión 2009. http://www.xlstat.com. [ Links ]
Altieri, M. A. 1995. Agroecology: The science of sustainable agriculture.First edition. Westview Press Boulder. United States.30-45 pp. [ Links ]
Altieri, M. A. y Nicholls, C. I. 2000. Teoría y práctica para una agricultura sustentable. Primera edición. Red de Formación Ambiental para América Latina y el Caribe. México. 13-43 pp. [ Links ]
Altieri, M. A. y Nicholls, C. I. 2005. Agroecology and the search for a truly sustainable agriculture. First edition. United Nations Environmental Programme, Environmental Training Network for Latin America and the Caribbean. México. 13-27 pp. [ Links ]
Altieri, M. A. y Nicholls, C. I. 2013. Diseños agroecológicos para incrementar la biodiversidad de entomofauna benéfica en agroecosistemas. Primera edición. SOCLA. Medellin, Colombia. 83 pp. [ Links ]
Andow, D. A. 1991. Vegetational diversity and arthropod population response. Annual review of entomology. 36:561-586. [ Links ]
Bahena-Juárez, F. y Velázquez-García, J. D. J. 2012. Manejo agroecológico de plagas en maíz para una agricultura de conservación en el valle de Morelia-Querendaro, Michoacán. http://biblioteca.inifap.gob.mx:8080/xmlui/handle/123456789/3541. [ Links ]
Barrientos-Lozano, L. 2001. El problema de la langosta (Schistocerca piceifrons piceifrons Walker) en México y Centro América.In: Barrientos Lozano, L. (ed.). Memoria del Curso 1 Internacional. Ecología, manejo y control de la langosta voladora (Schistocerca piceifrons piceifrons Walker). Altamira, Tamaulipas, México. 1-11 pp. [ Links ]
Barrientos-Lozano, L. 2004. Orthoptera. In: Llorente, J.; Morrone J.J.; Yañes O. y Vargas, I. (eds.). Biodiversidad, taxonomía y biogeografía de atrópodos de México: Hacia una síntesis de su conocimiento. Primera edición. Universidad Nacional Autónoma de México. México. 603-625 pp. [ Links ]
Barrientos-Lozano, L. y Almaguer-Sierra, P. 2009. Manejo sustentable de chapulines (Orthoptera: Acridoidea) en México. Vedalia(México). 13(2):51-56. [ Links ]
Bray, J. R. y Curtis, J. T. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological monographs(United States). 27(4):325-349. [ Links ]
Brechelt, A. 2004. El manejo ecológico de plagas y enfermedades. Primera edición. Red de acción de plaguicidas y sus alternativas para América Latina (RAP-AL). Chile. 36 pp. [ Links ]
Cano, S. Z. y Oyama, K. 1994. Wigandia urens (Hydrophyllaceae): un mosaico de recursos para sus insectos herbívoros. Acta Botánica Mexicana 28:29-39. [ Links ]
Cañedo, V. y Ames, T. 2004. Manual de laboratorio para el manejo de hongos entomopatógenos. Centro Internacional de la Papa.Primera edición. Lima, Perú. 1-60 pp. [ Links ]
Cerritos, R, y Cano-Santana, Z. 2008. Harvesting grasshoppers Sphenarium purpurascens in Mexico for human consumption: a comparison with insecticidal control for managing pest outbreaks. Crop Protection. 27(3) 473-480. [ Links ]
Del Val, E.; Arnés, E.; Gaona, J. A. y Astier, M. 2013. Incidencia de gallina ciega, sistemas de manejo campesino y variabilidad climática en la comunidad de Napízaro, Michoacán, México.Agroecología. 1(8):53-62. [ Links ]
Dent, D. 1999 Biological agents as biopesticides. CABI Bioscience Biopesticide Programme. Programme Briefing Paper. 15 p. [ Links ]
Díaz, M. P.; Macías, A. F.; Navarro, S. R. y De La Torre, M. 2006.Mecanismo de acción de los hongos entomopatógenos.Interciencia: Revista de Ciencia y Tecnología de América.31:856-860. [ Links ]
Díaz-Barriga, H.; Guevara-Fefer, F. y Valenzuela, R. 1988. Contribución al conocimiento de los macromicetos del estado de Michoacán.Acta Botánica Mexicana. 12(31):21-44. [ Links ]
Fontana, P.; Buzzetti, F. M. y Mariño-Pérez, R. 2008. Chapulines, langostas, grillos y esperanzas de México: guía fotográfica.México. Primera edición. Editado por asociación mundial de la biodiversidad. 266 p. [ Links ]
González, E. A.; Gutiérrez, J. I.; Calderón, A. E.; Vázquez, J. C. y Wood, S.2008. Impacto económico del mejoramiento genético del maíz en México: híbrido H-48. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Primera Edición. Coyoacán, Ciudad de México, México. 78-85 pp. [ Links ]
Grainge, M. y Ahmed, S. 1988. Handbook of plants with pest-control properties. First edition. Ilustrada Minnesota. 470 p. [ Links ]
INEGI (Instituto Nacional de Estadística y Geografía). 2009. Prontuario de información geográfica Municipal de los Estados Unidos Mexicanos: Erongarícuaro, Michoacán de Ocampo. http://www.inegi.org.mx/sistemas/mexicocifras/datos-geograficos. [ Links ]
Kevan, D. K. 1977. The American Pyrgomorphidae (Orthoptera). Revista de la Sociedad Entomológica Argentina. 36(4):3-28. [ Links ]
Manly, B. F. 2006. Randomization, bootstrap and Monte Carlo methods in biology. Third Edition. Chapman & Hall/CRC. New York.480 pp. [ Links ]
Mantel, N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Research (Gran Bretaña).2(27):209-220. [ Links ]
Marcos, Y. S.; Pacheco, R. P.; Pérez, G. E. G.; Manzanero, G. I. y Medina,G. R. O. 2015. Conocimiento tradicional y valor cultural de Sphenarium spp. en valles centrales de Oaxaca. Revista Mexicana de Agroecosistemas. 2(2):75-86. [ Links ]
Mariño, R.; Fontana P. y Buzzetti, F. M. 2011. Taxonomía y bioecología. In: García C. y Lozano J. (coords.). Control biológico de plagas de chapulín en el norte ~ centro de México. Primera edición.Universidad autónoma de Zacatecas. México. 13-56 pp. [ Links ]
McAleece, N. 1997. Bio Diversity Professional version 2. http://www.sams.ac.uk/peter-lamont/biodiversity-pro. [ Links ]
Monzón, A. 2001. Producción, uso y control de calidad de hongos entomopatógenos en Nicaragua. Manejo Integrado de Plagas.63:95-109. [ Links ]
Nicholls, C. I. 2008. Bases agroecológicas para diseñar e implementar una estrategia de manejo de hábitat para control biológico de plagas. Agroecología. 1:37-48. [ Links ]
Nicholls, C. I. y Altieri, M. A. 2006. Manejo de la fertilidad de suelo e insectos plaga: armonizando la salud del suelo y la salud de las plantas en los agroecosistemas. Manejo Integrado de Plagas y Agroecología. 77:8-16. [ Links ]
Pinheiro, J. C.; Bates, D. M. 2000. Mixed-effects models in S and S-Plus.Springer. 3-56 pp. [ Links ]
Pinheiro, J.; Bates, D.; DebRoy, S. y the R Core team. 2007. Linear and Nonlinear Mixed Effects Models. R package version 3. 1-83. [ Links ]
Rodríguez, H. C. y D. Nieto. 1997. Anonáceas con propiedades insecticidas. In: Rebouças, S. J.; A. I. Vilas Boas.; O. Magalhaes y Hojo R. (eds.). En Anonáceas, produção e mercado (pinha, graviola, atemóia e cherimólia). Primeira edição. Nobel. Bahía Brasil. 15-35 pp. [ Links ]
SAGARPA. 2010. Retos y oportunidades del sistema agroalimentario de México en los próximos 20 años. http://www.sagarpa.gob.mx/agronegocios/documents/pablo/retosyoportunidades.pdf. [ Links ]
Samson, R. A.; Evans, H. C. y Latgé, J. P. 1988. Atlas of entomopathogenic Fungi. Primer edición: Springer Science y Business Media. New York. 5-17 pp. [ Links ]
Sánchez, S. E. M.; Humber, R. A. y Freitas, A. L. 2011. El complejo Entomophaga grylli (Fresenius 1856) Batko (Zygomycetes: Entomophthorales) infectando saltamontes (Orthoptera: Acrididae) en Ilhéus (Bahia). Entomotropica (Brasil).24(2):71-81. [ Links ]
Su, J. C.; Debinski, D. M.; Jakubauskas, M. E. y Kindscher, K. 2004.Beyond species richness: Community similarity as a measure of cross‐taxon congruence for coarse‐filter conservation.Conservation Biology (United States). 1(18):167-173. [ Links ]
Tamayo-Mejía, F. 2009. Control biológico de Sphenairum purpurascens(Charpentier) y Melanoplus differentiales (Thomas)(Orthoptera: acrididae) con Metarhizium anisopliae (Metschnikoff) Sorokin. Guanajuato, México. Vedalia(México). 13(2):85-90. [ Links ]
Toledo, V. 2010. La biodiversidad de México: Inventarios, manejos, usos, informática, conservación e importancia cultural. Primera edición. FCE CONACULTA. México. 356 p. [ Links ]
Uribe-González, E., y Santiago-Basilio, M. Á. 2012. Contribución al conocimiento de enemigos naturales del chapulín (Orthoptera: Acridoidea) en el estado de Querétaro, México. Acta Zoológica Mexicana (México). 1(28):133-144. [ Links ]
Van Huis, A. 1997. Manipulating crop diversity to control pest. Entomology. 26th International Course on Integrated Pest Management. Wageningen, the Netherlands. 21-22 pp. [ Links ]
Vidal, J., Carbajal, A., Sisniegas, M., y Bobadilla, M. 2008. Efecto tóxico de Argemone subfusiformis Ownb. y Tagetes patula Link sobre larvas del IV estadio y pupas de Aedes aegypti L. Revista Peruana de Biología (Perú). 2(15):103-110. [ Links ]
Received: June 2017; Accepted: September 2017











 text in
text in 



