Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.6 Texcoco Aug./Sep. 2016
Articles
Nim biological activity on adult whitefly Trialeurodes vaporariorum (Aleyrodidae) West
1Campo Experimental Valle de México-INIFAP. Carretera los Reyes-Texcoco km 13.5, Coatlinchan, Texcoco, Estado de México, C. P. 56250. Tel: 01 800 088 22 22.
2ADAMA-México. Av. Insurgentes Sur 800 piso, 19 Col. Del Valle, Benito Juárez. C. P. 03100. Tel: 55 55 24 83 69.
3Postgrado en Fitosanidad, Entomología y Acarología. Colegio de Postgraduados. Carretera México-Texcoco, km 36.5. Montecillo, Texcoco, Estado de México. C. P. 56230. 595 9520200.
Chemical insecticides to control Trialeurodes vaporariorum West. have been virtually the only tool used, however the global problem of environmental pollution and insecticides selective pressure, has fostered a change in the way of handling pest to more environmentally friendly and efficient. Nim of Neem Azadirachta indica is an option as botanical insecticides, as its metabolites have already been marketed in different formulations; however it is necessary to evaluate its insecticide and insectistatic action. The objective of this research was to compare the effect on mortality, repellency and oviposition of formulated products of A. indica. In this study four formulations based on neem oil in adults of T. vaporariorum were tested. The products that showed the highest mortality were Neem Oil Spray and PHC Neeem, at a concentration of 0.6 mg mL-1. The best repellent effect was Neem Oil Spray (82.6%), PHC Neeem (72.3%), Biosave Neem (70.8%), and Neemix 4.5 (59.9%); the first two show greater persistence with similar effect the 3 days of evaluation. The best inhibitor of oviposition was Neem Oil Spray (99.6% using 1 mg mL-1), Biosave Neem (92.8%), PHC Neeem (82.6%), and Neemix 4.5 (57%). In contrast, Biosave Neem and Neemix 4.5 stimulated oviposition at concentrations of 0.01 to 0.3 mg mL-1 and PHC Neeem 0.035 mg mL-1. The oil concentration in the formulation is crucial in effect, as to larger proportion, the formulation becomes more persistent, otherwise makes it more polar and its systemic capacity increases.
Keywords: Trialeurodes; Azadirachta; mortality; oviposition; repellency
Los insecticidas químicos para controlar Trialeurodes vaporariorum West. han sido prácticamente la única herramienta utilizada, sin embargo la problemática global de contaminación ambiental y presión selectiva a insecticidas, ha fomentado un cambio en la forma de manejar las plagas más ecológicas y eficaces. El nim o neem Azadirachta indica es una opción como insecticidas botánicos, sus metabolitos ya se han comercializado en diferentes formulaciones, sin embargo es necesario evaluar su acción insecticida e insectistática. El objetivo de esta investigación fue comparar el efecto en la mortalidad, repelencia y oviposición de productos formulados de A. indica. En este estudio se probaron cuatro formulaciones a base de aceite de nim en adultos de T. vaporariorum. Los productos que mostraron la mortalidad más alta fueron Neem Oil Spray y PHC Neeem, a una concentración de 0.6 mg mL-1. El mejor efecto repelente fue de Neem Oil Spray (82.6%), PHC Neeem (72.3%), Biosave Neem (70.8%), y Neemix 4.5 (59.9%); los dos primeros muestran mayor persistencia con efecto similar a los 3 días de evaluación. El mejor inhibidor de la oviposición fue Neem Oil Spray (99.6% utilizando 1 mg mL-1), Biosave Neem (92.8%), PHC Neeem (82.6%), y Neemix 4.5 (57%). En contraste, Biosave Neem y Neemix 4.5 estimularon la oviposición en concentraciones de 0.01-0.3 mg mL-1 y PHC Neeem a 0.035 mg mL-1. La concentración de aceite en la formulación es determinante en el efecto, pues a mayor proporción, la formulación se hace más persistente, lo contrario la hace más polar y aumenta su capacidad sistémica.
Palabras clave: Trialeurodes; Azadirachta; mortalidad; oviposición; repelencia
Introduction
Plant extracts have been investigated throughout the world due to the great potential, as in the case of neem tree. Azadirachta indica Juss. (Meliaceae) has several terpenes and sulfur compounds at different doses, cause growth, feeding and breeding inhibition in more than 400 species of pests insect. It has been conducted research to determine its residual effect in the environmental (Gahucar, 2014), type of formulation (Boursiera et al., 2011) and its compatibility with some natural enemies and beneficial microorganisms (Huang et al., 2004; Mohan et al., 2007; Islam et al., 2010; Ahmed et al., 2012).
A pest that affects a large number of agricultural crops is the greenhouse whitefly, Trialeurodes vaporariorum West (Hemiptera: Aleyrodidae), in this have been conducted studies with different products based on neem determining different effects; Margosan-O, Nim 80 and Nim Oil, causing significant mortality (Abou-Fakhr Hammad et al., 2000); while others like Azatin EC, Neemazal and Neemazal-T/S are excellent repellents (Cubillo et al., 1999; Abou-Fakhr Hammad et al., 2000; Silva et al., 2003; Kumar and Poehling, 2006). Some of these products like Nim 80 are efficient in all three aspects already mentioned, Islam and Omar (2012) and Nzanza et al., (2012) report these same effects. Others like Nim Oil cause mortality, repellency and stimulate oviposition (Gomez et al., 1997; Salas and Mendoza, 2001, Chiasson et al., 2004). Other products water-based of seed 6%, Neem Rose Defense EC, Nim 20 and Sukrina are reported without repellent or toxic effect. The effects of products made of nim are diverse largely due to the quality and amount of oil present in the formulations, because it contains azadirachtin and at least six limonoids; the information of the phytochemicals still remains scattered in various scientific publications (Odeyemi et al., 2008).
Although several commercial products of nim are recommended for use in conventional farming, the real potential is not known for the diversity of responses that causes the same product, so it is necessary the evaluation in pests of economic importance and generate information that allows to compare its biological and insecticide effectiveness, thus support its use in alternative or organic agriculture. The aim of this study was to compare the effects of mortality, repellency and oviposition of four commercial products of nim, against adult whitefly T. vaporariorum.
Materials and methods
Insect breeding: the research was conducted in the Entomology and acarology green house from the Postgraduate College, Campus Montecillo, Texcoco, State of Mexico, from April 2007 to October 2008. T. vaporariorum adults from a susceptible breeding to organosintetic insecticides were used. These were introduced into entomological cages (60x40x60 cm) covered with organza fabric, containing bean plants Phaseolus vulgaris var. Bayomex six weeks old, the plants were placed in a mixture of vermicompost and soil (1:1) as support medium. Insects were maintained on plants for 7 d for oviposition and removed with a vacuum. Infested plants were transferred to another cage to await the emergence of adults in greenhouse under controlled conditions 25 ± 5 ° C, 12:12 L:O, 80% RH.
Product evaluation: four commercial products of nim were used as treatments and a minimum of six replicates. 20 adult whitefly of 3-6 days age, with fasting 2 hours and sex ratio 1:1were counted; a control to which was applied only distilled water was included. Based on azadirachtin content declared on the label of each product, calculation was performed to obtain a concentration of 10 mg mL-1 of azadirachtin, from which dilutions were made from 1 mg mL-1 and so successively azadirachtin concentrations of 0.1, 0.01, 0.001, 0.0001 and 0.00001 mg mL-1, to determine the biological timeframe response. Subsequently concentrations were intercalated between the response limits till conformation of seven concentrations, in order to assess the percentages of mortality, repellency or oviposition inhibition. All dilutions were prepared with distilled water and Tween 20 at 0.5% was added as adherent.
Mortality assessment: bean plants P. vulgaris var. Bayomex of 15-20 d age were used, of these plants, a leaflet was selected and immersed for 5 s in the product with calculated concentrations in the biological timeframe. After drying each treated leaflet was hanged inside a polyurethane entomological cage of 5 cm diameter and through a lateral hole 20 adult whitefly were introduced. Mortality was recorded 24 h after application and corrected with data observed in the control by Abbott´s (1925) equation. The replications were performed on different consecutive days, evaluating a product at a time in a brood chamber with conditions 28+5 °C, 12:12 D:O and 50+15% RH. For oviposition experiment, was calculated counting the number of eggs treated the leaves, and the percentage was determined in relation to that observed in the control. For repellency, the odor meter and the methodology described and modified by Ortega and Schuster (2000) were used. The arrangement of the odor meters was completely random; replications were performed on different consecutive days, evaluating a product at a time. Repellency percentage was obtained by subtracting the number of perch adults to introduced adults. Oviposition was calculated by counting the number of eggs in each leaflet treated and the percentage was determined in relation to that observed in the control.
Statistical analysis: mortality data corrected, repellence percentage and oviposition were transformed to function √x to calculate variances, subjected to Probit analysis to obtain the response line log dose-probit and concentration values required to eliminate 50% of the population (CL50's), concentration causing 50% repellency (CR50), and concentration required to inhibit 50% oviposition (CIO50) which is expressed in mg mL-1, using the Statistical Analysis program System (SAS, 1999).
Results and discussion
Mortality assessment and oviposition inhibition
Total mortality of adults was obtained with concentrations 0.6 mg mL-1 with Neem Oil Spray and PHC Neeem, with Biosave Neem was obtained with 1 mg mL-1; with a concentration of Neemix 4.5 of 1mg mL-1, mortality was 68.5%. CL50 values denote that there is no overlap between fiducial limits, indicating different toxicity categories in the formulations. Other nim products have also shown dissimilar effectiveness as for mortality refers, as Neemazal-T/S and Neem Rose Defense EC at respective concentrations of 0.5 and 0.7 mg mL-1 that caused 10 and 48.1% mortality in adults of T. vaporariorum (Von Elling et al., 2002; Chiasson et al., 2004), lower activity to the products evaluated in this research.
In contrast, the aqueous extracts made from Nim 25 at 0.06 mg mL-1 and ground seed (Nim 20) at 0.07 mg mL-1, caused 14-16.9 17-20.9% mortality in adult whitefly B. tabaci, respectively (Gomez et al., 1997, Fernandez et al., 2013), resulting with similar effectiveness to PHC Neeem from this work. Furthermore, ethanol extract of neem seed at 0.1 mg mL-1 caused 49% mortality in adults of T. vaporariorum, ethanolic extract has been reported as effective in other pests (Simmonds et al., 2002; Coria et al., 2008). The mortality obtained is intermediate between PHC Neeem and Neem Oil spray. Azatin EC at 0.02 and 0.1 mg ml-1 caused 37.8 and 65% mortality in adults of B. tabaci and T. vaporariorum respectively (Abou-Fakhr Hammad et al., 2000; Simmonds et al., 2002), resulting with similar activity to Neem Oil spray, unlike Margosan-O, Nim 80 and Nim Oil formulations that were more effective (Castillo-Sanchez et al., 2010) than the products evaluated in this study; Margosan-O at 0.01 mg mL-1 caused 15.5% mortality in adults of B. tabaci (Abou-Fakhr Hammad et al., 2000), and Nim 80 at 0.01 and Nim Oil at 0.03 mg mL-1 caused mortalities of 62 -65 and 33.8-80% in adults of B. tabaci, respectively (Gomez et al., 1997; Cubillo et al., 1999).
Neem Oil Spray totally inhibited oviposition at 1 mg mL-1, which, along with the concentration 0.6 mg mL-1, produced total mortality of adult, not observing correlation between mortality and oviposition, because in the first concentration females were removed before oviposition and in the second at least one female oviposited before dying. This product caused 50% inhibition of oviposition (CIO50) with 0.12 mg mL-1. On the other hand, Biosave Neem and PHC Neeem, caused total mortality of adults at concentrations of 1 and 0.6 mg mL-1, decreasing by 91.0 and 89.8% oviposition, respectively, denoting that both products eliminated adults after some of them oviposited. Both formulations inhibited by 50% oviposition (CIO50) with 0.61 and 0.13 mg mL-1; regarding Neemix 4.5, affecting less oviposition, with CIO50 of 1 mg mL-1. In contrast to the results obtained in this work, in other researches Neemazal-T/S at 0.5 mg mL-1, reduced by 35% oviposition in adults of T. vaporariorum (Von Elling et al., 2002), denoting similar effectiveness to Biosave Neem.
The aqueous extract of neem at 0.2 mg mL-1 inhibited by 78% oviposition in adults of B. tabaci resulting with the same level of toxicity than Neem Oil Spray and PHC Neeem; conversely the aqueous extract of cake (Nim 25) and Nim 80 at respective concentrations of 0.06 and 0.01 mg mL-1 inhibited in 34.6-54 and 31.7-68 % oviposition in adults of B. tabaci, respectively (Gómez et al., 1997; Cubillo et al., 1999), which resulted better than the products evaluated in this investigation.
Neem Biosave and Neemix 4.5 at concentrations of 0.1-0.2 and 0.1-0.15 and 0.3-0.4 mg mL-1 stimulated oviposition 12.8-35.9 and 41.7-52.8% and 2.8-22.2% respectively, probably in response to stress causing in female to sublethal concentrations, this can result in increased survival through enhanced oviposition rate, leading to the phenomenon of hormoligosis (Luckey, 1968), in Nim Oil 0.03 mg mL-1, caused increased oviposition of B. tabaci by 13% (Gomez et al., 1997).
Repellency and inhibition of oviposition: repellency manifested itself in three logarithmic ranges from 0.01 to 1.5 mg mL-1, with values of 0.8 to 90% from 24 to 72 h after application (Table 1). CR50 values were from 0.17 to 0.85 mg mL-1, expressing the highest effectiveness at 48 h, but does not differ in toxicity compared to 24 h in Biosave Neem, Neem Oil Spray and PHC Neeem. In contrast, Neemix 4.5 had different categories of toxicity, being less efficient at 24 h, moderately effective at 48 h and very biodegradable at 72 h, Biosave Neem was better at 24 and 48 h, with high biodegradation at 72 h; in both products, the 3rd day of evaluation CL50 was ≥ 1.0 mg mL-1, while Neem Oil Spray and PHCs Neeem showed overlap in their respective fiducial limits of CL50 in the three observation times, indicating similar effectiveness and strong persistence.
Table 1. Repellence percentage of whitefly adults T. vaporariorum at 24, 48 and 72 h with four nim products.
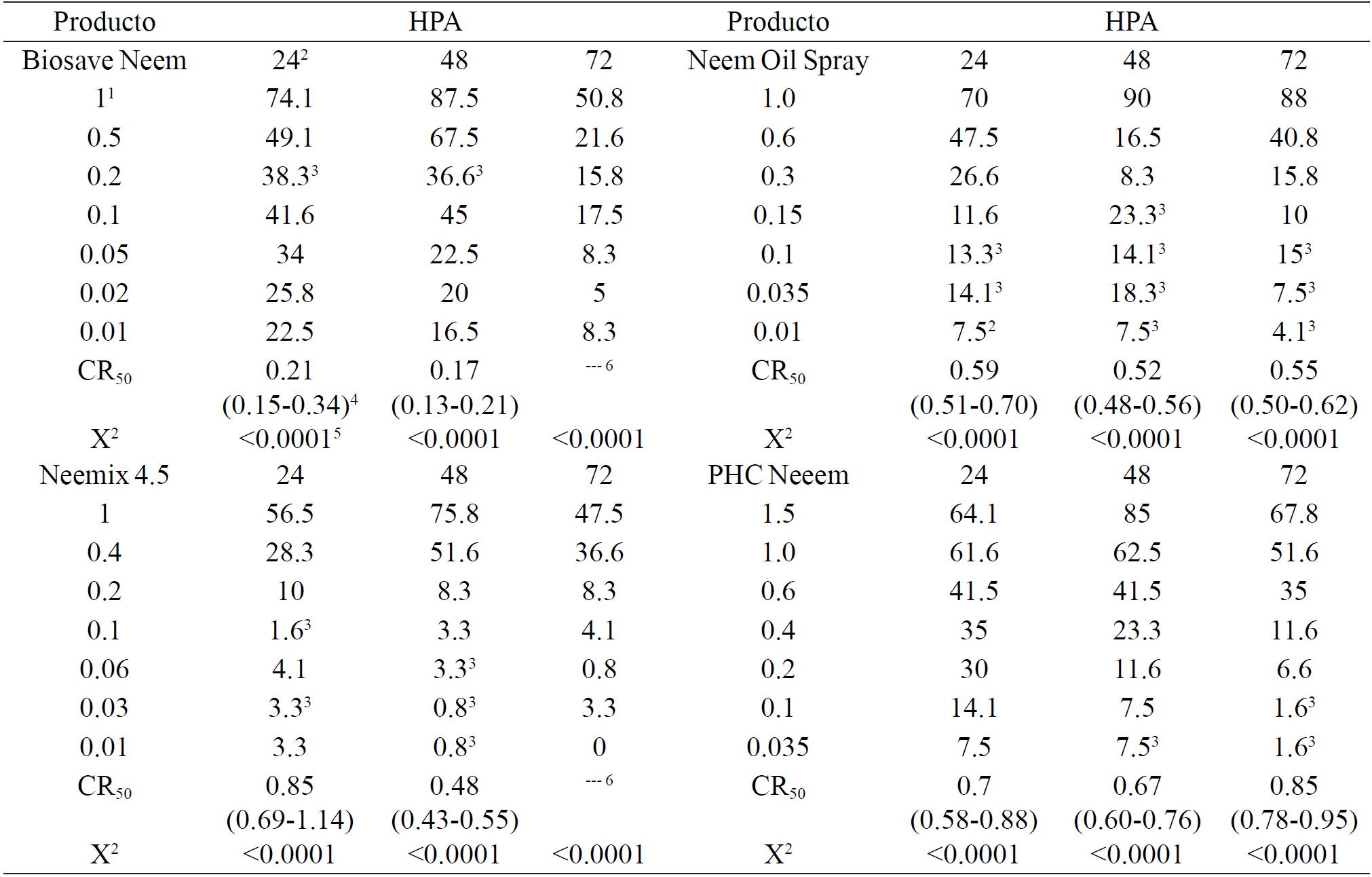
There are neem formulations than in similar experiments showed intermediate repellency between Biosave Neem and Neem Oil Spray, as Nim 20 and Nim 25 which prepared respectively at 0.07 and 0.06 mg mL-1 caused 17-18 and 9-13.5% repellency in adults of B. tabaci at 48 h, respectively (Gómez et al., 1997; Cubillo et al., 1999). While Margosan-O at 0.01 mg mL-1 was similar to Biosave Neem because it caused 18.4% repellency in adults of B. tabaci at 24 h (Abou-Fakhr et al., 2000).
In contrast, other products are better than those evaluated here; Neemazal-T/S at 0.01, 0.03, 0.05, 0.07 and 0.1 mg mL-1 caused 31.7, 52.4, 69.8, 80.3 and 86.7% repellency in adults of B. tabaci at 24 h, respectively (Kumar and Poehling, 2006); while Azatin EC at 0.02 mg mL-1 caused 40% repellency of B. tabaci at 24 h (Abou-Fakhr et al., 2000), as Nim 80 and Oil Nim that at concentrations of 0.01 and 0.03 mg mL-1 resulted in 30.6-61 and 16.9-76% repellency in adults of B. tabaci at 48 h, respectively (Gomez et al., 1997; Cubillo et al., 1999), while Neemazal at concentrations of 0.4 and 0.8 mg mL-1 caused 67.83 and 70.13% repellency respectively (Silva et al., 2003).
Neem Biosave and Neem Oil Spray products at concentration of 1 mg mL-1, totally inhibited oviposition at 48 h and 48-72 h, but it did not show full repellency. Neemix 4.5 and Neem Oil Spray formulations with 1 mg mL-1 inhibited from 98.0 to 98.5% oviposition at 24 and 48 h, respectively. PHC Neeem at 1.5 mg mL-1 also inhibited 98% at 48 h (Table 2). In comparison the fiducial limits of CIO50 at the three oviposition times, three levels of toxicity were determined, highlighting by its efficiency Biosave Neem and Neem Oil Spray, that at respective concentrations of 0.09 - 0.27 and 0.02 - 0.21 mg mL-1 inhibited oviposition by 50% from 24 to 72 h, while Neemix 4.5 and PHC Neeem showed less activity, which required of 0.21-> 1 and from 0.04 - 0.73 mg mL-1 to cause the same inhibition.
Table 2. Inhibition (%) of oviposition of whitefly T. vaporariorum at 24, 48 and 72 h with four nim products.
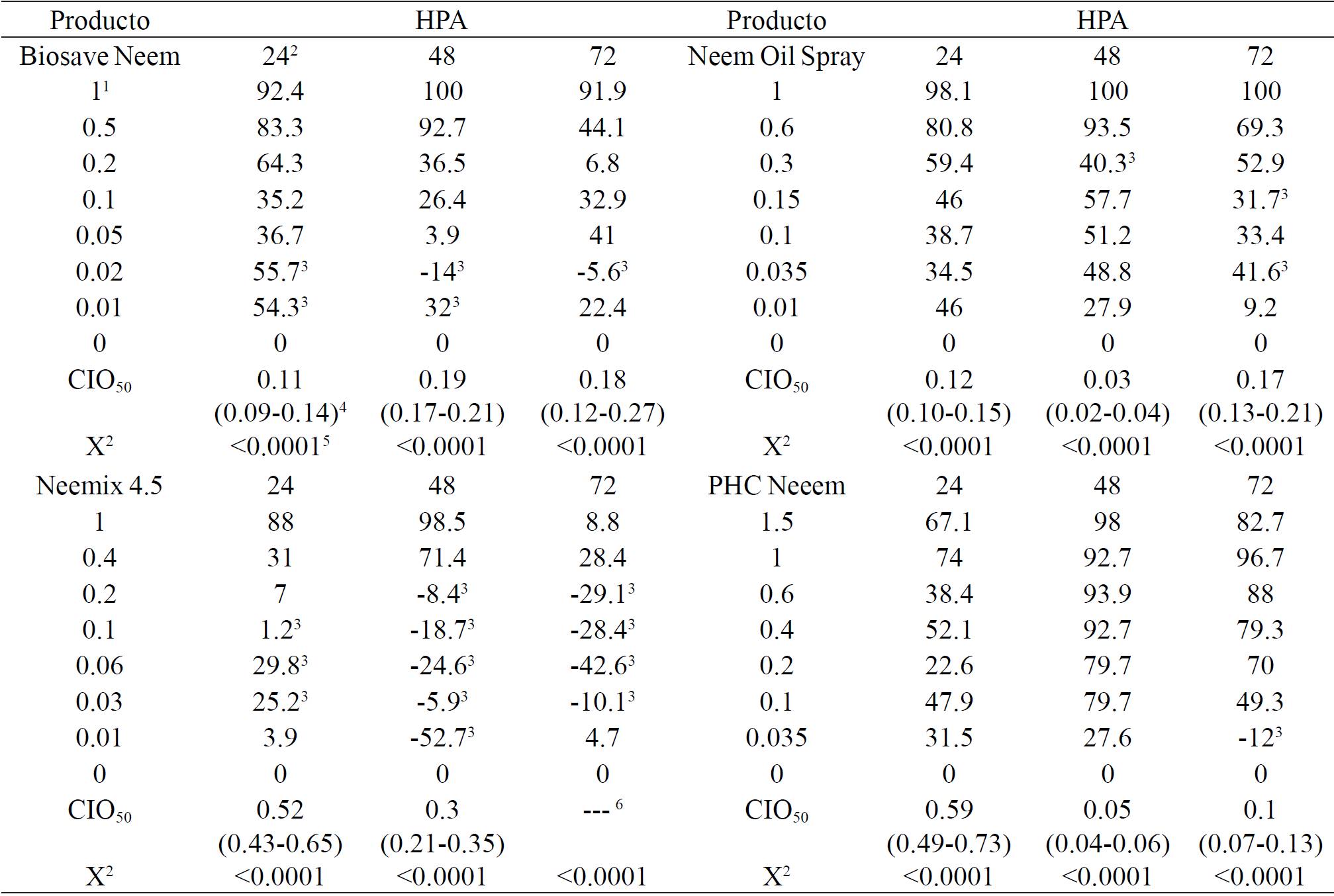
Females treated with Biosave Neem at 0.01 (24 and 48 h) and 0.02 mg mL-1 (from 24 to 72 h), Neemix 4.5 at concentrations of 0.03-0.1, 0.01-0.2 and 0.03-0.2 mg mL-1 at 24, 48 and 72 h, respectively and PHC Neeem at 0.035 mg mL-1 at 72 h, stimulate oviposition in a similar way to that observed with Nim 80 at 0.5 mg mL-1 in adults of B. tabaci (Cubillo et al., 1999).
The results of this research showed similarities with other studies; NeemAzal-T/S applied at 0.5 mg mL-1 inhibited in 29.4% oviposition in adults of T. vaporariorum at 72h (Von Elling et al., 2002), and Margosan-O, that at 48 h after application of 0.5 mg mL-1 inhibited oviposition in 83.59%, showing similar results to those obtained with Neemix 4.5.
The combined activity of the products showed that PHC Neeem caused total mortality at 0.6 mg mL-1 and even when it had a regular performance in repellency, it demonstrated persistence with toxic power from 24 to 72 h, in addition to inhibiting oviposition efficiently with values close to 100%; thus the commercial concentration in field (100 mL L-1), would cause from 90 to 95% mortality, 50% repellency and 80% inhibition of oviposition at 24, 48 and 48 h, respectively, so it is suggested that although causes hormoligosis at concentration of 0.035 mg mL-1, did not show phytotoxicity. Neem Oil Spray caused the highest mortality, repellency, oviposition inhibition and higher persistent, besides not causing hormoligosis; however, it was more phytotoxic at concentrations from 0.6 to 1 mg mL-1.
This may be partly due to the damage caused on the leaf, specifically at deterrent level in the selection of oviposition site (Rodriguez, 2000); while commercial dose (1.25 mL-1), would cause between 75 and 80% mortality, 92% repellency at 48 h and from 75 to 80% inhibition of oviposition. Biosave Neem, a mixture of extracts from two plants, was the third product in efficiency; however, it caused phytotoxicity. Full mortality occurred at 1 mg mL-1, which decreased significantly from 0.6 mg mL-1, was the best of the four products in repellency at 24 h, the second best at 48 h, and showed strong biodegradation at 72 h, and nevertheless strongly inhibited oviposition with 1 - 0.6 mg mL-1, stimulating it at concentrations of 0.2 - 0.01 mg mL-1, which is important in a mixture with high degradation like this, because in field high concentrations can be sublethal at 3 d.
As for the recommended commercial concentration (1.25 mg mL-1), this would cause 94-95% mortality, 80% repellency and 100% inhibition of oviposition at 24, 48 and 48 h. In contrast, the less efficient product was Neemix 4.5 which at 1 mg mL-1caused moderate mortality, repellency and inhibition of oviposition at 24, 48 and 48 h respectively; however, stimulated oviposition at concentrations of 0.4 to 0.01 mg mL-1, and even when the recommended commercial concentration could cause 95% of mortality, 90% repellency and 95% inhibition of oviposition at 24, 48 and 48 h, this product proved to be phytotoxic at such concentrations.
In general, two toxicological categories within concentrations evaluated in all products were observed, the first is between 0.4 and 1.5 mg mL-1, range in which causes more than 50% effectiveness in mortality, repellency and inhibition of oviposition, and the second from 0.01 to 0.3 mg mL-1 showing from 0.8 to 40% effect on mortality, repellency and inhibition of oviposition, and it is in this second category in which Biosave Neem and Neemix 4.5 show hormoligosis.
The persistent effect of formulations with oil can be considered an advantage due to lower concentrations are required to achieve the same effect than non-persistent; but the same implies greater permanence of the product in the environment and accelerates selection pressure, develops resistance and shortens product life, besides being phytotoxic. Authors note that products enriched with oil are more efficient, by the presence of insecticidal compounds and the persistence of these in the plant and the physical effect on the insect (Gómez et al., 1997; Cubillo et al., 1999). Stark and Walter (1995), mentioned that there is 62% more efficiency in four products with 5% neem oil, compared to three non-oily products, identified in the first six limonoids in addition to azadirachtin.
Taking into account the above, oily formulations in the form of emulsions (common in commercial products) should be considered the most appropriate schemes for inclusion in integrated pest management. Neem oil is an essential component in a commercial formulated product, since the addition of oil changes the polarity of the same, and in this context the formulations with lower proportion of oil and more emulsifiable can be absorbed more easily and have systemic effects up to 14 d, further demonstrating that the highest effectiveness of the products, after the first hours of application, is due to this effect (Silva et al., 2003), so it could behave as non-phytotoxic insecticides, with regular persistence . This is because azadirachtin and other limonoids decompose rapidly under the effect of light, UV rays, temperature, rain, HR, pH and microbial activity, within approximately 4 d (Mulla and Su, 1999). While applying crude extracts of oil turns efficient for at least 5 d, being able to increase up to 10 d, gradually decreasing, this depending on the solvent used in extraction (Abdel-Salam et al., 2005). This is because neem oil increases the toxicity of the formulations, either because it promotes penetration into insect cuticle or contains other limonoids that increase their insecticidal activity, in addition that just oil confers greater persistence to the mixture (Stark and Walter, 1995).
One more reason for the differential behavior of formulations, especially those like Biosave Neem, which are extract mixtures of two plants, is that azadirachtin concentration could cause loss of synergistic effect with other compounds, in addition that at low concentrations is possible to observe repellency and feeding inhibition, but these effects won’t be appreciated at high concentrations (Kumar et al., 2003).
Regarding stimulation behavior of oviposition of some nim products, another likely explanation (besides that it could show hormoligosis), is that these have a similar effect to Fenvalerate, which also induced hormoligosis in B. tabaci in cotton plants at concentrations of 25, 38 and 52 g ai ha-1. In the evaluation of various insecticides, it was found that this product does not cause reduction of total sugars in leaves after application, so that the insect does not consider the host as unsuitable for the development of their offspring (Abdullah et al., 2006).
However, studies in which the effect on fertility are determined and subsequent monitoring in the cycle of the insect are required. Considering other researches, in general, Neem Oil Spray and PHCs Neeem are more efficient than Neemazal-T/S and Neem Rose Defense EC (Von Elling et al., 2002; Chiasson et al., 2004); while Azatin EC and Margosan-O, exhibit similar activity to the first. On the other hand, it is suggested that Nim 80 and Nim Oil (Gomez et al., 1997; Cubillo et al., 1999), require lower concentrations than those evaluated in this research to produce similar effects.
Even though these products alone are not able to abate these populations, having a synergistic effect with some products of microbial origin such as entomopathogenic fungi, which can be used together, although its use should be subject to compatibility tests with natural enemies such as parasitoids, predators and pollinators (Morgan et al., 2009; Luna et al., 2011; Scudeler Santos, 2013).
Conclusions
The most efficient products to cause total mortality were Neem Oil Spray and PHCs Neeem; the best repellent was Neem Oil Spray (82.6%), PHC Neeem (72.3%), Biosave Neem (70.8%), and Neemix 4.5 (59.9%); the first two show greater persistence with similar effect at 3 days of evaluation, while the two remaining strongly degrade at 72 hours. The best inhibitor of oviposition was Neem Oil Spray (99.6%), Biosave Neem (92.8%), PHC Neeem (82.6%), and Neemix 4.5 (57%). In contrast, Biosave Neem and Neemix 4.5 stimulated the ovoposition in concentrations of 0.01 to 0.3 mg mL-1 and PHC Neeem 0.035 mg mL-1.
In order of effectiveness Neem Oil Spray was the best but more residual and phytotoxic; while PHC Neeem, followed in efficacy and was less persistent and phytotoxic; Biosave Neem and Neemix 4.5, had moderate values of mortality, repellency and inhibition of oviposition, but at sublethal concentrations stimulated oviposition. The results suggest that Neem Oil Spray and PHCs Neeem showed better insecticidal effect while Biosave Neem and Neemix 4.5 may work best as insectistatics.
Literatura citada
Abbott, W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18:265-267. [ Links ]
Abdullah, N. M. M.; Singh, J. and Sohal, B. S. 2006. Behavioral hormoligosis in oviposition preference of Bemisia tabaci on cotton. Pest. Biochem. Phys. 84:10-16. [ Links ]
Abou-Fakhr, H. E. M.; Nemer, N. M.; Hawi, Z. K. and Hanna, T. 2000. Responses of the sweet whitefly, Bemisia tabaci, to the chinaberry tree (Melia azederach L.) and its extracts. Ann. appl. Biol. 137(8):79-88. [ Links ]
Ahmed, N.; Ansari, M. S. and Hasan, F. 2012. Effects of neem based insecticides on Plutella xylostella (Linn.). Crop Prot. 34:18-24. [ Links ]
Andel-Salam, S. E.; Canyon, D; Faried-Younes, M. W.; Abdel-Wahab, H. and Mansour, A. H. 2005. A review of botanical phytochemicals with mosquitocidal potential. Envir. Inter. 31(8):1149-1166. [ Links ]
Boursiera, C. M.; Bosco, D.; Coulibaly, A. and Negre, M. 2011. Are traditional neem extract preparations as efficient as a commercial formulation of azadirachtin A? Crop Protec. 30(3):318-322. [ Links ]
Castillo-Sánchez, L. E.; Jiménez-Osornio, J. J. and Delgado-Herrera, M. A. 2010. Secondary metabolites of the Annonaceae, Solanaceae and Meliaceae families used as biological control of insects. Tropical and Subtropical Agroecosystems. 12(3):445-462. [ Links ]
Chiasson, H.; Vincent, C. and Bostanian, N. J. 2004. Insecticidal properties of a Chenopodium - Based Botanical. J. Econ. Entomol. 97(4):1378-1383. [ Links ]
Coria, C.; Almiron, W.; Valladares, G.; Carpinella, C.; Ludueña, F.; Defago, M. and Palacios, S. 2008. Larvicidae and oviposition deterrent effects of fruit and leaf ext from Melia azedarach (L.) on Aedes aegypti (L) (Diptera: Culicidae) Bioresour. Technol. 99:3066-3070. [ Links ]
Cubillo D.; Sanabria, G. y Hilje, L. 1999. Evaluación de la repelencia y mortalidad causada por insecticidas comerciales y extractos vegetales sobre Bemisia tabaci. Man. Inte. Plagas. 53:65-71. [ Links ]
Fernández, J. A.; Ghiggia, L. I.; Macián, A. J.; Arce, O; Jaime A. P. y Paz, M. R. 2013. Dos sistemas de manejo de un cultivo comercial de pimiento (Capsicum annuum L.) bajo carpa plástica para el control de Bemisia tabaci (Gennadius) en Lules, Tucumán. Paz Rev. Agron. N. O. Argent. 33(1):21-24. [ Links ]
Gahucar, R. T. 2014. Factors affecting content and bioefficacy of neem (Azadirachta indica A. Juss.) phytochemicals used in agricultural pest control. Crop Prot. 62:93-99. [ Links ]
Gómez, P.; Cubillo, D.; Mora, G. A y Hilje, L. 1997. Evaluación de posibles repelentes de Bemisia tabaci: I. Productos comerciales. Man. Inte. Plagas. 46:9-16. [ Links ]
Huang, Z.; Shi, P.; Dai, J and Du, J. 2004. Protein metabolism in Spodoptera litura (F.) is influenced by the botanical insecticide azadirachtin. Pest. Biochem. Physiol. 80:85-93. [ Links ]
Islam, M. T.; Castle, S. J. and Ren, S. X. 2010. Compatibility of the insect pathogenic fungus Beauveria bassiana with neem against sweetpotato whitefly, Bemisia tabaci, on eggplant. Entomol. Exp. Appl. 134:28-34. [ Links ]
Islam, M. T. and Omar, D. B. 2012. Combined effect of Beauveria bassiana with neem on virulence of insect in case of two application approaches. J. An. Plant Sci. 22(1):77-82. [ Links ]
Kumar, A. R. V.; Jayadevi, H. C.; Ashoka, H. J and Chandrashekara, K. 2003. Azadirachtin use efficiency in commercial neem formulations. Curr. Sci. 84(11):1459-1464. [ Links ]
Kumar, P. and Poehling, H. M. 2006. Persistence of soil and foliar azadirachtin treatments to control whitefly Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) on tomatoes under controlled (laboratory) and field (netted greenfouse) conditions in the humid tropics. J. Pest Sci. 79(4):189-199. [ Links ]
Luckey, T. D. 1968. Insecticide hormoligosis. J. Econ. Entomol. 61(1):7-12. [ Links ]
Luna-Cruz, A.; Lomelí-Flores, J. R.; Rodríguez-Leyva, E; Ortega-Arenas, L. D. y Huerta-de La Peña, A. 2011. Toxicidad de cuatro insecticidas sobre Tamarixia triozae (Burks.) (Hymenoptera: Eulophidae) y su hospedero Bactericera cockerelli (Sulc.) (Hemiptera: Triozidae). Acta Zool. Mex. (n.s.) 27(3):509-526. [ Links ]
Mohan, M. C.; Narasimha, P.; Reddy, N. P.; Devi, U. K.; Kongara, R. and Sharma, H. C. 2007. Growth and insect assays of Beauveria bassiana with neem to test their compatibility and synergism. Biocontr. Sci. Tech., 17: 1059-1069. [ Links ]
Mordue, A. J.; Morgan, E. D. and Nisbet, A. J. 2005. Azadirachtin, a natural product in insect control. In: Mordue, A. J.; Morgan, E. D. and Nisbet, A. J. Comprensive Molecular Insect Science-Control. Elsevier, Oxford, United Kingdom. 2875 p. [ Links ]
Morgan, E. D. 2009. Azadirachtin, a scientific gold mine. Bioorganic Medicinal Chem. 17:4096-4105. [ Links ]
Mulla, M. S. and Su, T. 1999. Activity and biological effects of neem products against arthropods of medical and veterinary importance. J. Amer. Mosquito Control Assoc. 15(2):133-152. [ Links ]
Nzanza, B. P. and Mashela, W. 2012. Control of whiteflies and aphids in tomato (Solanum lycopersicum L.) by fermented plant extracts of Neem leaf and wild garlic. Afr. J. Biotechnol. 11(94):16077-16082. [ Links ]
Odeyemi, O. O.; Masika, P. and Afolayan, A. J. 2008. A review of the use of phytochemicals for insect pest control. Afr. Plant Prot. 14:1-7. [ Links ]
Ortega, A. L. D. and Schuster, D. J. 2000. Repellency to silverleaf withefly adults. Gulf coast research and education center. University of Florida. Bradenton, Fl., USA. 2 p. [ Links ]
Rodríguez, H. C. 2000. Inhibición de la alimentación en insectos plaga. In: métodos de investigación en las ciencias ambientales. López, O. J. F.; Aragón, G. A. y Valera, M. A. P. (Eds). Benemérita Universidad Autónoma de Puebla (BUAP). Puebla, México. Publicación Especial. 75-97 pp. [ Links ]
Salas, J. y Mendoza, O. 2001. Evaluación de un extracto de nim en le control de Bemisia tabaci y Liriomyza sativae en tomate. Agron. Trop. 51(2):221-234. [ Links ]
SAS. 1999. Procedures Guide. Release 8. Cary, North Carolina, USA. 1643 p. [ Links ]
Scudelera, E. L. and Carvalho dos Santos, D. 2013. Effects of neem oil (Azadirachta indica A. Juss) on midgut cells of predatory larvae Ceraeochrysa claveri (Navás, 1911) (Neuroptera: Chrysopidae). Micron. 44:125-132. [ Links ]
Silva, L. D.; Bleicher, E. e Araújo, A. C. 2003. Eficiência de Azadiractina no controle de mosca-blanca em meloeiro sob condiςoes de casa de vegetaςao e de campo. Hort. Brasil. 21(2):198-201. [ Links ]
Simmonds, M. S. J.; Manlove, J. D.; Blaney, W. M. and Khambay, B. P. S. 2002. Effects of selected botanical insecticides on the behavior and mortality of the glasshouse whitefly Trialeurodes vaporariorum and the parasitoid Encarsia formosa. Ent. Exp. Appl. 102(1):39-47. [ Links ]
Stark, D. J. and Walter, J. F. 1995. Neem oil and neem oil components affect the efficacy of commercial neem insecticides. J. Agric. Food Chem. 43(2):507-512. [ Links ]
Von Elling, K.; Borgemeister, C.; Sétamou, M. and Poehling, H. M. 2002. The effect of NeemAzal-T/S®, a commercial neem product, on different developmental stages of the common greenhouse whitefly Trialeurodes vaporariorum Westwood (Hom. Aleyrodidae). J. Appl. Ent. 126(1):40-45. [ Links ]
Received: June 2016; Accepted: August 2016











 text in
text in 


