Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.7 Texcoco Sep./Nov. 2016
Articles
Water relations in corn's High Valleys of Central Bureau of Mexico in drought conditions and nitrogen fertilization
1Campo Experimental Edzná-INIFAP. Carretera Campeche-Pocyaxum, km 15.5. Mpio. Campeche Campeche, México. C. P. 24520. Tel: 98 81 39748. Ext. 100. villalobos.antonio@inifap.gob.mx;
2Colegio de Postgraduados-Genética. Carretera México-Texcoco km 36.5 Montecillo, Texcoco, Estado de México. C. P. 56230. Tel: 01 595 9520200. Ext. 1587. (clc@colpos.mx; smiranda@colpos.mx; aheber@colpos.mx; mobel@colpos.mx).
The objective was to study the response of a group of hybrid and creoles corns in the water relations of the plant, the leaf area development, flowering, grain yield and its components under irrigation, drought and nitrogen deficiencies in a greenhouse of the Graduate College, Campus Montecillo in 2013. The treatments were: S1 unirrigated 10 d (30-40 dds); S2 unirrigated 20 d (50-70 dds); S3 unirrigated 30 d (70-100 dds), with high (AN, 160-40-20) and low N (BN, 80-40-20) applied at planting. The decrease of the soil water content (CHE) was reflected in a significant reduction (p ≤ 0.05) of the leaf water potential (ψ), osmotic (π) and turgidity (P) and relative water content (CRA) in S1, S2 and S3 with respect to irrigation AN and BN at the end drought treatments; hybrids showed higher ψ, π, P and CRA that the creoles. In all materials tested water stress reduced the expansion of leaf area (EAF), anthesis and silking was delayed (IA-FF), it decreased grain yield (RG), number of grains cob-1 (NGNM) and weight of 100 grains (P100G). In S1 and S2 hybrids showed higher EAF and produced more RG, NGNM, and had a shorter IA-FF than the creoles with AN and BN. In R and S1, with BN and S2 with AN and BN hybrids they showed higher P100G. In the S3 did not occur in grain.
Keywords: corn; creoles; hybrid; water stress; yield
El objetivo fue estudiar la respuesta de un grupo de maíces híbridos y criollos en las relaciones hídricas de la planta, el desarrollo del área foliar, la floración, el rendimiento de grano y sus componentes, en condiciones de riego, sequía y deficiencias de nitrógeno en un invernadero del Colegio de Postgraduados, Campus Montecillo en 2013. Los tratamientos fueron: S1 sin riego 10 d (30-40 dds); S2 sin riego 20 d (50-70 dds); S3 sin riego 30 d (70-100 dds), con alto (AN, 160-40-20) y bajo N (BN, 80-40-20) aplicado en la siembra. La disminución del contenido hídrico edáfico (CHE) se reflejó en una reducción significativa (p≤ 0.05) de los potenciales hídrico foliar (ψ), osmótico (π) y de turgencia (P), y contenido relativo de agua (CRA) en S1, S2 y S3 con respecto a riego con AN y BN, al finalizar los tratamientos de sequía; los híbridos presentaron mayor ψ , π, P y CRA que los criollos. En todos los materiales evaluados el estrés hídrico redujo la expansión del área foliar (EAF), se retrasó la antesis y floración femenina (IA-FF), disminuyó el rendimiento de grano (RG), número de granos mazorca-1 (NGNM) y peso de 100 granos (P100G). En S1 y S2 los híbridos presentaron mayor EAF y produjeron mayor RG, NGNM, y presentaron un IA-FF más corto que los criollos con AN y BN. En R y S1 con BN, y S2 con AN y BN los híbridos presentaron mayor P100G. En S3 no se produjo grano.
Palabras clave: criollos; híbridos; estrés hídrico; maíz; rendimiento
Introduction
The maize cultivation in rainfed conditions is exposed to wide variations in quantity and distribution of rainfall during growth; these variations in moisture availability often cause water stress, which in turn decreases the accumulation of dry matter in the aerial organs of the plant, the leaf area development and pimple formation, factors that ultimately reflected in the loss of performance. When moisture deficiencies occur in different periods of the crop cycle (intermittent drought), drought can cause water stress in seedling stage and reduce crop establishment; in more advanced phenological stages, stress can affect the development of leaf area and photosynthesis. If the drought ( 'drought midsummer' or 'heat wave') is presented prior to flowering and/or stages of flowering and grain filling, the water deficit reduces the production of cobs and grains during the two weeks of flowering due to a decrease in the rate of photosynthesis during grain filling and rapid induction of leaf senescence; grain yield can also be affected by competition for photo-assimilated among the aerial organs of the plant and roots as an adaptive response to drought (Bánziger et al., 2012).
In general, leaf elongation dynamically depends on the availability of water in the soil; and corn is no exception. With an adequate supply of water in the soil, elongation remains constant, however, when the soil water potential decreases, the elongation is also reduced. The changes in the water status point to direct role that water has on the growth; absorption provides physical strength to the elongation of the cells (Acevedo et al, 1971).
Aphenomenon commonly observed under drought imposed in bloom is lengthening female anthesis-flowering interval (Bolaños and Edmeades, 1990). This usually occurs by delay in the emergence of baby corn in relation to the emergence of the anthers, the latter being less affected by drought. In this context, the anthesis-flowering interval female is considered more valuable as diagnostic character behavior genotype date silking per se, because the anthesis-flowering interval female is quite independent of differences in physiological maturity between varieties (Edmeades et al, 1989). The availability of nitrogen is one of the limiting factors of growth and yield in rainfed conditions; if plants experience stress by deficiencies of this element, the photosynthetic rate is reduced because the leaf area development is decreased and leaf senescence accelerates (Bánziger et al, 2012).
In corn, a low level of nitrogen reduces the capacity of grain growth during filling stage (Bánziger et al, 2000), in conditions of water stress and nitrogen deficiencies in breeding could, include agronomic characteristics related to phenology, photosynthetic rate, biomass accumulation and partitioning photo-assimilated into the cob (Bolaños and Edmeades, 1993). Therefore, the objective ofthis rescobch was to study the response of hybrids and landraces of maize, irrigation and drought and two levels of nitrogen fertilization in leaf area development, flowering, grain yield and its components, under greenhouse conditions.
Materials and methods
Experimental site and plant material
The experiment was conducted in greenhouses in the area of drought resistance, the Postgraduate College in Montecillo, State of Mexico (19° 21' North latitude, 98° 55' west longitude and 2 250 masl). The gases used for this experiment had roof transparent plastic material. Four hybrid corn were used produced by the National Institute of Forestry, Agriculture and Livestock (INIFAP), these hybrids are characterized by being developed for rainfed areas of the High Valleys of the Central Bureau of Mexico and two creoles materials collected in Españita, Tlaxcala. The genetic material of INIFAP, representing commercial hybrids produced between 1960 and 2000. Hybrids released between 1961 and 1973 are classified as old hybrids and released in 1999 as modern hybrids (Table 1).
Table 1 Hybrids and landraces of maize, evaluated under different conditions of drought and nitrogen fertilization.

†Híbrido de cruza doble, 'Híbrido trilineal; §Germoplasma recolectado en el Municipio de Españita, Tlaxcala, por el Dr. Cándido López-Castañeda, Programa de Postgrado en Recursos Genéticos y Productividad-Genética, Colegio de Postgraduados. Montecillo, Estado de México en el ciclo otoño-invierno, 2013.
Experimental design
The design randomized complete block design was used in 4 x 2 factorial arrangement, with two repetitions, corresponding to four treatments of soil moisture and two fertilization levels for all materials. The seeds were sown in a PVC tube 4'' in diameter and 1 m long, with a cylindrical plastic bag of the same dimensions inside, containing sterilized soil; the soil used was crum-loam (34% sand, 56% silt and 10% clay) texture to which he determined its field capacity (CC) of 33.9%, a percentage permanent wilting (PMP) 21.1 % and bulk density of 1.08 g cm3 (soil physics laboratory, graduate College, Montecillo, Texcoco, State of Mexico). The planting took place on May 5, 2013, seeds were used with a weight of 350±5 mg in each experimental unit. The four treatments of soil moisture (HE) were evaluated: T1=irrigation (R) was to maintain the HE ncob field capacity (CC) to maturity; T2= drought 1 (S1) without R for 10 days (d) when plants were 30 d after sowing (dds); T3= drought 2 (S2) without R for 20 d when the plants were 50 dds, and T4= drought 3 (S3) for 30 d without R for 70 dds plant; at the end of periods of drought irrigation Recovery (RR) was applied in each treatment of soil water deficiencies with the application of subsequent risks to maturity. The nitrogen fertilization treatments were applied at planting time were also evaluated; High doses of nitrogen (AN) with 160-40-20 and low dose (BN) with 80-40-20, as nitrogen source, phosphorus and potassium were used: urea, calcium triple superphosphate and potassium chloride, respectively. To control aphids and whiteflies was applies 11 L ha-1 Engeo® at 38 dds.
Quantified variables and statistical analysis
The leaf water potential (ψ, bar) pump pressure or Scholander in the middle portion of fully expanded and exposed to solar radiation leaves was measured, between 11:00 and 13:00 was 30, 35 and 40 dds T2= S1; 50, 55, 60, 65 and 70 dds in T3= S2, and 70, 75, 80, 85, 90, 95 and 100 dds in T4= S3 with AN and BN, and 41, 71 and 101 dds (one day after RR) in all treatments.
The osmotic potential (π, bars) was determined in leaf samples used to measure ψ on all treatments of HE and N soil, and on the dates indicated above; subsequently these samples were placed in plastic vials and 5 mL were placed in a flask with N liquid (-195 °C) for two minutes, to break the cell wall and removing the sap; the π was measured with a Osmometer Wescor brand 5100C model in the laboratory (Barrios-Gómez et al, 2011).
The turgor potential (P, bars) was calculated as the difference between the potential y and π (p= ψ - π), for each sample analyzed (Begg and Turner, 1976). The relative contended water (CRA,%) was determined in all plants, extracting 10 discs of 5 mm diameter of the same portion of the blade used to measure ψ, π and p; discs were weighed immediately to obtain fresh weight (Pf, mg) and placed in petri dishes to remain suspended in distilled water for 10 hours under illumination, for weight to complete saturation of the tissue (Psat, mg) and subsequently , the weight of dry matter (Ps, mg), to dry the samples leaf disk in an oven at 70 °C for 48 h, [CRA=(Pf-Ps)/(Psat-Ps)100].
The expansion of leaf area (EAF) per plant (EAF= L*A*0.75, cm2), was determined by measuring the increase in leaf area elongating every five days in all treatments. The number of days to anthesis or tasseling (FM); it was determined when 50% of anthers had dehiscence spikelets and exposed outside the glumes. The number of days to silking (FF); it determined when the plant presented the jilote or female inflorescence with exposed stigmas. The grain yield per plant (RG, g) was also determined and its components normal number of grains (GN) per cob and weight (g) of 100 grains.
The edaphic water content (CHE) was determined by weighing the tubes three times a week in all treatments HE and N; in R the amount of water lost through perspiration weighing between successive dates added, keeping moisture (H) ncob CC to physiological maturity (MF). The RR was applied in S1, S2 and S3 at the end of treatments drought and subsequent risks after RR were applied to MF. The total amount of water transpired was calculated by adding water losses estimated in each tube during the experiment.
Statistical analysis
The analysis of variance for all variables using SAS for Windows version 9.0 (SAS, 2002) was calculated individually. The comparison of means was performed with the Tukey test (p≤ 0.05).
Results and discussion
Soil water content
In the S1 was suspended R between 30 and 40 dds and exploitable H decreased 75% without reaching the PMP (Figure 1a); in S2 the R was suspended between 50 and 70 dds and exploitable and the H dropped below PMP between 60 and 70 dds (Figure 1b), and S3 are stopped applying R between 70 and 100 dds and humidity soil decreased below the PMP between 88 and 100 dds (Figure 1c).
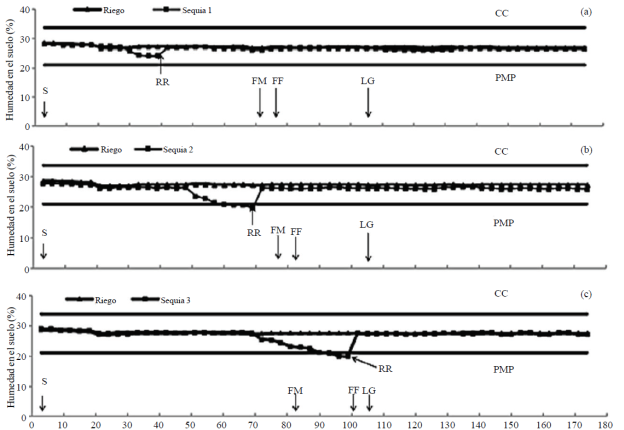
Figure 1 Water content in the soil in irrigation and drought 1 (a), irrigation and drought; 2 (b) and irrigation and drought 3 (c) greenhouse, summer-autumn cycle 2013. Montecillo, State of Mexico. CC= field capacity; PMP= percentage permanent wilting; S= seed; FM= male flowering; FF= silking; LG= grain filling; RR= risk of recovery.
The reduction in the CHE was reflected in a considerable decrease in leaf ψ in S1 (ψ < -12 bars), S2 (ψ <-25 bars) and S3 (ψ < -30 bar) with AN and BN with respect to R (ψ > -5 bars), with more negative values for π and lower levels for P and CRA, at the end of treatments soil water deficiencies. In S2 plants showed severe symptoms of wilting, but were more pronounced in S3, where drought coincided with the FM, FF and the beginning of grain filling. A study showed that bean soil water deficiencies during flowering, severely reduce the water content of the plant, significantly reducing seed yield, biomass, number of pods (Barrios-Gómez et al., 2010).
Water relations
For irrigation treatment with two levels of fertilization, values presented ψ, π and p, and CRA between -1 to -5, -10 to -13 and 8 to 11 bars, and 78 to 83%, respectively and showed no significant differences between maize evaluated materials presented no water stress in any measurement date. In the environment of drought (S1) with AN (Figures 2 a, b, c and d) and BN Figures 2 e, f, g and h), the following ranges of variation were: ψ (-3 to -16 bars), π (-14 to -19 bars) and p (2to 12 bars) and CRA (50 to 80%).

Figure 2 Water potential (a and e), osmotic (b and f) and turgidity (c and g) and relative water content (d and h) in dry 1 with high dose (a, b, c, d) and low nitrogen (e, f, g and h) under greenhouse, summer-autumn cycle 2013. Montecillo, State of Mexico. [* (p≤ 0.05); ns= not significant].
In S2 the ψ, π and p, and CRA with AN (Figures 3a, b, c) and BN (Figures 3e, f, g y h) ranged from -4 to -29, -12 to -20 to -8 bars 9, 20 to 85%, respectively.

Figure 3 Water potential (a and e), osmotic (b and f) and turgidity (c and g) and relative water content (d and h) in dry 2 high dose (a, b, c, d) and low nitrogen (e, f, g and h) under greenhouse conditions, summer-autumn cycle 2013. Montecillo, State of Mexico. [* (p≤ 0.05); ns= not significant].
For S3 with AN (Figure 4 a, b, c and d) and BN (Figures 4 e, f, g and h), the variation ranges were: ψ (-6 to -30 bars), π (-12 to -24) and p (6 -5 bars), CRA and 18 to 85%. For all treatments the ψ drought, π and p, and CRA were different (p ≤ 0.05) between genotypes in some measurement dates and very low values were achieved off over the period of drought.

Figure 4 Water potential (a and e), osmotic (b and f) and turgidity (c and g) and relative water content (d and h) in drought 3 with high dose (a, b, c, d) and low nitrogen (e, f, g and h) under greenhouse conditions, summer-autumn cycle 2013. Montecillo, State of Mexico. [* (p≤ 0.05); ns= not significant, FM= male flowering; FF= silking. ]
The drought decreased the ψ to -18, -22 and -15 bars in different genotypes of maize, sorghum and snuff at noon in the upper leaves of the crop canopy; the π also declined but not to the same degree as did the ψ, so that the p was zero values on some leaves; π decreased in the highest leaves the crop canopy, it resulted in these sheets have high p more than a given ψ; stomatal resistance top sheets increased to more negative values ψ in three crops used and was also observed that higher plants of snuff leaves withered to more negative values ψ the lower leaves of crop canopy (Turner, 1974).
When comparing the behavior of a hybrid corn Sorghum halapense under irrigation (75% CC) and water deficit (23.5-25% CC) for 24 d, was determined that the Sorghum halapense made a continuous absorption of water, which will he allowed achieve greater ψ with respect to hybrid corn and keep the CRA above 70% in conditions of low water availability, which was reflected in an active gas exchange (Acciaresi et al., 2012). Avendaño et al. (2005) observed that improved varieties of corn Zacatecas-58 (cycle 19 mass selection) and CAFIME (Cycle 16 mass selection), had lower y and π than the original varieties (Zacatecas 58 and CAFIME) under drought conditions edaphic (10 d in soil PMP).
In the present study it was observed that the physiological behavior of germplasm under conditions of water stress and nitrogen deficiencies (DN) in the soil was similar in the vegetative stage (S1) before the start of flowering (S2) and during flowering and the beginning of grain filling (S3) without any observed differences in ψ, π and P, and CRA between plants with AN and BN. Also, it observed that genotypes maintained values, π and P, and CRA higher at the end of treatment drought, irrigation reacted faster recovery.
Leaf area expansion
The greatest reduction in EAF plant was observed in drought treatments (14.2%) compared with irrigation (Table 2). In the vegetative stage were observed the lowest values of leaf area expansion (17.1%) (S1, 30- 40 dds) compared with the other phenological stages of the crop: start flowering and grain growth. The largest expansion of leaf area (EAF) the presented three of the six materials tested: H-50, H-48 and white creole, in the four treatments evaluated.
Table 2 Expansion of leaf area accumulated in six genotypes of maize under irrigation, drought 1, drought 2 and 3 with high doses drought (AN) and low nitrogen (BN) at 40, 70 and 100 days after planting, under greenhouse conditions. Cycle summer-autumn 2013. Montecillo, Texcoco, State of Mexico.
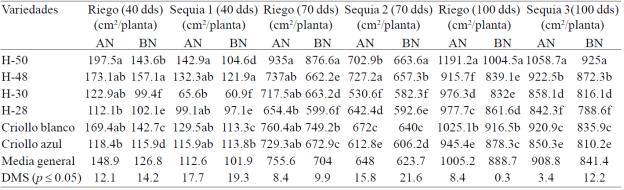
The greatest effect of water stress on the expansion of leaf area in the vegetative stage (S1) was due to leaf area of plants at the beginning of the cycle was in full expansion and this phenological stage, water deficiency has greater effect in reducing the elongation speed of leaves when plants are fully extended leaf area (S2) including the flag leaf (S3) where the effect of water deficiency has less effect on leaf elongation; Bánziger et al. (2012) found that drought during the period prior to flowering in corn reduces leaf area development and photosynthetic rate, and these reductions in the size of photosynthetic area and photosynthetic activity of the plant, they are reflected in a drastic reduction production of cobs and grains during the two weeks of flowering. Differences in EAF were also observed between treatments AN and BN; EAF decreased 19.4, 3.8 and 7.4% due to the BN in S1 S2 and S3 (Table 2), and 14.8, 6.8 and 11.6% in the respective treatments of R (Table 2).
Vos et al. (2005), by subjecting plants of the hybrid Lincoln corn N doses ranging from 0.5 to 6 g per plant under greenhouse conditions, they observed that the low N reduced to 29% total leaf area and 16% the end area the largest leaf of the plant, confirming the importance of N in corn EAF with deficient N.
Flowering
In R S1 and S2 the H-48 and H-50 with AN and BN had fewer days A and FF than other genotypes and S3 the blue creole had fewer days to FM and FF than the other genotypes ( Table 3); similar differences between hybrids and creoles were observed under field conditions with R, drought and high low N, where the creoles under drought had fewer days A hybrids, with no difference between creole and hybrids for the number of days FF and the interval anthesis-flowering female (IA-FF) (Serrem et al., 2009). The greatest effects of water deficiency are manifested in floral development and failures in fertilization and abortion of zygotes (Saini and Westgate, 2000), stigma receptivity (Bassetti and Westgate, 1993) and the pollination process (Sawhney and Shukla, 1994).
Table 3 Days to anthesis (A) and silking (FF) in six genotypes of maize under irrigation, drought 1, drought 2 and drought 3 high (AN) and under nitrogen (BN), under greenhouse conditions. Cycle summer-autumn 2013. Montecillo, Texcoco, State of Mexico.

¶Sin floración.
The drought delayed (p 0.05) on average A and FF at 0.5, 7.5 and 11 d, and 0.5, 7.5 and 21 d with respect to irrigation S1, S2 and S3, respectively. The nitrogen deficiency anthesis delayed a day at S1 and S2, the FF was delayed a day at S1 and S2 and two d in S3, without presenting changes in irrigation. The effects of the soil water content were also observed in the IA-FF in S3; the IA-10 D FF it lengthened in S3 with respect to R, without presenting changes in R, S1 and S2; this elongation in the IA-FF was so important that prevented the formation of grain on all corn plants in S3; nitrogen deficiencies had only effect on plants in S3, where the IA-FF was nine days longer than in R with AN and 11 days longer than in R with BN. In S3 the jilote the H-30 and AN white creole, and H-30, H-28 and BN white creole with no stigmas issued due to water stress (Table 3).
The number of days to A and FF was delayed seven days on average corn plants with BN eight d in plants with AN in S2, and 11 and 20 d with AN, and 11 and 22 d with BN S3. On average the IA-FF in the presence of severe EH (S3) was higher with AN (15 d) and BN (17 d) in R with AN and BN (six d); S1 with AN and BN (six d); and S2 with AN (six d) and BN, where the asynchrony between the blooms was higher (15 d with AN and 17 d BN) in R (six d with AN and BN), S1 (six d with AN and BN ) and S2 (six d with AN and BN). Studies under field conditions show that the drought delayed 1.5 d A three d FF with respect to R and IA-FF rose three d lines S1 of creole Ibarrilla, the effect of drought (Reyes-Ramones, 2000) and in drought conditions in greenhouses, it was determined that the drought delayed 19 d a and 18 d FF average of four varieties of maize (Original CAFIME Zacatecas 58 original, CAFIME SM16 and Zacatecas 58 SM19), without significant changes in the IA-FF (Avendaño et al., 2008).
Grain yield and its components
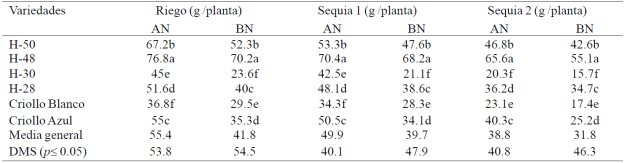
In S1 and S2, the EH reduced 8 and 27% the RG and reduced nitrogen deficiencies RG 25, 20 and 18% in R, S1 and S2; H-48 scored higher RG (p≤ 0.05) than the other genotypes in R and S1 with AN and BN; H-48 and H-50 obtained greater RG (p≤ 0.05) than the rest of the genetic material in S2 with AN and BN (Table 4). In S3 it did not occur in any of the grain materials evaluated.
Table 4 Grain yield six maize genotypes evaluated under irrigation, drought 1 and drought 2 with high (AN) and under nitrogen (BN), under greenhouse conditions. 2013 summer-autumn cycle Montecillo, Texcoco, State of Mexico.
The number of normal grains cob-1 (NGNM) also decreased as a result drought although this reduction was smaller in magnitude than the RG; the NGNM decreased 8 to 15% by S1 and S2, and 2.3, 5.7 and 3.5% by effect of BN in R, S1 and S2. Deficiencies of nitrogen increased the number of grains per cob aborted (NGAM) 32% in S1 and 2% in S2. The weight of100 grains (P100G) was reduced by drought in 13 and 40% in S1 and S2, and 29, 27 and 20% due to low N in R, S1 and S2, respectively (Table 5).
Table 5 Number of normal grains cob-1 (GN) and weight of 100 grains (P100G) in six maize genotypes evaluated under irrigation, drought 1 and drought 2 with high (AN) and under nitrogen (BN) under greenhouse conditions, summer-autumn cycle 2013. Montecillo, Texcoco, State of Mexico.

The response of materials corn to drought and nitrogen deficiency in the RG and its components varied with the phenological stage, duration and intensity of the EH in different treatments EH; in S1 (30-40 dds) reduction in the RG and its components NGNM and P100G, the effect of drought (Grant et al, 1989) and nitrogen deficiencies was much lower than in S2 (50-70 dds) and especially S3 (70 100 dds) where corn plants did not produce grain. The EH reduced RG and its components in greater deficiencies of nitrogen in all genotypes; RG is reduced when plants suffer severe EH during the flowering period (Nielsen, 2013); the kernel abortion and cobs at the start of grain filling and reduce photosynthesis (Bánziger et al, 2012).
Conclusions
The hybrids maintained and turgidity increased water potential and relative water content until the end of the drought treatments, had higher resilience to water stress and greater expandability leaf area under high and low nitrogen. Drought delayed the male and female flowering, and reached anthesis-flowering interval female, reduced grain yield and its components number of grains cob-1 and grain size, and this effect was greater in drought three when it coincided with the anthesis and silking, causing corn plants produced no grain yield in high and low nitrogen. The hybrids outperformed the creoles in grain yield and normal grains cob-1. Hybrids outperformed the Creoles in the P100G in drought conditions with nitrogen deficiencies. High and low in N the creoles reached anthesis and silking in less time than hybrids and had a anthesis-flowering interval longer than hybrids drought female ten days. Hybrids reached anthesis and silking in less time than the creoles and had an anthesis-flowering interval shorter female Creoles when drought was applied before flowering.
Literatura citada
Acciaresi, H. A.; Zuluaga, M. S.; Yanniccani, M. E. and Guiamet, J. J. 2012. Zea mays and Sorghum halepense water competition and their impact on leaf gas exchange. Ecosistemas 21:103-109. [ Links ]
Acevedo, E.; Hsiao, T. C. and Henderson, D. W. 1971. Immediate and subsequent growth responses of maize leaves to changes in water status. Plant Physiol. 48:631-636. [ Links ]
Avendaño, A. C. H.; Trejo, L. C.; López, C. C.; Molina, G. J. D.; Santacruz, V. A. y Castillo, G. F. 2005. Comparación de la tolerancia a la sequía de cuatro variedades de maíz (Zea mays L.) y su relación con la acumulación de prolina. Interciencia. 30:560-564. [ Links ]
Avendaño, A. C. H.; Molina, G. J. D.; Trejo, L. C.; López, C. C.; Cadena, I. I. 2008. Respuesta a altos niveles de estrés hídrico en maíz. Agron. Mesoam. 19:27-37. [ Links ]
Bánziger, M.; Edmeades, G. O.; Beck, D. and Bellon, M. 2000. Breeding for drought and nitrogen stress tolerance in maize: From theory to practice. In: CIMMYT (Ed.). De la teoría a la práctica. México, D.F.: CIMMYT. 68 p. [ Links ]
Bánziger, M.; Edmeades, G. O.; Beck, D. y Bellon, M. 2012. Mejoramiento para aumentar la tolerancia a sequía y a deficiencia de nitrógeno en el maíz: In: CIMMYT. (Ed.). De la teoría a la práctica. México, D. F., CIMMYT. 61 p. [ Links ]
Barrios, G. E. J.; López, C. C.; Kohashi, S. J.; Acosta, G. J. A.; Miranda, C. S. y Mayek, P. N. 2010. Rendimiento de semilla y sus componentes en frijol Flor de Mayo en el centro de México. Agrociencia 44: 481-489. [ Links ]
Barrios, G. E. J.; López, C. C. y Kohashi, S. J. 2011. Relaciones hídricas y temperaturas altas en frijol del tipo "Flor de Mayo". Agron. Costar. 35:131-145. [ Links ]
Bassetti, P. and Westgate, M. E. 1993. Water deficit affects receptivity of maize silks. Crop Sci. 33:279-282. [ Links ]
Begg, J. E. and Turner, N. C. 1976. Crop water deficits. Adv. Agron. 28:161-217. [ Links ]
Bolaños, J. and Edmeades, G. O. 1990. CIMMYTs' strategies in breeding for drought tolerance in tropical maize: In: Unger, P. W.; Jordan, W. R.; Sneed, T. V. and Jensen, R. W. (Eds.). Challenges in dryland agriculture - a global perspective. Proceedings of the International Conference on Dryland Farming. Texas Agricultural Experiment Stations, College Station, Texas, USA. 752- 754 pp. [ Links ]
Bolaños, J. and Edmeades, G. O. 1993. Eight cycles of selection for drought tolerance in lowland tropical maize. I. Responses in grain yield, biomass, and radiation utilization. Field Crops Res. 31:233-252. [ Links ]
Edmeades, G. O.; Bolaños, J.; Lafitte, H.R.; Rajaram, S.; Pfeiffer, W. and Fischer, R. A. 1989. Traditional approaches to breeding for drought resistance in cereals. In: Baker, F. W. G. (Ed.). Drought resistance in cereals. CAB International, U. K. 27-52 pp. [ Links ]
Grant, R. F.; Jackson, B. S.; Kiniry, J. R. and Arkin, G. F. 1989. Water deficit timing effects on yield components in maize. Agron. J. 81:61-65. [ Links ]
Nielsen, R. L. 2013. Effects of stress during grain filling in corn. Corny news network articles. Department of Agronomy, Purdue University. http://www.kingcorn.org/news/timeless/StalkHealth.html. [ Links ]
Reyes, R. R. E.; Rodríguez, O. J. L. f y López, C. C. 2000. Resistencia a sequía de líneas S1 derivadas de la variedad de maíz criollo de Ibarrilla. Agric. Téc. Méx. 26:159-172. [ Links ]
Saini, H. S. and Westgate, M. E. 2000. Reproductive development in grain crops during drought. Adv. Agron. 68:59-96. [ Links ]
Statistical Analysis System (SAS). 2002. The SAS System Release 9.0 for Windows, SAS Institute. [ Links ]
Sawhney, V. K. and Shukla, A. 1994. Male sterility in flowering plants: Are plant growth substances involved? Amer. J. Bot. 81:1640-1647. [ Links ]
Serrem, C. K.; López, C. C. y Kohashi, S. J. 2009. Efecto del nivel de humedad y nitrógeno en el suelo en el comportamiento de maíces híbridos y criollos de los Valles Altos de México. Agron. Costar. 33:103-120. [ Links ]
Turner, N. C. 1974. Stomatal behavior and water status of maize, sorghum, and tobacco under field conditions. II. At low soil water potential. Plant Physiol. 53:360-365. [ Links ]
Vos, J.; van der Putten, P. E. L. and Birch, C. J. 2005. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Res. 93:64-73. [ Links ]
Received: January 2016; Accepted: April 2016











 text in
text in 


