Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.5 Texcoco Jun./Aug. 2016
Articles
Alternatives for the control of Colletotrichum spp.
1Departamento de Postgrado- Universidad Politécnica de Francisco I. Madero. Domicilio conocido Tepatepec, Hidalgo. C. P. 42660. (nlanderova@conacyt.mx, fmlaravi@conacyt.mx, pandrade@upfim.edu.mx, gaguado@upfim.edu.mx).
2Postgrado en Fitopatología-Colegio de Postgraduados. Carretera México-Texcoco, Montecillo, Texcoco, km 36.5, Estado de México. C. P. 56230. (aguilar.luis@colpos.mx).
Colletotrichum gloeosporioides is a pathogen that causes a disease known as anthracnose, which occurs in plants and fruits. The fruit from their training and development on the ground, and even post-harvest is damaged by this pathogen, causing losses of up to 100%, depending on weather conditions prevailing in a region. Fungicides preharvest or postharvest are the main way to reduce losses from this disease. However, indiscriminate use of these has resulted in resistance of pathogenic microorganisms. On the other hand, its use is largely restricted due to concerns about toxic waste and the risks posed to health. Therefore, there is a need to manage the disease friendly alternatives with the environment, including plant extracts, microorganisms as biological control agents, hydrothermal treatments, genetic manipulation and induced resistance. The aim of this study was to analyze the different alternatives applied to the management control of Colletotrichum spp.
Keywords: Trichoderma; anthracnose; biological control; hosts; postharvest
Colletotrichum gloeosporioides es un patógeno que causa una enfermedad conocida como antracnosis, la cual se presenta en plantas y frutos. El fruto desde su formación y desarrollo en la planta, y hasta la poscosecha sufre daños por este patógeno, ocasionando pérdidas hasta de 100%, dependiendo de las condiciones climáticas que prevalezcan en una región. Los fungicidas en precosecha o poscosecha constituyen la principal forma de reducir las pérdidas por esta enfermedad. Sin embargo, el uso indiscriminado de éstos ha tenido como consecuencia la resistencia de los microorganismos patógenos. Por otro lado, su uso está ampliamente restringido debido a la preocupación por los residuos tóxicos y los riesgos ocasionados a la salud. Por ello, existe la necesidad de manejar la enfermedad con alternativas amigables con el medio ambiente, entre ellas los extractos de plantas, los microorganismos como agentes de control biológico, tratamientos hidrotérmicos, manipulación genética y resistencia inducida. El objetivo del presente trabajo fue analizar las diferentes alternativas de control aplicadas al manejo de Colletotrichum spp.
Palabras clave: Trichoderma; antracnosis; control biológico; hospedantes; poscosecha
Introduction
Colletotrichum is a fungus is a cosmopolitan distribution and predominantly in tropical and subtropical regions (Xiao et al., 2004). Mainly it comprises pathogens of plants and fruits, including more than 100 species responsible for causing anthracnose, for this reason it is essential to identify the species to improve control of the disease (Crounch et al., 2014). Colletotrichum gloeosporioides is considered the most challenging kind of resolve, includes the widest range of guests of all species of Colletotrichum (Du et al., 2005; Prabhakaran, 2010).
In Figure 1A we see the symptoms in green pepper (Capsicum annuum var. annuum) and Figure 1B and C are appreciated fruit of start fruit (Averrhoa carambola L.) and mango (Mangifera indica L.) with typical symptoms of the disease; Figure 2A shows the symptoms of anthracnose in the fruit of papaya (Carica papaya L.), other affected species are banana (Musa paradisiaca L.), coffee (Coffea arabica L.), avocado (Persea americana Mill.), pigeon pea (Cajanus cajan L. Mill sp.), strawberry (Fragaria vesca L.), apple (Malus domestica Borkh.), almond (Prunus dulcis Mill.) and cherimoya (Annonna cherimola Mill.); ornamentals such as violets (Viola odorata L.), orchids (Orchidaceae) from different genera and species, golden shower (Cassia fistula L.); other plants such as soybean (Glycine max (L.) Merr.), peppermint (Menta piperita L.), lentil (Lens culinaris L.) and cotton (Gossipium hirsutum L.) (Freeman et al., 2001; Freeman, 2008); this species can cause high losses of fruits in postharvest (Gomes et al., 2013).
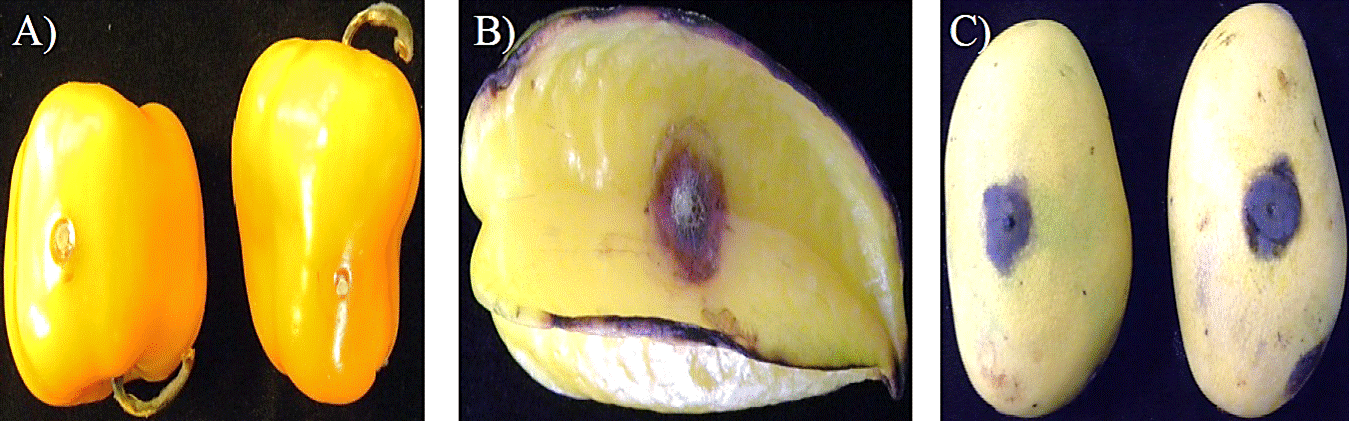
Figure. 1 Symptom anthracnose caused by Colletotrichum gloeosporioides inoculated in A) green pepper Capsicum annuum var. annuum; B) star fruit (Averrhoa carambola L.); and C) mango (Mangifera indica L.).
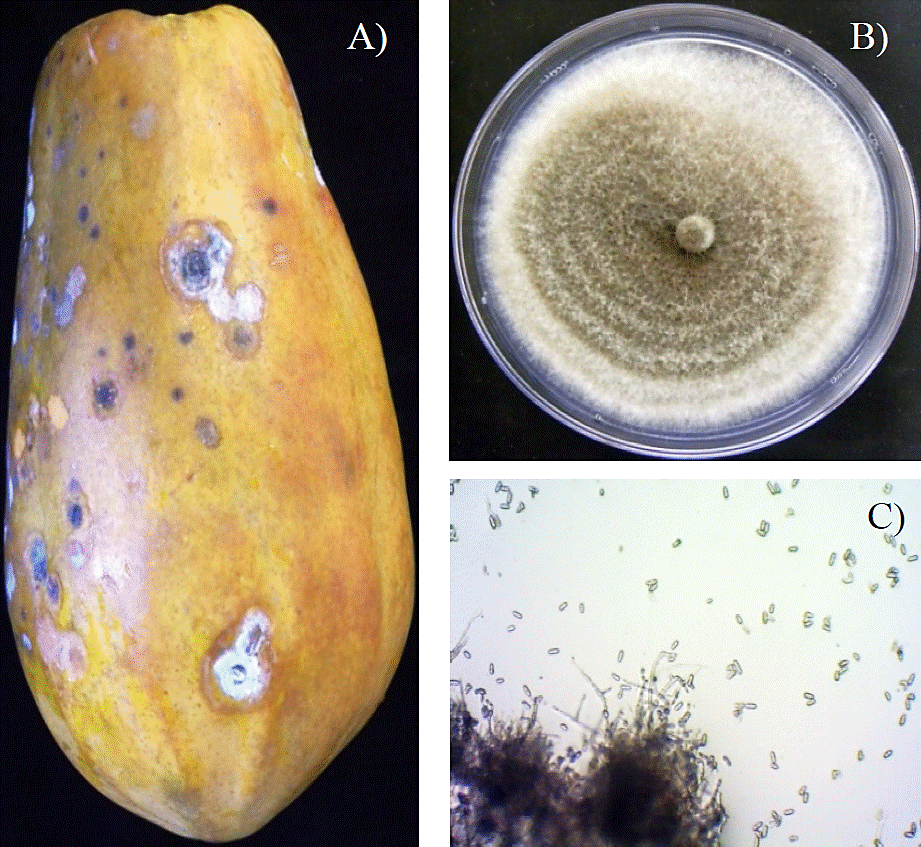
Figure 2 Colletotrichum gloeosporioides A) Papaya fruit with anthracnose caused by Colletotrichum gloeosporioides; B) Colletotrichum gloeosporioides. Growing PDA culture medium; C) spores Colletotrichum gloeosporioides.
Currently control alternatives for Colletotrichum spp. they are varied because the most common strategy, pesticides, have caused resistance in pathogenic organisms. Methods of control of the pathogen in preharvest and postharvest are using hot air, hydrothermal treatments, modified atmospheres (Karabulut and Baykal, 2004; Gutiérrez et al., 2004; Ragazzo et al., 2015), ultraviolet light, ozone (Cia et al., 2009), plant extracts (Bautista-Baños et al., 2003) and micro-biological control agents that act as antagonism against various pathogens such as Rodhotorula minuta, Bacillus subtilis, Trichoderma spp., to name a few (Janisiewics and Korsten, 2002; Spadaro and Gullino, 2004; Pérez, 2006; Vos et al., 2014).
Biorational against Colletotrichum gloeosporioides. At present, natural products are widely accepted and increasingly replacing synthetic products or materials generated artificially. In response to this trend it has been a growing interest in investigating the possible use of essential oils, plant extracts and others. Therefore, an alternative to the use of synthetic chemical molecules to control harmful organisms in agriculture is the application of biorationals, which are defined as produced by microorganisms, plants or minerals, which decompose within a few hours after application and they are specific for the organism to be controlled (O’Farril, 2008).
Regarding the use of plants with antimicrobial properties, Lara et al. (2014) found that glucosinolates broccoli florets isolated at concentrations of 1.54 and 0.92 µg/ µL-1 fully inhibited spore germination of Colletotrichum gloesporioides isolated from mango (Mangifera indica L.). The Gliricida sepium root extract against C. gloeosporioides was tested by reducing the severity of anthracnose on fruits 94% in Hawaiian papaya in postharvest chemical control while the reduced 84% (Loaiza and Rivera, 2000). Another extract has been used to control in papaya C. gloeosporioides postharvest seed it is the same fruit, alone and mixed with chitosan, the latter inhibiting mycelial growth of C. gloeosporioides at concentrations of 2.5 and 3%. The in vivo results showed that at a concentration of 1.5% chitosan, the severity was 1% (Bautista-Baños et al., 2003).
This shows that the chitosan has fungal potential against different pathogens belonging to the fungi kingdom, which is due to having polycationic load, while the walls of fungi are negatively charged deactivating germinating spores Botryosphaeria parva and C. gloesporioides (Everett et al., 2005).
Another biorational to use are plant extracts and essential oils of various plant species with antimicrobial properties, they affect the development of fungi both in vitro and in vivo of horticultural products (Molina et al., 2010). One of these species is Thyme vulgaris L., which has been found essential oil antibacterial and antifungal is, tested on several pathogenic microorganisms (Hammer et al., 1999; Vanneste and Boyd, 2002; Rassooli and Mirmostafa, 2003; Yang and Clausen, 2007).
When the biological effectiveness of the oil was tested against C. gloeosporioides isolated from papaya postharvest had a fungicidal effect, the development of the fungus was reduced as concentrations increased (Molina et al., 2010). Similarly Landero et al. (2013) found that cinnamon oil had a better effect on mycelial inhibition when increased concentrations; however, these were low (0.0015, 0.0025 and 0.005%) compared with garlic extract that inhibited 100% of fungal growth when used 10% concentration. This indicates that due to the effectiveness of the oils used at low concentrations could be considered more promising potential for use commercially.
Biological control of Colletotrichum gloeosporioides. The biological control of anthracnose has been reported in apples (Janisiewicz et al., 2003), papaya (Gamagae et al., 2004), avocado (Korsten et al., 1995) and handle (Carillo et al., 2005). Most of the experiments postharvest biocontrol diseases were made by using the antagonists in controlled conditions of humidity and temperature. Few studies has been done with application of microorganisms under field conditions, where the effectiveness of the antagonists is affected by other factors such as changes in temperature, humidity, ultraviolet light and interaction with other microorganisms. Paradoxically, one of the biggest obstacles to the development of biological control in postharvest is its relative inability to control preharvest established infections, such as latent infections (Spadaro and Gullino, 2004). However, Ippolito and Nigro (2000) state that the application in the field of biological control agents can lead to the colonization of the fruit surface and prevent the establishment of latent infections in producing the fruits of the orchard.
In anthracnose of mango, Juárez (2001) reported an extensive search of antagonistic microorganisms to the causative agent of this disease, where 120 strains were isolated (5 yeast and 115 bacteria) of the phyllosphere mango, of which two strains, a bacterium (Bacillus subtilis) and a yeast (Rodhotorula minuta) were those that showed higher antagonistic activity in vitro assays against C. gloeosporioides.
Meanwhile, Baños-Guevara et al. (2003) used antagonistic bacteria and plant extracts with fungitoxic properties, to evaluate the effects of anthracnose on some physical, chemical and physiological characteristics of papaya fruit red Maradol. The results showed that two strains B. firmus and four Pseudomonas fluorescens reduced growth in vitro of C. gloeosporioides. Examples of antagonistic microorganisms used successfully to control postharvest diseases are: B. licheniformis against C. gloeosporioides in mango (Govender et al., 2005).
Trichoderma spp. (Figure 3A, B, C, D, E and F) is considered the most studied antagonist for the control of phytopathogenic. Infante et al. (2009) studied and made a summary of the modes of action. The integration of the results mentioned that the mechanisms of action that allow control of pathogens Trichoderma spp., competition is the substrate, parasitism (Figure 3A, B, C, D and F), antibioses, disabling enzymes pathogen penetration (Figure 3F) and induced resistance, mainly.
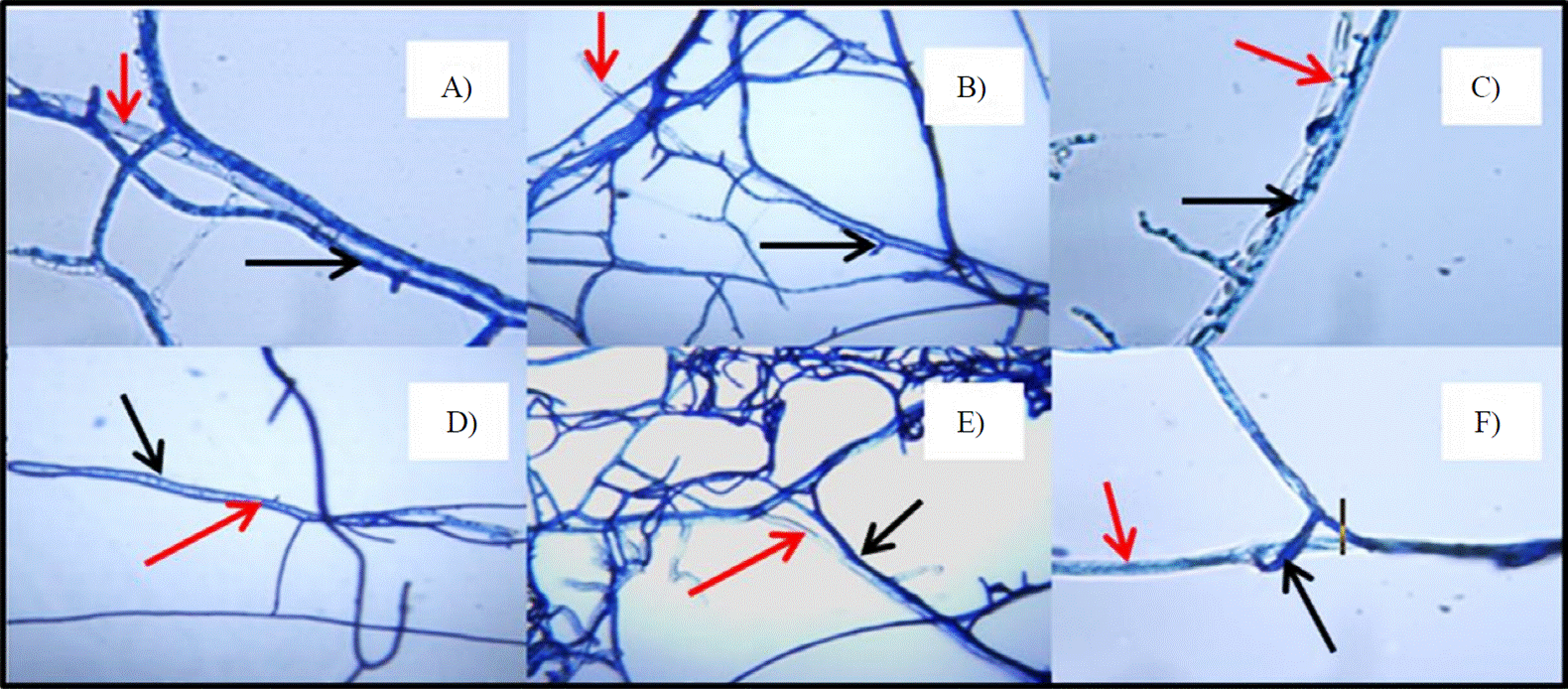
Red arrows indicate the hyphae of Colletotrichum, black arrows indicate the hyphae of Trichoderma.
Figure 3 Colletotrichum gloeosporioides, hyphae interacting with A) Trichoderma viride; B) Trichoderma longibrachiatum; C) Trichoderma asperellum 1; D) Trichoderma asperellum 2; E) and F) Trichoderma harzianum parasitizing, lysing or deforming the mycelium of Colletotrichum gloeosporioides.
Colletotrichum spp. control through molecular manipulation. The manipulation of genes by genetic engineering techniques has reduced ethylene synthesis, hormone considered maturity in plant species, Colletotrichum spp. Develops in the fruit precisely when it begins its maturation. In order to decrease the synthesis of ethylene they were carried out work where plants were developed which have been silenced them the ACC oxidase genes showing various alterations (Wi and Park, 2002). Fruits in postharvest also have been subjected to these techniques, delaying maturity in the case of tomatoes, in addition to stop the decomposition due to the presence of C. gloeosporioides, whose infection was detained until the application of external ethylene (Cooper et al., 1998). Another species to which it successfully silenced many genes is Nicotiana benthamiana (Robertson, 2004) which is also susceptible to infection by Colletotrichum orbicularie (Shen et al., 2001).
The main sources of resistance to anthracnose have been identified in Capsicum baccatum L. and C. chinense Jacq. By the Centre for Development and Asian Vegetable Research in 1999, and researchers have used these sources to study the inheritance of resistance to anthracnose (Pakdeevaraporn et al., 2005; Kim et al., 2010; Lee et al., 2010). Genetic analysis of segregating populations showed that the patterns of inheritance of resistance vary depending on the species and isolation of Colletotrichum, the source of resistance, and also the stage of maturity of the fruit. Considering Colletotrichum acutatum, the resistance came from C. chinense 'PBC932' in línea '0038-9155 '. The pathogen was controlled by two complementary dominant genes in green fruits, but two recessive genes in red fruits (Lin et al., 2007).
Colletotrichum capsici was another species on which has worked to control it, because it can cause postharvest losses up to 30% (Lakshmesha et al. (2002). Therefore, Lakshmesha et al. (2007) reduced cellulase activity (42.86%) and pectinase (40%) of this pathogen, exposing for three days the mature mycelia under ultraviolet (312 nm wavelength at a distance of 12 inches) during different time, finding that 45 min C. capsici exposure was less virulent. the reduction in the activity of enzymes resulted in 3.5 days late in the manifestation of anthracnose. for the specific case of mature chili interaction with C. gloeosporioides was found that the esterase gene chile (PepEST) is highly expressible during incompatible interactions and the gene expression is specific to the fruit in response to pathogen inoculation also can be regulated by injuries and treatment with jasmonic acid during ripening. This expression of genes PepEST not present in unripe fruits (Ko et al., 2005).
Inducers of resistance to Colletotrichum spp. Currently the induction of resistance to pathogens in fruits harvested using elicitors, physical, chemical and biological is becoming a promising approach to control postharvest diseases as an alternative to fungicides (Terry and Joyce, 2004). The non-proteinaceous β-aminobutyric acid (BABA), a rare compound found in nature, has been shown to induce resistance against a wide range of pathogenic organisms, including fungi, viruses, bacteria and nematodes in plants (Zimmerli et al., 2001; Cohen, 2002; Van der Ent et al., 2009; Quaglia et al., 2011). Other inducers that have been tested against C. gloeosporioides are salicylic acid, 2,6-dichloroisonicotinic acid, benzo1,2,3-tiadiazole-7-carbothioic acid S methyl ester and jasmonic acid, salicylic acid was found that a concentration of 1 mM on immature fruits of pepper no disease symptoms presented, as in mature fruits untreated (Lee et al., 2009) stage in which the resistance to phytopathogenic increases (Oh et al., 1999).
Conclusions
The main way to control Colletotrichum spp. Is through the use of synthetic chemicals. Resistance to fungicides and regulatory pressure regarding pesticide residues and concern for human health are increasing. The biorracionales products such as plant extracts have increased their importance and application in the past three decades, not a fashion, but by the knowledge of their chemical properties necessary to defend efficiently microorganisms and insects, properties that man has used to their benefit, without adversely affected the environment.
Another alternative are biofungicides, widely studied and often have proven to be compatible with other products, and operate through a variety of mechanisms. Regarding the compatibility of beneficial microorganisms with other products is limited, so we have the case of fungi are biofungicides only tolerate mixed with certain synthetic chemical fungicides, it is necessary that the producer knows the different chemical groups that belong fungicides and to make effective and efficient use of bio-fungicides. Inducers of plant defense have proven effective in suppressing Colletotrichum spp. Both alone and in combination with other products.
Genetic manipulation is one of the most viable alternatives today; however, is an expensive strategy that requires more knowledge to safely consider also getting an engineered product can take years to achieve, which involves expenditure of various resources.
Significantly, the need to control postharvest diseases is what has allowed us to develop strategies that contribute to the success of sustainable agriculture. The integration of various activities aims to reduce dependence on chemical control. In addition, the integration of these activities is intended to promote a production model system that benefits the producer and at the same time enabling the consumer to have a product that meets the quality requirements, without negatively impacting the environment.
Literatura citada
Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E. and Wilson, C. L. 2003. Effect of chitosan and plant extracts on growth of Colletotrichum gloeosporioides anthracnose level and quality of papaya fruit. Crop Protection. 22:1087-1092. [ Links ]
Carrillo, F. J. A.; García, E. R. S.; Muy, R. M. D.; Sañudo, B. A.; Márquez, Z., I.; Allende, M. R.; de la Garza, R. Z.; Patiño, V. M. y Galindo, F. E. 2005. Control biológico de antracnosis [Colletotrichum gloeosporioides (Penz.) Penz. y Sacc.] y su efecto en la calidad poscosecha del mango (Mangifera indica L.) en Sinaloa, México. Rev. Mex. Fitopatol. 23:24-32. [ Links ]
Cia, P.; Aparecida, B. E.; De Toledo, V. S. R.; Delgado de Almeida Anjos, V.; Scolfaro, P. F.; Sanches, J. e Monteiro, T. M. 2009. Radiação ultravioleta no controle pós-colheita de Colletotrichum gloeosporioides em uva ‘Niagara rosada’. Bragantia, Campinas. 68:1009-1015. [ Links ]
Cohen, Y. 2002. β-Aminobutyric acid-induced resistance against plant pathogens. Plant Dis. 86:448-457. [ Links ]
Cooper, W.; Bouzayen, M.; Hamilton, A.; Barry, C.; Rossall, S. and Grierson, D. 1998. Use of transgenic plants to study the role of ethylene and polygalacturonase during infection of tomato fruit by Colletotrichum gloeosporioides. Plant Pathol. 47:308-316. [ Links ]
Crouch J.; O’Connell, R.; Gan, P.; Buiate E.; Torres, M.; Beirn, L.; Shirasu, K.; Vaillancourt, L. 2014. The genomics of Colletotrichum. In: Dean, R. A.; Lichens-Park, A. and Kole, C. (Eds.). Genomics of plant-associated fungi and oomycetes: Monocot pathogens. Springer-Verlag, Berlin Heidelberg. 69-102 pp. [ Links ]
Du, M.; Schardl, C. Nuckles, E. and Vaillancourt, L. 2005. Using mating-type gene sequences for improved phylogenetic resolution of Collectotrichum species complexes. Mycologia. 97:641-658. [ Links ]
Everett, K. R.; Owen, S. G. and J. M. Cutting. 2005. Testing efficacy of fungicides against postharvest pathogens of avocado (Persea Americana cv. Hass). New Zealand Plant Protection. 58:89-95. [ Links ]
Freeman, S. 2008. Management, survival strategies, and host range of Colletotrichum acutatum on strawberry. Hort Sci. 43:66-68. [ Links ]
Freeman, S. S.; Horowitz, S. and Sharon, A. 2001. Pathogenic and non-pathogenic lifestyles in Colletotrichum acutatum from strawberry and other plants. Phytopathology. 91:986-992. [ Links ]
Gamagae, S. U.; Sivakumar, D. and Wijesundera, R. L. C. 2004. Evaluation of postharvest aplication of sodium bicarbonate incorporated wax formulation and Candia oleophila for the control of antracnose of papaya. Crop Protection. 23:575-579. [ Links ]
Gomes, M. S. R.; Osama, T. F. A.; Massola, J. N. S. 2013. Histopathology of Colletotrichum gloeosporioides on guava fruits (Psidium guajava L.). Rev. Bras. Frutic. 35-2:657-664. [ Links ]
Govender, V.; Korsten, L. and Sivakumar, D. 2005. Semicommercial evaluation of Bacillus licheniformis to control mango postharvest diseases in South Africa. Postharvest Biol. Technol. 38:57-65. [ Links ]
Gutiérrez, A. J. G.; Gutiérrez A. O.; Nieto A. D.; Téliz O. D.; Zavaleta, M. E. y Delgadillo, S. F. 2004. Manejo integrado de la antracnosis [Colletotrichum gloeosporioides (Penz.) Penz. y Sacc.] del mango (Mangifera indica L.) durante la postcosecha. Rev. Mex. Fitopatol. 22:395-402. [ Links ]
Hammer, K. A.; Carson, C. F. and Riley, T. V. 1999. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 86:985-990. [ Links ]
Infante, D.; Martínez, B.; González, N. y Reyes, Y. 2009. Mecanismos de acción de Trichoderma frente a hongos fitopatógenos. Protección Vegetal. 24:14-21. [ Links ]
Ippolito, A. and Nigro, F. 2000. Impact of preharvest aplication of biological control agent on postharvest diseases of fresh fruit and vegetables. Crop Protection. 19:610-619. [ Links ]
Janisiewicz, W. J. and Korsten, L. 2002. Biological control of postharvest disease of fruits. Ann. Review Phytopathol. 40:411-441. [ Links ]
Janisiewicz, W. J.; Leverentz, B.; Conway, W. S.; Saftner, R. A.; Reed, A. N. and Camp, M. J. 2003. Control of bitter rot and blue mold of apple integrating heat and antagonistic treatments on 1-MCP treated fruits stored under controlled atmosphere conditions. Postharvest Biol. Technol. 20:129-143. [ Links ]
Juárez, R. C. 2001. Microorganismos antagonistas para el control de antracnosis en mango cultivado en Sinaloa. Tesis de maestría en ciencias de la producción agrícola. Facultad de agronomía de la Universidad Autónoma de Sinaloa. Culiacán, Sinaloa, México. 78 p. [ Links ]
Karabulut, O. A. and Baykal, N. 2004. Integrated control of postharvest disease of peaches with a yeast, hot water and modified atmosphere packaging. Crop Protection 23:431-435. [ Links ]
Kim, S.; Kim, K. T.; Kim, D. H.; Yang, E. Y.; Cho, M. C.; Jamal, A.; Chae, Y.; Pae, D. H.; Oh, D. G. and Hwang, J. K. 2010. Identification of quantitative trait loci associated with anthracnose resistance in chili pepper (Capsicum spp.). Korean J. Hortic. Sci. Technol. 28:1014-1024. [ Links ]
Ko, M. K.; Jeon, W. B.; Kim, K. S.; Lee, H. H.; Seo, H. H.; Kim, Y. S. and Oh, B. J. 2005. A Colletotrichum gloeosporioides-induced esterase gene of nonclimacteric pepper (Capsicum annuum) fruit during ripening plays a role in resistance against fungal infection. Plant Mol. Biol. 58:529-541. [ Links ]
Korsten, L.; De Jager, G. S.; De Villiers, E. E.; Lourens, A.; Kotzé, J. M. and Wehner, F. C. 1995. Evaluation of bacterial epiphytes isoloted from avocado leaf and fruits surfaces for biocontrol of avocado postharvest diseases.79:1149-1156. [ Links ]
Lakshmesha, K. K.; Mallikarjuna, A. S. and Lakshmidevi, N. 2002. Postharvest management of anthracnose disease on capsicum. Asian Cong. Mycol. Plant Pathol. 252 p. [ Links ]
Laksmesha, K. K.; Lakshmidevi, N. and Majikarjuna, A. 2007. Changes in pectinase and cellulase activity of Colletotrichum capsici mutants and their effect on anthracnose disease on capsicum fruit. Arch. Phytopathol. Plant Protection. 4:267-279. [ Links ]
Landero, V. N.; Nieto, A. D.; Téliz, O. D.; Alatorre, R. R.; Orozco, S. M. y Ortíz, G. C. F. 2013. Potencial antifúngico de extractos de cuatro especies vegetales sobre el crecimiento de Colletotrichum gloeosporioides en papaya (Carica papaya) en poscosecha. Rev. Venezolana de Ciencia y Tecnología de Alimentos. 4:47-62. [ Links ]
Lara, V. F. M.; Nieto, A. D.; Nava, D. C.; Gutiérrez, A. G.; Ayala, G. J. O.; Aguilar, P. L. A. y Martínez, T. D. 2014. Efecto del glucorafano aislado de floretes de brócoli sobre la germinación de esporas de Colletotrichum gloeosporioides. Rev. Fitotec. Mex. 37:141-147. [ Links ]
Lee, J.; Hong, J.; Do, J.; Yoon, J.; Lee, J. D.; Hong, J. H.; Do, J. W. and Yoon, J. B. 2010. Identification of QTLs for resistance to anthracnose to two Colletotrichum species in pepper. J. Crop Sci. Biotechnol. 13:227-233. [ Links ]
Lee, S.; Hong, J. C.; Jeon, W. B.; Chung, Y. S.; Sung, S.; Choi, D.; Joung, Y. H. and Oh, B. J. 2009. The salicylic acid-induced protection of non-climacteric unripe pepper fruit against Colletotrichum gloeosporioides is similar to the resistance of ripe fruit. Plant Cell Reports. 28:1573-1580. [ Links ]
Lin, S.W.; Gniffke, P. A. and Wang, T. C. 2007. Inheritance of resistance to pepper anthracnose caused by Colletotrichum acutatum. Acta Hortic. 760:329-334. [ Links ]
Loaiza, J. E. and Rivera, G. 2000. Potencial biocida de extractos de Gliricida psepium contra patógenos del cultivo de la papaya. Agron. Costarric. 1:29-36. [ Links ]
Molina, B. E.; Ronquillo, E. J.; Bautista, B. S.; J. R.; Verde, C. J. R. and Morales, L. J. 2010. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol. Technol. 57:132-137. [ Links ]
O’Farrill, N. H. S. A. 2008. Insecticidas biorracionales. <http://academic.uprm.edu/ofarrill/HTMLobj-323/biorational.pdf>. [ Links ]
Oh, B. J.; Kim, K. D. and Kim, Y. S. 1999. Effect of cuticular wax layers of green and red pepper fruits on infection by Colletotrichum gloeosporioides. J. Phytopathol. 147:547-552. [ Links ]
Pakdeevaraporn, P.; Wasee, S.; Taylor, P. W. J. and Mongkolporn, O. 2005. Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breed. 124:206-208. [ Links ]
Prabhakaran, N. K. P. 2010. The agronomy and economiy of important tree crops of the developing world. (Eds.). Elsevier. USA. 58 p. [ Links ]
Quaglia, M.; Ederli, L.; Pasqualini, S. and Zazzerini, A. 2011. Biological control agents and chemical inducers of resistance for postharvest control of Penicillium expansum Link. on apple fruit. Postharvest Biol. Technol. 59:307-315. [ Links ]
Ragazzo-Sánchez, J. A.; Coria-Tellez; Ramírez de León, J. A.; OrtízBasurto, R. I.; Cabanillas-Beltrán, H. and Calderon-Santoyo, M. 2015. Control of antracnose in mango (Mangifera indica L.) var. ataulfo by high hydrostatic pressure combined with moderated temperature and the biocontrol agent candidat famata. Sylwan. 159:448-460. [ Links ]
Rassooli, I. and Mirmostafa, S. A. 2003. Bacterial susceptibility to and chemical composition of essential oils from Thymus kotschyanus and Thymus persicus. J. Agric. Food Chem. 51:2200-2205. [ Links ]
Robertson, D. 2004. VIGS vectors for gene silencing: many targets, many tools. Annual Review Plant Biol. 55:495-519. [ Links ]
Shen, S.; Goodwin, P. H. H. and Siang, T. 2001. Infection of Nicotiana species by the anthracnose fungus, Colletotrichum orbiculare. Eur. J. Plant Pathol. 107:763-773. [ Links ]
Spadaro, D. and Gullino, M. L. 2004. State of the art and future prospects of the biological control of postharvest fruit disease. Int. J. Food Microbiol. 91:185-194. [ Links ]
Terry, L. A. and Joyce, D. C. 2004. Elicitors of induced disease resistance in harvested horti-cultural crops: a brief review. Postharvest Biol. Technol. 32:1-13. [ Links ]
Van der Ent, S.; Van Hulten, M.; Pozo, M. J.; Czechowshi, T.; Udvardi, M. K.; Pieterse, C. M. J. and Ton, J. 2009. Priming of plant innate immunity by rhizobacteria and β-aminobutyric acid: differences and similarities in regulation. New Phytologyst. 183:419-431. [ Links ]
Vanneste, J. L. and Boyd, R. J. 2002. Inhibition of Erwinia amylovora and potential antagonistic bacteria by essential oils and natural compounds. Acta Hortic. 590:315-317. [ Links ]
Vos, C. M.; Yang, B.; De Coninck, B. and Cammue, B. P. A. 2014. Fungal (-like) biocontrol organism in tomato disease control. Biological Control. 74:65-81. [ Links ]
Wi, S. J. and Park, K. Y. 2002 Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Molecular Cells. 13:209-222. [ Links ]
Xiao, C. L.; MacKenzie, S. J. and Legard, D. E. 2004. Genetic and pathogenic analyses of Colletotrichum gloeosporioides isolates from strawberry and noncultivated host. Phytopathology. 94:446-453. [ Links ]
Yang, V. W. and Clausen, C. A. 2007. Antifungal effect of essential oils on southern yellow pine. Int. Biodeter. Biodegrad. 59:302-306. [ Links ]
Zimmerli, L.; Metraux, J. P. and Mauch-Mani, B. 2001. β-Aminobutyric acid-induced protection of Arabidopsis against the Necrotrophic Fungus Botrytis cinerea. Plant Physiology. 126:517-523. [ Links ]
Received: April 2016; Accepted: June 2016











 text in
text in 


