Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 n.6 Texcoco Aug./Sep. 2017
Articles
Culture medium and agar substitutes for in vitro growth of orchids
1Colegio de Postgraduados-Campus Montecillo. Carretera México-Texcoco km 36.5. Montecillo, Texcoco, Estado de México. CP. 56230. Tel. (595) 20200, ext. 1581.(floresh.luis@colpos.mx; morisonia-77@hotmail.com).
The in vitro culture is a technique that has allowed the spread of different species of orchids, but the slow growth of this group of plants, and the cost of the minerals and the gelling agent (agar) used in the culture media, can limit its application. The aim of this research was to understand the effect of the composition of the culture medium and the use of substrates as agar substitutes for in vitro growth of Laelia anceps Lindl. And Epidendrum sp. Adventitious buds of both species were cultured in vitro in different substrates mixtures (perlite, volcanic rock and coir) and agar with different concentrations of Murashige and Skoog basal salts (50 and 100%) and gibberellic acid (AG3) (0 and 1 mg L-1) to evaluate its length, number of leaves, number of roots and fresh weight in an experimental design with three factors and 15 repetitions, each repetition consisted in a flask with 5 adventitious shoots. The in vitro behavior was based on the genotype L. anceps Lindl. that responded better tan Epidendrum sp. Outbreaks grown on perlite-volcanic rock or coir-volcanic rock, and médiums with 50% salts and AG3 showed no significant difference in terms of length, number of leaves, number of roots and fresh weight, with respect to those grown in agar, 100% salts without AG3. The culture médium with diluted with salts without AG3 and substrates as substitutes for agar, allowed the in vitro growth of L. plants anceps Lindl. And Epidendrum sp. plants with a significant 60% costs reduction.
Keywords: Laelia anceps Lindl; Epidendrum sp.; culture medium substrates; gibberellic acid; in vitro culture
El cultivo in vitro es una técnica que ha permitido la propagación de distintas especies de orquídeas, pero el lento crecimiento de este grupo de plantas, así como el costo de las sales minerales y del agente gelificante (agar) empleados en los medios de cultivo, pueden limitar su aplicación. El objetivo del presente trabajo, fue conocer el efecto de la composición del medio de cultivo y el uso de sustratos como sustitutos del agar en el crecimiento in vitro de Laelia anceps Lindl. y Epidendrum sp. Brotes adventicios de ambas especies se cultivaron in vitro en distintas mezclas de sustratos (perlita, tezontle y fibra de coco) y agar con diferentes concentraciones de las sales basales de Murashige y Skoog (50 y 100%) y ácido giberélico (AG3) (0 y 1 mg L-1) para evaluar su longitud, número de hojas, número de raíces y peso fresco en un diseño experimental con tres factores y 15 repeticiones, cada repetición consistio en un frasco con 5 brotes adventicios. El comportamiento in vitro, estuvo en función del genotipo; L. anceps Lindl. respondió mejor que Epidendrum sp. Los brotes crecidos sobre perlita-tezontle o fibra de coco-tezontle, y medios con sales al 50% y AG3 no mostraron diferencias significativas en cuanto a su longitud, número de hojas, número de raíces y peso fresco, con respecto a aquellos que lo hicieron en agar, sales al 100% y sin AG3. Los medios de cultivo con sales diluidas sin AG3 y sustratos como sustitutos del agar, permitieron el crecimiento in vitro de las plantas de L. anceps Lindl. y Epidendrum sp. con una reducción significativa del 60% de los costos.
Palabras clave: Laelia anceps Lindl.; Epidendrum sp.; ácido giberélico; cultivo in vitro; medio de cultivo; sustratos
Introduction
México has a great diversity in animal and vegetal species, to take care and to preserve such diversity is very important. In this regard, México is home to a remarkable wealth of orchids, which has been recorded in 1 260 species and 170 genera (Soto and Salazar, 2004; Hágsater et al., 2005). Of these, 181 are included in some risk category in the official norm in force NOM-059-ECOL-2001 (Diario Oficial de la Federación, 2002), 72 are endemic, 58 are in the threatened category, 107 require special protection, 15 are endangered and one species is already extinct in the wild (Laelia gouldiana Rchb. F.) (Diario Oficial de la Federación, 2002).
Laelia anceps Lindl. And Epidendrum sp. are two orchids species that due to their striking flowers have been subjected to a high collection pressure. This pressure, coupled with habitat destruction has led to the rapid decline in their populations (Halbinger and Soto, 1997; Romero-Tirado et al., 2007).
With respect to its reproduction, germination in orchids represents one of the major limitations to its survival, since the endosperm is reduced in some species, while in others it is absent, so to ensure its germination it is necessary that the seeds are associated with mycorrhizal fungi that provide them with nutrients (Téllez, 2011).
The in vitro culture is a technique which facilitates the germination and propagation of virtually any orchid, as it is done aseptically, in the presence of a controlled source of nutrients and physical conditions, which potentiates its reproduction and growth ability (Zettler et al., 2001; Salazar et al., 2013). However, the cost of the materials used in the preparation of the culture media is high, especially the gelling agent (agar or phytagel) which can represent up to 70% of the cost of the plants. In addition, the agar may reduce the oxygen concentration and disable the nutrient diffusion in the medium (Fujiwara et al., 1993; Ichimura and Oda, 1998).
Furthermore, for some species (Laelia halbigeriana Salazar & Soto Arenas, Agave cocui Trelease) it has been documented that the full concentration of the Murashige and Skoog (1962) medium salts, commonly used in tissue culture, exceeds the tissue nutrimental requirements (Raya-Montaño et al., 2011; González et al., 2012). Also, the slow growth of most orchids, contributes to increased production costs, having to keep plants in vitro longer until they reach a size that allows to transfer them to the greenhouse.
The reduction of production costs could be achieved with the use of hydroponic substrates (perlite, vermiculite, coir), as they may be an alternative to replace expensive gelling agents. In this regard, there is evidence that the use substrates during in vitro phase has allowed growth in species like Limonium latifolium Lindl., Ipomoea batatas L., and Myrtus communis L. (Afreen-Zobayed et al., 1999; Afreen-Zobayed et al., 2000; Lucchesini et al., 2006; Xiao y Kozai, 2006).
Similarly, including phytoregulators as gibberellic acid in the culture medium has had a positive effect on stem elongation and the number of formed leaves in Cattleya loddigesii Lindl., Manihot esculenta Crantz, Cynodon dactylon Pers. and Cuscuta chinensis Lam. (Maheshwari et al., 1980; Bhagwat et al., 1996; Li and Qu, 2002; Ávila and Salgado-Garciglia, 2006; Rodrigues et al., 2009).
Given the importance of taking care and preserve the diversity of Mexican orchids and to propose alternatives to reduce production costs of it in vitro propagation, the objective of this research was to determine the effect of the composition of the culture medium and the use of substrates as substitutes for agar, in the in vitro growth of Laelia anceps Lindl. And Epidendrum sp. Based on the assumption that diluted culture means supplemented with gibberellic acid, and substrates would enable the in vitro growth of both species.
Materials and methods
Culture mediums
The culture mediums tested for the growth of adventitious buds of Laelia anceps Lindl. and Epidendrum sp. of 1.1 ±0.1 cm, previously regenerated in vitro, containing nutrient salts of Murashige and Skoog (MS) 50 and 100%, 30 g L-1sucrose, 0 and 1 mg L-1 gibberellic acid (AG3), agar (7 g L-1) or 30 ml of substrate mixtures: pearlite-volcanic rock (PT) and coir-volcanic rock (FCT) in 3:1 ratio (0.5 mm particle size). The médium pH was adjusted to 5.7-5.8.
Planting of crops
Adventitious shoots were placed in 250 ml glass vials containing 30 ml of the culture medium described above plus the PT, FCT or agar substrates (Table 1). Previously, the flasks were sterilized in an autoclave for 15 minutes at 120 C before the establishment of the shoots (explants).
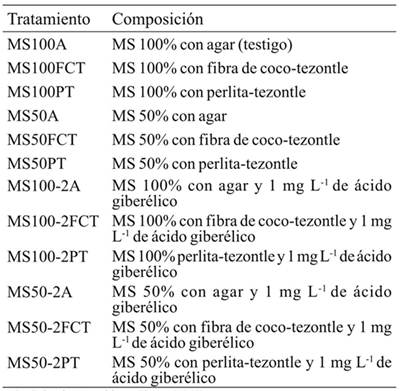
MS= Sales de Murashige y Skoog (1962).
Table 1 Tested treatments in the in vitro culture of Laelia anceps Lindl. and Epidendrum sp.
Cultures were incubated in a growth room at 26 ±2 °C and a photoperiod of 16 h provided by cold white fluorescent lamps (25 µmol m-2 s-1 light intensity).
Evaluated variables
After 45, 90 and 135 days of initiating the crop, the length of the shoot (from the beginning of the root to the tip of the longest leaf), number of leaves and number of roots were evaluated; and the fresh weight was evaluated at 60 days using an analytical balance.
Experimental design
The experiment was carried out in the Laboratory of Biotechnology and Pathology of Seeds of Campus Montecillo, Colegio de Postgraduados. A completely randomized experimental design was used with three factors: species (2), substrates (3) and médium culture (4) resulting in 24 treatments. Each treatment had 15 replicates and each replicate consisted of one flask with five adventitious shoots. The data of each variable were analyzed by analysis of variance (ANOVA) and the comparison of means by the Tukey test (≤ 0.05). Both procedures were performed with the SAS v.9.0 statistical program (SAS Institute, 2002).
Results and discussion
Effect of the species
The analysis of the results showed that the species had a significant effect on shoot length (45, 90 and 135 days), number of leaves (45 days) and number of roots (90 and 135 days) (Table 2). After 45 days of culture, the Epidendrum sp species, showed better response for shoot length; however, after this time it was Laelia anceps Lindl. which showed the highest values (Table 3). The number of leaves of the shoots of Laelia anceps Lindl. after 45 days was higher than Epidendrum sp. but at 90 and 135 days both species behaved in a similar way.
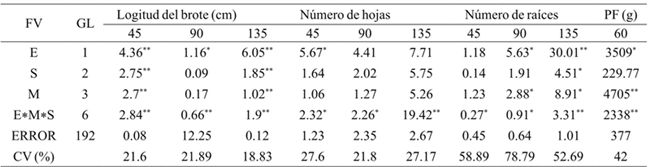
**= p≤ 0.01; *= p≤ 0.05; CV= coeficiente del variación; E= especie; M= medio; S= sustrato; PF= peso fresco.
Table 2 Squares sum of variance analysis for the effect of the species, substrates and culture medium on shoot length, number of leaves, number of roots and fresh weight of Laelia anceps Lindl. and Epidendrum sp. after 45, 90 and 135 days of culture.
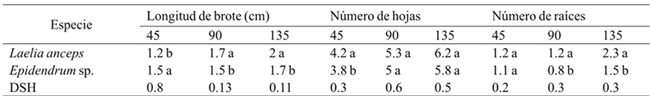
DSH= diferencia significativa honesta. Letras distintas en una columna indican diferencias estadísticas significativas (Tukey≤ 0.05).
Table 3 Effect of the species on shoot length, number of leaves and number of roots at 45, 90 and 135 days after starting the in vitro cultivation.
Moreover, for the number of roots there were no statistical differences during the first 45 days between the two species, but after this period L. anceps Lindl. showed significantly higher values tan Epidenderum (Table 3). Regarding these results, the genotype effect on the in vitro growth was also observed in Cattleya aurantiaca Bateman, Encyclia adenocaula La Llave & Tex., Laelia speciosa Kunth., Epidendrum radicans Pav. Ex Lindl., Euchile citrina La Llave ex Lex., Laelia albida Bateman ex Lindl., Laelia autumnalis (Lex.) Lindl., Oncidium cavendishianum Bateman and Oncidium tigrinum La Llave & Lex. (Ávila and Salgado-Garciglia, 2006). Also, Abdoli and Moieni (2003) found that genotype greatly influenced the induction of organogenesis in sunflower (Helianthus annuus L.).
Effect of substrates
The substrates had a significant effect on shoot length (45 and 135 days) and number of roots at 135 days (Table 2). After 45 days of cultivation, shoot length was statistically higher in perlite-volcanic rock (PT), but at 135 days the highest values were recorded in shoots grown on agar, while no significant differences were found in treatments with coir-volcanic rock (FCT) and PT (Table 4) (Figure 1). With respect to the number of leaves, no significant differences were found between the shoots grown on different substrates and agar during the 135 days when shoots were kept in vitro.
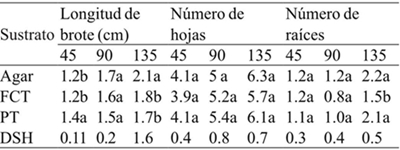
FCT= fibra de coco-tezontle; PT= perlita-tezontle; DSH= diferencia significativa honesta. Letras distintas en una columna indican diferencias estadísticas significativas (Tukey≤ 0.05).
Table 4 Effect of the substrates on shoot length, number of leaves and number of roots at 45, 90 and 135 days after cultivation.
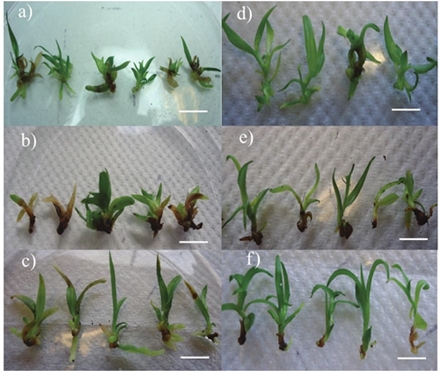
Figure 1 Adventitious buds of Laelia anceps Lindl. (a, b, c) and Epidendrum sp.(d, e, f) 90 days after initiation of in vitro culture. Agar (a, d), coir-volcanic rock (b,e) and perlita-volcanic rock (c, f). Bar 1 cm.
It was also observed that the number of roots was statistically similar in agar, FCT and PT at 45 and 90 days of culture; however, after 135 days FCT-grown shoots had significantly fewer root numbers than those grown on agar or PT (Table 4). In this regard, Keatmetha and Suksa-Ard (2004) found that the use of vermiculite and peat moss during the in vitro culture of Anthurium andraeanum L. did not promote more roots compared to the use of phytagel.
Meanwhile, Afreen-Zobayed et al. (2000) used various combinations of vermiculite and paper pulp to replace agar for in vitro culture of Ipomoea batatas L. obtaining the best results with vermiculite with 30% paper pulp. Also, Labrousse et al. (2012) evaluated Sorbarod (buffer cellulose), peat and paper pulp as alternative supports to agar in micropropagation for Nemesia denticulata (Benth.) Grant ex Fourc., they found that substrates promoted better root development and increased acclimation survival. Also, Hazarika (2006) indicates that the roots of Brassica oleracea L. developed in agar have low functionality, limiting the survival of plants during the acclimatization process. Similarly, Kozai (2010) and Oh et al. (2012) found that substrates of vermiculite, paper pulp and perlite increase water conductivity, which favors the absorption of nutrients by plants.
Effect of culture medium
It was possible to observe significant differences in shoot length (45 and 135 days), and number of roots after 90 and 135 days of cultivation by medium effect (Table 2). At 45 days after starting the cultivation the shoots grown on MS medium with 50% AG3 (MS50-2) showed the greatest length, but at the end of the evaluation this medium and the one containing MS salts at 100% without AG3 showed no statistically differences (Table 5). In this regard, Coello et al. (2010) found that gibberellic acid was the factor that promoted shoot elongation of Guarianthe skinneri Bateman. On the other hand, the number of leaves was not significantly different between the different treatments throughout the crop. In contrast, Rodrigues et al. (2009) found that the use of AG3 Cattleya loddigesii Lindl. growth promoted a greater number of leaves. Also, only the number of roots of shoots grown in MS at 100% was significantly lower than those remaining in MS medium at 50% and AG3 after 90 and 135 days after starting the culture (Table 5).
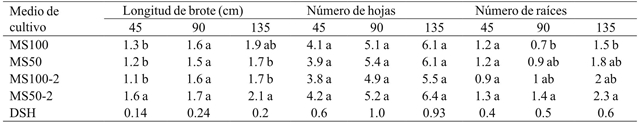
MS= sales de Murashige y Skoog; MS100= MS al 100%; MS50= MS al 50%; MS100-2= MS al 100% con 1 mg L-1 de ácido giberélico; MS50-2= MS al 50% con 1 mg L-1 de ácido giberélico; DSH= diferencia significativa honesta. Letras distintas en una columna indican diferencias estadísticas significativas (Tukey ≤ 0.05).
Table 5 Effect of culture medium on shoot length, number of leaves and number of roots after 45, 90 and 135 days of cultivation.
Meanwhile, Jara et al. (2007) used four mediums (MS 50%, MS 100%, Morel medium, Knudson medium for in vitro germination of Chloraea virescens (Willd.) Lindl., Chloraea lamellata Lindl. and Gavilea araucana Phil., finding that the MS 50% medium induced the best results in the studied species. Similarly, Dalzotto (2013) reported that plant height, number of roots and dry matter at 95 days of in vitro culture of Oncidium bifolium (Sims) Dumort. were higher in the MS 50% medium. The results of this paper show that less concentrated media containing MS salts at 50% did not limit growth of shoots of Laelia anceps Lindl. and Epidendrum sp. which is consistent with the results of Pervin (1997), who observed that orchids of the genera Vanda, Dendrobium, Aerides, Acampe and Apathoglottis can grow in media with few nutrients (peptone, inositol, banana extract or coconut water).
Flores-Escobar et al. (2008) observed that shoots of Oncidium stramineum Lindl. cultured in MS medium supplemented with organic extracts (coconut water, peptone, activated charcoal, polyvinylpyrrolidone) reached a length of 2.05 cm in 90 days, which is a very close to that obtained in the medium containing 50% of the MS salts and 1 mg L-1 of AG3 (MS50-2) in a similar time interval.
Effect of treatments
Significant differences were found for shoot length, number of leaves and number of roots at 45, 90 and 135 days in the interaction factors (Table 2). After 135 days of cultivation, the shoot length in L. anceps Lindl. in the treatments of MS salts at 50%, AG3 and agar (MS50-2A) and 100% MS salts with agar (MS100-A) was statistically higher than the other treatments. However, at 90 days only statistical differences between treatments MS50-2A and MS50A (MS 50% without AG3) and MS100-2PT (MS100%, AG3 and perlite-volcanic rock) (Table 6) were observed.
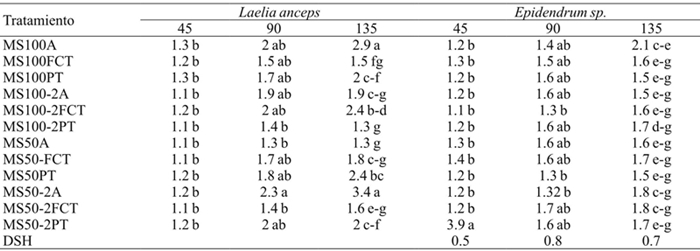
DSH = diferencia significativa honesta. Letras distintas en una columna indican diferencias estadísticas significativas (Tukey ≤ 0.05).
Table 6 Effect of treatments on shoot length of Laelia anceps Lindl. and Epidendrum sp. at 45, 90 and 135 days of culture.
This indicates that outbreaks of L. anceps Lindl. can grow properly in both agar and perlite-volcanic rock or vermiculite-perlite, and MS salts at 50 or 100% with or without AG3 for 90 days, and after this time the growth is better in the media gelled with agar, despite the concentration of salts or AG3. These results agree with those of Martínez et al. (2006) who evaluated the germination, multiplication and rooting of Citrus volkameriana Ten & Pasq., Citrumelo Swingle (Citrus Paradise Macfad cv. Duncan x Poncirus Trifolia L.) and Citrange in vermiculite, perlite and volcanic rock, without finding significant statistical differences between these and the treatments with agar.
These authors also mention that survival during acclimatization was greater in plants coming from substrates, since the agar ones suffered stress caused by the rupture of roots, causing their death. Afreen-Zobayed et al. (2000) found that treatments based on agar in the micropropagation of Ipomoea batatas L. were below the treatments based on vermiculite and paper pulp.
Unlike what was observed in L. anceps Lindl. in Epidendrum sp. no significant differences were found in the length of the shoots submitted to the different treatments tested throughout the growing period (Table 6).
On the other hand, there were no significant differences in the number of leaves formed in shoots of L. anceps Lindl. and Epidendrum sp. after 45 and 90 days, but at 135 days only Epidendrum sp. of the MS50A treatment (50% MS agar) had a statistically different number leaves, while for L. anceps Lindl. the treatment MS50 PT (MS 50% perlita-volcanic rock) induced the formation of a larger number of leaves (Table 7).
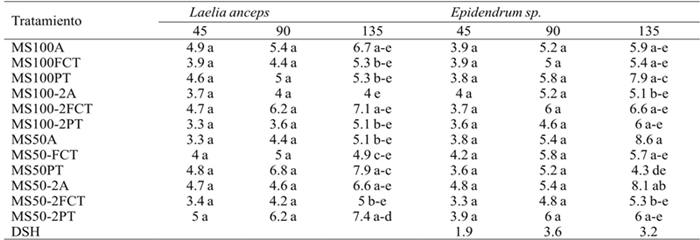
DSH= diferencia significativa honesta. Letras distintas en una columna indican diferencias estadísticas significativas (Tukey ≤ 0.05).
Table 7 Effect of treatments on the number of leaves of Laelia anceps Lindl. y Epidendrum sp. at 45, 90 and 135 days after the beginning of the experiment.
These results agree with those of Martínez-Hernández et al. (2009) who evaluated vermiculite as a substitute for agar in Citrus volkameriana Ten & Pasq., Citrumelo swingle (Citrus paradise Macfad cv. Duncan × Poncirus trifolia L.) and C-35 and found that the number of leaves did not show statistically significant differences in the first eight weeks of in vitro growth.
On the other hand, Xiao and Kozai (2006) tested a porous material (Florialite) alternative to agar for Limonium latifolium Lindl. growth and found significant statistical differences in leaf area, dry and fresh weight during the first 25 days of cultivation. In this regard, the results obtained in this research indicate that growing outbreaks of L. anceps Lindl. and Epidendrum sp. in media with 50% of the salts and mixtures of perlite-volcanic rock or perlite-coir without AG3 promotes a similar number of sheets to those grown in the MS salts at 100% and agar.
After 135 days it was observed that only the shoots of L. anceps Lindl. which were cultivated in the MS50-A treatment formed significantly fewer roots than those in the MS50-FCT treatment (Table 8). For Epidendrum sp. the MS100FCT treatments and MS50-2FCT induced a lower number of roots. At that same time interval, the shoots of Epidendrum sp. cultivated in the treatment MS100A-2A showed to be significantly more efficient to form roots than those of the MS50-PT, MS50-A, MS100-2PT, MS100-2FCT, MS100-FCT y MS100-A treatments (Table 8).
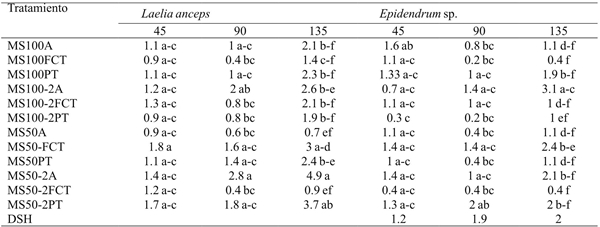
DSH= diferencia significativa honesta. Letras distintas en una columna indican diferencias estadísticas significativas (Tukey ≤ 0.05).
Table 8 Effect of treatments on the number of roots of Laelia anceps Lindl. y Epidendrum sp. after 45, 90 and 135 days of culture.
In this regard, Xia and Kozai (2006) found that the shoots of Limonium latifolium Lindl. formed a greater number of roots in Florialite than in agar. On the other hand, Mohan et al. (2004) used organic sugar cane residues as a substitute for agar in the in vitro rooting of apple trees (Malus prunifolia Borkh.) and obtained a 63% increase in the number of roots.
It has been documented that roots formed in mediums with agar are usually thin and fragile, which are damaged during transplantation, leading to the loss of individuals (Debergh and Maene 1981; Roberts and Smith 1990).
Table 2 shows significant differences for the fresh weight of shoots at 60 days of cultivation. Also, significant differences between L. anceps Lindl control shoots (MS100A) and treatments MS100-PT, MS100-2PT, MS100A for the fresh weight variable, were found (Table 9). While for Epidendrum sp. the fresh weight of the control shoots was statistically similar to the treatments MS50-FCT, MS50-PT, MS50-2FCT, MS50-2PT. In this regard, when studying the effect of vermiculite as a substitute for agar in Carica papaya L. Kataoka (1994) it was found that fresh root weight was higher in the explants developed in agar. In this research the use of substrates, diluted mediums and addition of gibberellic acid (AG3) had no negative effect on the fresh weight of L. anceps Lindl. and Epidendrum sp.
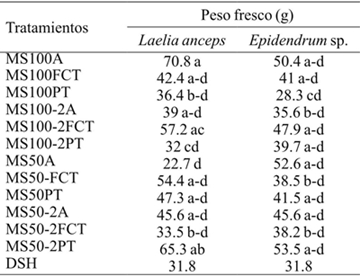
DSH= diferencia significativa honesta. Letras distintas en una columna indican diferencias estadísticas significativas (Tukey ≤ 0.05).
Table 9 Fresh weight of Laelia anceps Lindl. And Epidendrum sp. Shoots after 60 days of in vitro culture.
In this research, the best evaluated treatments could become a protocol in which diluted culture mediums and substrates are used as substitutes for the agar. This protocol represents an alternative to the in vitro propagation of L. anceps Lindl. and Epidendrum sp. Which is efficient and has a lower cost. This decrease in cost is attributed to the reduction in the concentration of nutrient salts and AG3, but especially to the use of inexpensive substrates, which unlike agar, represents only 13 to 17% of the cost.
Conclusions
The use of less concentrated culture medium without AG3 and substrates as support, allowed the growth of L. anceps Lindl. and Epidendrum sp. plants with the same or greater efficiency than the conventional gelled with agar culture method. Replacing agar with substrata and use of culture medium with diluted salts without AG3, not only allows the in vitro growth of shoots of orchid species under study, but may reduce the production costs of plants propagated by this technique.
Literatura citada
Abdoli, M. and Moieni, A. H. D. 2003. Effects of genotype and cotyledon section on organogenesis in sunflower. Ir. J. Biotechnol.1:134-138. [ Links ]
Afreen, Z. F.; Zobayed, S. M. A. C.; Kubota, T.; Kozai, O. and Hasegawa, A. 1999. Supporting material affects the growth and development of in vitro sweet potato plantlets cultured photoautotrophically. In vitro Cellular Develop. Biol. Plant.35: 470-474. [ Links ]
Afreen, Z. F.; Zobayed, S. M. A. C.; Kubota, T.; Kozai, O. and Hasegawa, A. 2000. Combination of vermiculite and paper pulp supporting material for the photoautotrophic micropropagation of sweet potato. Plant Sci. 157: 225-231. [ Links ]
Ávila, D. I. y Salgado, G. G. R. 2006. Propagación y mantenimiento in vitro de orquídeas mexicanas, para colaborar en su conservación. Biológicas. 8:138‐149. [ Links ]
Bhagwat, B.; Vieira, L. G. E. and Erickson, L. R. 1996. Stimulation of in vitro shoot proliferation from nodal explants of cassava by thidiazuron, benzyladenine and gibberellic acid. Plant Cell Tissue Organ Culture. 46:1-7. [ Links ]
Coello, C. Y.; Miceli, C. L.; Orantes, C.; Dendooven, L. F. and Gutiérrez, A. 2010. Plant growth regulators optimization for in vitro cultivation of the orchid Guarianthe skinneri (Bateman)Dressier & W. E. Higgins. Gayana Bot. 67:19-26. [ Links ]
Dalzotto, C. A. 2013. Efecto de medios de cultivo en el crecimiento in vitro de Oncidium bifolium sims. “federal”. Rev. Científ. Agrop. 17:7-15. [ Links ]
Debergh, P. C. and Maene, L. J. 1981. A scheme for comercial propagation of ornamental plants by tissue culture. Sci. Hortic.14:335-345. [ Links ]
DOF (Diario Oficial De La Federación). 2002. Norma Oficial Mexicana.NOM- 059-ECOL. 2001. Segunda Sección, Anexo narrativo II.Secretaría de Medio Ambiente y Recursos Naturales. 95-190 pp. [ Links ]
Flores, E. G.; Legaria, S. J. P.; Gil, V. I. y Colinas, L. M. T. 2008.Propagación in vitro de Oncidium stramineum lindl. Una orquídea amenazada y endémica de México. Rev. Chapingo Ser. Hortic.14:347-353. [ Links ]
Fujiwara, K. C.; Yamauchi, T. and Kozai, K. 1993. Effects of culture media components on the oxygen diffusion coefficient in liquid and gelled media. Abstr. Japan Plant Cell Tissue Culture Meeting, Kyoto. Japan. 40 p. [ Links ]
González, M.; Mogollón, N.; Alvarado, G.; Giménez, A. y Capote, T. 2012. Efecto del medio de cultivo in vitro y la fuente nitrogenada sobre el crecimiento del Cocuy (Agave cocui TRELEASE). Bioagro. 24:39-44. [ Links ]
Hágsater, E.; Soto, A. M. A.; Salazar, C. G. A.; Jiménez, M. M. A.; López, R. Y. y Dressler, R. L. 2005. Las orquídeas de México. Instituto hhinoín. México. 304 p. [ Links ]
Halbinger, F. M. and Soto, A. M. A. 1997. Laelias of México. Orquídea(Méx.) México, D. F. 15: 160 p. [ Links ]
Hazarika, B. N. 2006. Morpho-physiological disorders in in vitro cultureof plants. Sci. Hortic. 108:105-120. [ Links ]
Ichimura, K. and Oda M. 1998. Stimulation of root growth of several vegetables by extracts from a commercial preparation of agar. J. Japan Soc. Hortic. Sci. 67:341-346. [ Links ]
Jara, G.; Seemann, P.; Durán, C. y Soto, S. 2007. Multiplicación in vitro y caracterización citológica de dos especies de orquídeas nativas(Chloraea y Gavilea) de la provincia de valdivia, Chile. Agro Sur. 35:43-44. [ Links ]
Kataoka, I. 1994. Influence of rooting substrates on the morphology of papaya root formed in vitro. Japanese J. Tropical Agric.38:151-157. [ Links ]
Keatmetha, W. and Suksa, A. P. 2004. Effects of rooting substrates on in vitro rooting of Anthurium andraeanum L. cv. avanti. Walailak J. Sci. Technol. 1:49-55. [ Links ]
Kozai, T. 2010. Photoautotrophic micropropagation-environmental control for promoting photosynthesis. Propagation ornamental plants. 10:188-204. [ Links ]
Labrousse, P.; Delmail, D.; Decou, P.; Carlúe, M.; Lhernould, S. and Krausz, P. 2012. Nemesia root hair response to paper pulp substrate for micropropagation. The Sci. World J. 1:1-7. [ Links ]
Li, L. and Qu, R. 2002. In vitro somatic embryogenesis in turf-type bermudagrass: roles of abscisic acid and gibberellic acid, and occurrence of secondary somatic embryogenesis. Plant Breed.121:155-158. [ Links ]
Lucchesini, M.; Monteforti, G.; Mensuali, S. A. and Serra, G. 2006.Leaf ultrastructure, photosynthetic rate and growth of myrtle plantlets under different in vitro culture conditions. Biol. Plant.50:161-168. [ Links ]
Maheshwari, R.; Shailini, C.; Veluthambi, K. and Mahadevan, S. 1980.Interaction of gibberellic acid and indole-3-acetic acid in the growth of excised cuscuta shoot tips in vitro. Plant Physiol.65:186-192. [ Links ]
Martínez, H. M. J.; Alonso, L. A.; Osorio, A. F.; Gallardo, L. F.; López, M. H. y Mata, M. R. M. 2006. Cultivo in vitro de patrones de cítricos tolerantes al virus de la tristeza, empleando sustratos inertes alternativos al agar. Interciencia. 38:616-619. [ Links ]
Martínez, H. M. J.; Alonso, L. A.; Osorio, A. F.; Gallardo, L. F.; López, M. H. y Mata, R. M. 2009. Evaluación de diferentes fuentes de carbohidratos y medios de soporte, para la multiplicación in vitro de portainjertos de cítricos tolerantes a la tristeza. Agron. Trop. 59:343-350. [ Links ]
Mohan, R.; Soccol, C. R.; Quoirin, M. and Pandey, A. 2004. Use of sugarcane bagasse as an alternative low-cost support material during the rooting stage of apple micropropagation. In vitro Cellular Develop. Biol-Plant. 40:408-411. [ Links ]
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant.15:473-497. [ Links ]
Oh, M. M.; Seo, J. H.; Park, J. S. and Son, J. E. 2012. Physicochemical properties of mixtures of inorganic supporting m aterials affect growth of potato (Solanum tuberosum L.) plantlets cultured photoautotrophically in a nutrient-circulated micropropagation system. Horticulture Environ. Biotechnol.53:497-504. [ Links ]
Pervin, S. 1997. Studies on large scale plantlets development in different orchid species and hybrid through in vitro culture. M. Sc.Thesis, Univ. Dhaka. Dhaka, Bangladesh. 101. [ Links ]
Raya, M. Y. A.; Carrillo, G. C.; Pedraza, S. M. E.; Corona, T. T.; Carrillo, S. A. y Alcántar, G. G. 2011. Propagación in vitro de Laelia halbingeriana. Rev. Mex. Cienc. Agríc. 3:539-553. [ Links ]
Roberts, A. V. and Smith, E. F. 1990. The preparation in vitro of chrysanthemum for transplantation to soil. I. Protection of roots by cellulose plugs. Plant Cell, Tissue Organ Culture. 21:129-132. [ Links ]
Rodrigues, S. J. D.; Gomes, A. I. A.; Pasqua, M.; Almendagna, R. F. e Aparecida, A. F. 2009. Concentrações de sais do meio knudson C e de ácido giberélico no crescimento in vitro de plântulas de orquídea. Ciência Rural, Santa Maria. 39:772-777. [ Links ]
Romero, T. R.; Luna, R. B. S. y Barba, A. A. 2007. Uso de complejos comerciales como sustitutos de componentes del medio de cultivo en la propagación in vitro de Laelia anceps.Lankesteriana. 7: 353-356. [ Links ]
Salazar, M. A. S.; Amaya, N. Z. A. y Barrientos, R. F. 2013. Evaluación de diferentes medios de cultivo in vitro en el desarrollo de híbridos de Phalaenopsis (Orchidaceae). Rev. Colomb. Biotecnol.15:97-105. [ Links ]
SAS, Institute. 2002. User’s guide of SAS (Statistical Analysis System).SAS Institute Inc. Cary, N. C. USA. 550. [ Links ]
Soto, A. M. A. y Salazar, G. A. 2004. Orquídeas. Biodiversidad de Oaxaca.García, M. A. J.; Ordóñez M. J. y Briones-Salas, M. (Eds.).World Wildlife Fund. México. 271-295 pp. [ Links ]
Téllez, V. M. A. 2011. Diagnóstico de la familia Orchidaceae en México.Universidad Autónoma Chapingo (UACH). Chapingo, Estado de México. 181. [ Links ]
Xiao, Y. and Kozai, T. 2006. Photoautotrophic growth and net photosynthetic rate of sweet potato plantlets in vitro as affected by the number of air exchanges of the vessel and type of supporting material. Tsinghua Sci. Technol. 11:481-489. [ Links ]
Zettler, L. W.; Stewart, S. S.; Bowles, L. M. and Jacobs, A. K. 2001.Mycorrhizal fungi and cold-assisted symbiotic germination of the federally threatened eastern prairie fringed orchid,Platanthera Leucophaea (Nuttall) Lindley. The Am. Midland Nat. 145:168-17. [ Links ]
Received: March 2017; Accepted: June 2017











 text in
text in 


