Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.5 Texcoco Jun./Aug. 2016
Articles
Essential oil of Cynnamomum zeylanicum: control alternative for Penicillium expansum on pear in postharvest
1Universidad Politécnica de Francisco I. Madero. Conocido, Tepatepec, Hidalgo, México, C. P. 42660. Tel: 738 7241174. Conocido, Tepatepec, Hidalgo, México, C. P. 42660. (nlanderova@conacyt.mx; gjaguadoro@conacyt.mx; pandrade@upfim.edu.mx; eadali_081@hotmail.com; da_estra_4@hotmail.com).
The major postharvest pear infection is caused by the fungus Penicillium expansum, resulting in significant global economic losses. This study aimed to evaluate the effect of different concentrations of essential oil of cinnamon (Cinnamomum zeylanicum) on the development of Penicillium expansum in vitro and in vivo on postharvest pear fruit. The cinnamon essential oil was tested at three different concentrations (60, 120 and 300 µL L-1), considering variables that were mycelial growth, sporulation and severity of the disease produced in pear fruit for eight days. The results of the in vitro experiment showed that when the concentration of cinnamon oil was 300 µL L-1 pathogen mycelial growth was inhibited 81 h after being sown in the culture medium. The generated mathematical models estimate that allowed spore production decreases in concentrations above 135 µL L-1. In the experiment in vivo development of the disease it was statistically similar when the pathogen was growing on pear fruit sprinkled with 300 µL L-1 essential oil of cinnamon compared to the development of the disease on pear fruit sprinkled with Imazalil. This supports the idea that cinnamon oil can be a compound useful to maintain the shelf life of fruit postharvest pear.
Keywords: cinnamon; pears diseases; postharvest; natural extracts
La principal infección de pera en poscosecha es causada por el hongo Penicillium expansum, resultando en pérdidas económicas globales significativas. El presente trabajo tuvo como objetivo evaluar el efecto de diferentes concentraciones de aceite esencial de canela (Cinnamomum zeylanicum) sobre el desarrollo de Penicillium expansum in vitro, e in vivo sobre frutos de pera en poscosecha. Aceite esencial de canela fue probado en tres concentraciones diferentes (60, 120 y 300 µL L-1), considerando variables que fueron crecimiento micelial, esporulación y severidad de la enfermedad producida en frutos de pera durante ocho días. Los resultados del experimento in vitro mostraron que cuando la concentración de aceite de canela fue de 300 µL L-1 el crecimiento micelial del patógeno se inhibió 81 h después de haber sido sembrado en el medio de cultivo. Los modelos matemáticos generados permitieron estimar que la producción de esporas disminuye en concentraciones superiores a 135 µL L-1. En el experimento in vivo el desarrollo de la enfermedad fue estadísticamente similar cuando el patógenos estuvo creciendo sobre frutos de pera asperjados con 300 µL L-1 de aceite esencial de canela comparado con el desarrollo de la enfermedad sobre frutos de pera asperjados con Imazalil. Lo anterior soporta la idea de que el aceite de canela puede ser un compuesto de utilidad para mantener la vida de anaquel de frutos de pera en poscosecha.
Palabras claves: canela; enfermedades en peras; extractos naturales; poscosecha
Introduction
The post-harvest diseases can cause high economic losses due to the disposal of fruit not reach their final market. In developed countries it is estimated that up to 25% of the fruit may be affected by pathogens, while in developing country the percentage of loss doubles (Sharma et al., 2009). The pear is no exception, the main infection in these fruits that are stored for long periods of time is caused by Penicillium expansum and the presence of this fungus can result in significant global economic losses, why this pathogen is considered the most important postharvest pear fruits (Rosenberger, 1990).
The application of synthetic chemical fungicides is the first tactical control of this fungus in packing pear and other fruits in order to reduce the incidence (Hao et al., 2010); however, repeated application of these products has generated a strong selection pressure on pathogens, limiting the success in controlling infections caused by Penicillium (Baraldi et al., 2003).
In fact, for several years, there are reports of Penicillium expansum strains resistant to benzimidazoles and in vitro studies demonstrated cross-resistance to benomyl, carbendazim, and thiabendazole thiophanato (Koffmann et al., 1978). However, the use of synthetic chemical fungicides is becoming ever more restricted, not only by the emergence of resistant strains, but because of the harmful effects caused to the environment and public health (Lima et al., 2011). Therefore, consumer awareness about safer, more nutritious and environmentally friendly food increased. They have also explored alternative methods to control postharvest diseases.
One such alternative is the use of compounds of extracts from plants with antimicrobial activity, which are known to be effective, also with less environmental effects and do not harm or even improve human health (Azzouz, 1982; Amadioha, 2000; Paranagama et al., 2003). Essential oils are widely used compounds for this purpose with the advantage that some of them are volatile so they do not leave residues in fruits, as in the case of oil of cinnamon (Ayala et al., 2008), whose main component it is the cinnamaldehyde (China Pharmacopeia Commission, 2010).
The essential oil of cinnamon has been found to possess antimicrobial and antifungal properties (Chang et al., 2001; Kim et al., 2004; Singh et al., 2007). The severity caused by fungal diseases in strawberry fruits decreased after treatment with vapors of cinnamon oil, compared to untreated fruits (Tzortzakis, 2007). It has also been shown its antifungal effectiveness against Fusarium moniliforme (Paran et al., 1996) and Fusarium proliferatum, the latter isolated from banana fruits and in which case was required a lower concentration of essential oil of cinnamon (0.05% v/v) that the fungicide benomyl (1% v/v) to achieve the minimum dose required to inhibit fungal growth (Ranasinghe et al., 2002).
On the other hand we have studied the effect of essential oil of cinnamon mycotoxin production from Aspergillus flavus, finding a significant reduction in aflatoxin B1 with a concentration of essential oil of cinnamon 2%, placed on disks polypropylene which volatiles from the oil came off, total inhibition of aflatoxin B1 production was achieved with a concentration of 4% of essential oil of cinnamon (Manso et al., 2014). Other studies report the possible application of essential oil of cinnamon in packaging due to antifungal effect on pathogens affecting fruit of various species in postharvest (Nielsen and Ríos, 2000).
Considering the above, the present work was to evaluate the effect of cinnamon oil at different concentrations in the development of the fungus Penicillium expansum on pear fruit postharvest.
Materials and methods
The research was conducted at the premises of the Polytechnic University of Francisco I. Madero, in the municipality of Tepatepec, Hidalgo, specifically in the analysis laboratory and chemistry.
Pathogen isolation and pathogenicity tests
The fungus Penicillium expansum was isolated pear fruit with symptoms characteristic of the disease, which were obtained from the central supply of Mexico City. The fragments of about 5 x 5 mm were used, with 20% of infected tissue and 80% of healthy tissue seeded in Petri dishes with Potato Dextrose Agar (PDA) and incubated at room temperature (26 ± 2 ºC) for 10 days. A portion of mycelia was transferred to another Petri dish with PDA to purify the strains. After morphologically confirmed the identity of Penicillium expansum by morphological keys Seifer et al. (2011), the strain was seeded on PDA culture medium was developed where for a period of 7 days, at a temperature of 28 °C.
When the colony had an age of more than a week, spores were scraped and a suspension was prepared by adjusting a concentration of 106 conidium mL-1. These conidium were inoculated on pear fruits var. Anjou (Pyrus communis) that were selected from a fruit trade in the municipality of Actopan, Hidalgo, Mexico. All fruits to be used in the trial were in consumption maturity and free of damage, signs of infection and uniform in size. Each of the fruits of pear were washed and disinfested with a solution of sodium hypochlorite 11% v/v and performed two wounds of about 2 mm diameter by 2 mm depth caused by a needle sterile dissection, in 20 µL of that pathogen spores at a concentration of 106 conidium mL-1 were added to reproduce the above symptoms, in order to corroborate the pathogenicity of P. exapansum. A control fruits were placed PDA without the fungus. Strains caused the development of symptoms were preserved in mineral oil and PDA for later use.
In vitro control of Penicillium expansum with Cinnamomum zeylanicum
For the preparation of each treatment were mixed, 100 mL of PDA medium (before gelling) with each of the emulsions of cinnamon oil (60, 120 and 300 µL L-1), with 5 replications of each treatment in addition commercial witness (Imazalil 500 mg L-1) and untreated control, which was not given any treatment. The gelation of each of the treatments was allowed, and immediately P. expansum was transferred in the center of Petri dishes. In Petri dishes are incubated at 28 °C for 8 days.
The efficacy of each treatment was determined by the measurement of radial growth of the fungus colony every 24 h, the growth rate of the colony in the culture medium, and sporulation when applied different treatments; for this variable, the concentration of conidium was determined after 10 days when the maximum sporulation was achieved. Each petri dish was rinsed with sterile distilled water (15 mL), the surface was scraped with a glass rod and filtered through a sterile cotton mesh. The aliquots of spore suspension with a volume of 0.5 mL of each box were transferred to a chamber Newbauer for counting the number of conidium. The experiment was repeated twice, the second set at 48 h after the first.
Antifungal effect in vivo
The fruits pear var Anjou were selected of healthy appearance, without damage or infection, to be artificially inoculated with Penicillium expansum.
Each fruit was disinfected with sodium hypochlorite 2% by immersion for 3 min, then rinsed with sterile distilled water and dried at room temperature on absorbent paper. In parallel, was prepared an aqueous spore suspension of P. expansum at 106 conidium mL-1 concentration, and cinnamon oil concentrations of 60, 120 y 300 µL L-1. Such emulsions of essential oil were sprayed on 25 fruits (5 replicates for 5 treatments) 24 h prior to inoculation with P. expansum, a chemical control was included (Imazalil at a concentration of 500 mg L-1) and one absolute (distilled water sterile). Inoculation of the fruits were wounded by two 2 mm deep sterile dissection needle, placed over said wound 20 µL of an aqueous suspension of 106 conidium mL-1 with micropipette.
The fruits were placed in groups of two in sealed plastic containers that maintained a temperature of 26 ± 2 ºC and 90% relative humidity. The diameter of the lesion was determined every 24 hours by analyzing digital images obtained from diseased fruits by means of a camera. The fruits in the humid chamber were photographed daily by Fuji® of 12-megapixel camera brand, positioned at a constant height of 30 cm from the edge of the humid chamber. The images obtained were processed by the software Photoshop CS6. The diameter of the lesion that caused the pathogen was determined in number of pixels.
Statistical analysis
The data obtained from in vitro experiment regression analysis were performed for each treatment, generating an equation describing a curve representing each. Each curve generated under it an area that was calculated with the method previously reported by polygons (Liengme, 2002). The units resulting from this method are dimensionless. The results were subjected to analysis of variance using a completely randomized design and multiple tests Tukey mean separation using SAS v.9 program for Windows®.
The increase in colony size was calculated by subtracting the colony size of a given day to the size of the colony the day before and the results obtained were subjected to regression analysis. For in vivo results the area under the curve of progress of the disease by the method described above. The minimum values mycelial growth and spore production maximum was estimated from the regression equation generated by the model using the Solver command of Microsoft Excel®.
Results and discussion
In vitro experiments
Mycelial growth
The data showed a decrease in the growth of mycelium of P. expansum at the highest concentration of cinnamon extract. The mycelial growth in this concentration was statistically the same as in growth when the pathogen was developed in the presence of chemical control. In contrast the effects of concentrations less than 300 µL L-1 were equal to those observed in the control treatment (Table 1).
Table 1 Effect of essential oil of cinnamon on mycelial growth of the fungus Penicillium expansum eight days after sowing and overall growth.
| Concentración de Canela (Porcentaje de extracto v/v) | Promedio de crecimiento 8 días después de la siembra (cm)† | Área bajo la curva del crecimiento micelial†† |
| Testigo absoluto | 1.02 a | 6.47 a |
| Extracto de canela 60 µL L-1 | 1.16 a | 6.22 a |
| Extracto de canela 120 µL L-1 | 1.44 a | 5.78 a |
| Extracto de canela 300 µL L-1 | 0.81 b | 1.95 b |
| Imazalil (500 mg L-1) | 0 b | 0 b |
| DMS* | 0.95 | 2.05 |
†Letras iguales entre columnas no indican diferencias estadísticas significativas (Tukey, p= 0.05). DMS= diferencia mínima significativa. ††El cálculo del área bajo la curva arroja números adimensionales.
The mycelial growth in Petri dishes containing cinnamon extract was lower compared to the control. Mathematical models allowed us to estimate that in the presence of the essential oil of cinnamon to a concentration of 300 µL L-1, the pathogen stopped growing 81 h after seeding, while the fungus growing in medium free of essential oil of cinnamon never completely stopped its growth; however, he showed a minimal increase of size 90.3 h after plating, that is, about eight hours after treatment with 300 µL L-1 essential oil (Figure 1). In the present experiment the essential oil of cinnamon showed growth inhibition of the pathogen; however, after a few hours that growth was restored, so a fungistatic effect was observed.
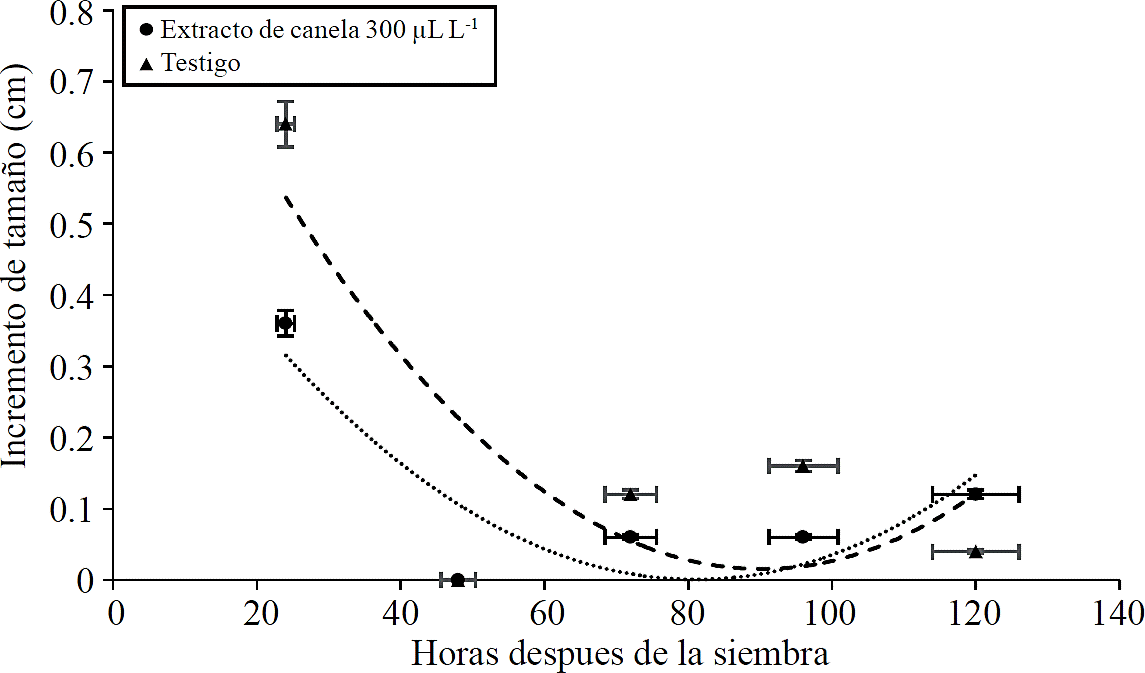
Figure 1 Effect of cinnamon essential oil to 300 µL L-1 on the increase in the mycelial diameter of Penicillium sp. compared to the control treatment (mycelium growing in agar potato-dextrose) in the first five days after planting.
The increase in the mycelial diameter of Penicillium growing in cinnamon extract was adjusted to an equation of the form y= 9.6726 X 10-5 X2 + -0.015 X + 0.636; where y= mycelial growth and X= hours after seeding. While the witness was adjusted to an equation of the form y= 0.0001 X2 -0.0215 X + 0.984. The coefficient of determination was 0.7 and 0.6 respectively.
The antimicrobial effect of cinnamon extracts previously reported by (Tzortzakis, 2009), which managed to inhibit the growth of the colony of two species of Colletotrichum and the genus Rhizopus affecting fruit in postharvest, and the production of spores a species of Botrytis and other of genus Aspergillus. For some years it is known that the essential oil of cinnamon contains high concentrations of cinnamaldehyde and eugenol compounds that may be responsible for inhibiting the mycelial growth (Helander et al., 1995). Recently it used the essential oil of cinnamon to impregnate films that coat fruits for control of Penicillium (Montero-Prado et al., 2011). In this paper the results showed that cinnamon oil achieved a significant decrease in mycelial growth of the pathogen. Some authors have reported that the effectiveness of applications cinnamon extracts by immersion or spraying are effective because the hydrophobic compounds present in the extracts bind to cellular hydrophobic compounds affecting the activity of cell membranes (Avila et al., 2012).
Sporulation
The results showed that the essential oil of cinnamon caused an increase in the amount of spores produced by P. expansum. The mathematical models to estimate the maximum allowed value of production (618.08 spores mL-1) when the fungus was placed in 135 µL L-1 essential oil of cinnamon. The same mathematical model allowed us to observe that increasing the concentration of cinnamon extract above that value (135 µL L-1) the amount of spores produced decreased to completely inhibit spore production with 312.38 µL L-1 (Figure 2). Other authors have found similar behavior in some other physiological processes fungi when exposed to different concentrations of essential oils of cinnamon, for example (Tzortzakis, 2009), found an increase in spore germination and size of the germ tube Aspergillus niger when these were placed in presence of 25 or 50 ppm cinnamon extract, in contrast to higher than 100 ppm both variables showed lower values in relation to the control concentration. Other genres such as Colletotrichum coccodes and Rhizopus stolonifer showed a decrease in germination of spores so it is possible that this response is characteristic of each species.
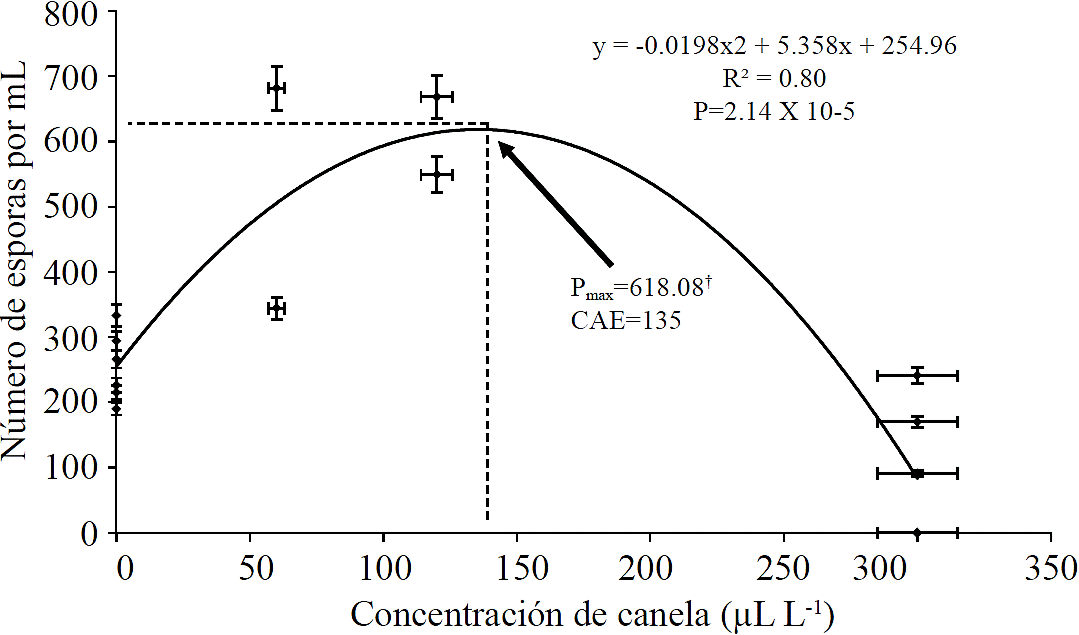
Donde: †Pmax= producción máxima de esporas por mililitro; CAE= concentración de aceite esencial de canela en µL L-1; ambos parámetros fueron estimados a partir de ecuación de la forma y= ax2 + bx + c; y= Número de esporas por mililitro, x= concentración de aceite esencial de canela. Los coeficientes de la ecuación aparecen en la figura.
Figure 2 Effect of cinnamon extract on spore production in Penicillium expansum.
The production of spores, conidium, sclerotia and cleistothecia is influenced by factors related to the compounds present in the growth medium or environmental factors (Calvo et al., 2002). In the case of Penicillium necessary for spore production conditions are much more restrictive than those required for vegetative growth (Sekiguchi and Gaucher, 1977). This is an indication that this physiological process is much more prone to changes mycelial growth.
In this paper the mycelial growth was inhibited when the fungus grew in the presence of 300 µL L-1 essential oil of cinnamon (Figure 1), on the other hand, spore production was increased in the presence of lower doses at 135 µL L-1. When the fungus grew doses above this value decreased to completely inhibited (Figure 2).
In vivo experiments
The fruit treated with 300 µL L-1 essential oil of cinnamon oil showed development similar to the fruit treated with Imazalil at 500 mg L-1 condition. In both cases the development of the disease was lower than the control treatment. In contrast doses of 60 and 120 µL L-1 essential oil of cinnamon showed no statistically significant differences compared to the control treatment (Table 2).
Table 2 Effect of different concentrations of cinnamon essential oil and imazalil on the development of the disease for eight days produced by Penicillium expansum on pear fruit postharvest.
| Dosis de aceite de canela (µL L-1) e Imazalil (mg L-1) | Área bajo la curva del progreso de la enfermedad† |
| 0 | 5327.3 a |
| 60 | 4886.5 a |
| 120 | 4409.6 ab |
| 300 | 3425.9 b |
| Imazalil (500 mg/L) | 3784.1 b |
| DMS* | 1000.6 |
†Letras iguales entre tratamientos no indican diferencias estadísticas significativas (Tukey, p= 0.05). El cálculo del área bajo la curva arroja números adimensionales.
*DMS= diferencia mínima significativa.
During the first days after inoculation, the fruits witness as well as those treated with essential oil of cinnamon 300 µL L-1 or imazalil at 500 mg L-1, they showed similar in the development of disease behavior, however from the day number five, the same fruits showed a development of symptoms much slower relative to untreated fruits.
Although both imazalil as cinnamon essential oil showed a significant effect on the development of the disease, Penicillium expansum continued to grow in the fruits treated with both products, although at a much slower pace than untreated fruits. On the last day of assessment the area under the curve of progress of disease of untreated fruit was 1 032 units, whereas for fruit treated with essential oil of cinnamon 300 µL L-1 was 513 units, which a decrease in disease progression 49.7%, on the other hand the fruits treated with imazalil showed a decrease in disease progression 47.8% (Figure 3).
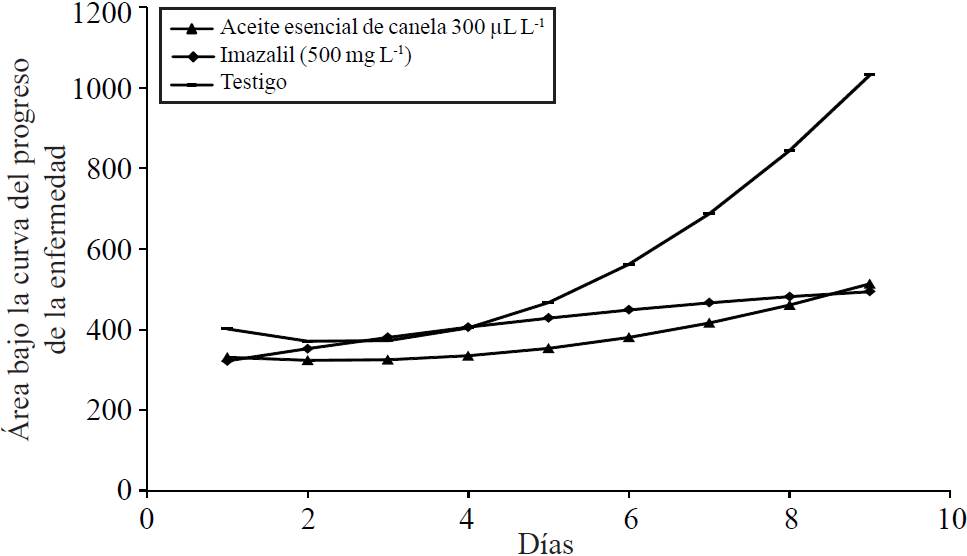
Figure 3 Area under the curve of progress of the disease in pear fruit treated with essential oil of cinnamon 300 µL L-1, imazalil at a concentration of 500 mg L-1 and untreated fruit.
The regression model adjusted for cinnamon essential oil was; y= -20.31549 x + 4.31269624x 2 + 347.0032, R2=0.74; for Imazalil was y= 34.382655 x - 1.281755 x 2 + 288.6624, R2=0.71; fruits for untreated (control) was the model; y= -77.11011 x +15.594707 x 2 + 462.82721 R2=0.98; where: y= days after inoculation fruit with Penicillium, x= area under the curve of disease progression.
For some time it is known that the essential oil of cinnamon has a strong biological effectiveness against certain microorganisms (Mahdi-Ojagh et al., 2010). Extracts of essential oil of cinnamon is used to control sprinkling them pathogens on fruit or by immersing the fruits directly in the extract thereby increasing shelf life (Ranasinghe et al., 2003); however, in most research extracts are used in combination with other control methods; for example, (Kyu Kyu Win et al., 2007) found a statistically significant decrease in the severity fruits of bananas when inoculated with C. musae and Fusarium spp however, these authors fruits stored at 13 °C, so the observed effect on the severity is due not only to extract, but its interaction with the temperatures used. In other work results with the same trend are reported using similar methodologies (Melgarejo-Flores et al., 2013) found a significant decrease in the severity of disease caused by fungus grape fruits treated with different concentrations of compounds obtained from the cinnamon; however, in the above research fruits they were stored at 15 °C.
In this work the fruits were stored at ambient temperature (25 ± °C) and high humidity (+ 90%) which could increase the respiration rate of the fruit, with the consequent increase in CO2 production and ethylene (Taiz and Zeiguer, 2010). Some authors (Chilea et al., 1985) have pointed out that this gas can act as a pathogenic factor in infections caused by Penicillium. Because of this, the conditions under which this study was able to provide the pathogen a significant advantage to cause damage to the fruits of pear, despite the above conditions, the essential oil of cinnamon was as efficient as imazalil to slow the progress of the disease compared to the control, with the advantage that the essential oil of cinnamon provides minimal risk to the health of humans, since it is used as a typical ingredient of some foods.
Conclusions
The results of the in vitro experiment showed that the essential oil of cinnamon to a concentration of 300 µL L-1 was able to inhibit the mycelial growth of Penicillium expansum. From 135 µL L-1 spore production was decreased; however, at lower at this dose, the amount of spores increased. In the in vivo experiment Penicillium expansum mycelial growth showed a statistically equal when it grew on pear fruit sprinkled with 300 µL L-1 essential oil of cinnamon compared to the pathogen to grow in the presence of chemical control (imazalil). This supports the idea that cinnamon oil can be a compound useful to maintain the shelf life of fruit postharvest pear.
Literatura citada
Amadioha, A. C. 2000. Controlling rice blast in vitro and in vivo with extracts of Azadirachta indica. Crop Protection. 19:287-290. [ Links ]
Avila, S. R.; Palou, E.; Jiménez, M. M. T.; Nevárez, M. G. V.; Navarro, C. A. R. and López, M. A. 2012. Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. Int. J. Food. 153:66-72. [ Links ]
Ayala, Z. J. F.; Del Toro, S. L.; Alvarez, P. E.; Soto, V. H.; Martin, B. O.; Ruiz, C. S. and González, A. G. 2008. Natural antimicrobial agents incorporated in active packaging to preserve the quality of fresh fruits and vegetables. Stewart Postharvest Review. 4:1-9. [ Links ]
Azzouz, M. A. and Bullerman, L. B. 1982. Comparative antimycotic effects of selected herbs, spices, plant components and commercial antifungal agents. J. Food Protection. 45:1298-1301. [ Links ]
Baraldi, E.; Mari, M.; Chierici, E.; Pondrelli, M.; Bertolini, P. and Pratella, G. C. 2003. Studies on thiabendazole resistance of Penicillium expansum of pears: pathogenic fitness and genetic characterization. Plant Pathol. 52:362 [ Links ]
Calvo, A. M.; Wilson, R. A.; Woo, B. J. and Keller, N. P. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 3:447-459. [ Links ]
China Pharmacopeia Commission, 2010. Pharmacopoeia of the People’s Republic of China 2010. Chinese Medical Science and Technology Press, Beijing, China. 63-127 pp. [ Links ]
Chang, S. T.; Chen, P. F. and Chang, S. C. 2001. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 77:123-127. [ Links ]
Chilea, O.; Fuchs, Y.; Chalutz, E. and Rot, I. 1985. The contribution of host and pathogen to ethylene biosynthesis in Penicillium dig&turn-infected citrus fruit. Physiol. Plant Pathol. 27:55-63. [ Links ]
Goubran, F. H. and Holmes, R. J. 1993. The development of alternative fungicides from essential oils. Victoria, Australia: Institute for Horticultural Development, Knoxfield, Department of Agriculture. 45 p. [ Links ]
Hao, W.; Zhong, G.; Hu, M.; Luo, J.; Weng, Q. and Rizwan-ul-Haq, M. 2010. Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol. Technol. 56:39-43. [ Links ]
Helander, I. M.; Alakomi, H. L. and Latva-Kala, K. M. 1995. Characterization of the action of selected essential oil components on Gramnegative bacteria. J. Agric. Food Chem. 46:3590-3595. [ Links ]
Kim, H. O.; Park, S. W. and Park, H. D. 2004. Inactivation of Escherichia coli O157:H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiol. 21:105-110. [ Links ]
Koffmann, W.; Penrose, L. J.; Menzies, A. R.; Davis, K. C.; Kaldor, Jill. 1978. Control of benzimidazole-tolerant Penicillium expansum in pome fruit. Sci. Hortic. 9:31-39. [ Links ]
Kyu Kyu Win, N.; Jitareerat, P.; Kanlayanarat, S. and Sangchote, S. 2007. Effects of cinnamon extract, chitosan coating, hot water treatment and their combinations on crown rot disease and quality of banana fruit. Postharvest Biol. Technol. 45:333-340. [ Links ]
Liengme B. V. 2002. A guide to microsoft excel for scientists and engineers. 2 (Ed.). Butterworth-Heinemann. Oxfordshire, England. 271 p. [ Links ]
Lima, G.; De Curtis, F; Castoria, R. and De Cicco, V. 2011. Activity of the yeasts Cryptococcus laurentii and Rhodotorula glutinis against post-harvest rots on different fruits. Bio. Sci. Technol. 8:257-267. [ Links ]
Mahdi-Ojagh, S.; Rezaei, M.; Hadi-Razavi, S. and Hashem-Hosseini, S. M. 2010. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 122:161-166. [ Links ]
Manso, S.; Pezo, D. and Gómez, L, R. 2014. Diminution of aflatoxin B1 poduction caused by an active packaging containing cinnamon essential oil. Food Control. 45:101-108. [ Links ]
Melgarejo, F. B. G.; Ortega, R. L. A.; Silva, E. V. C.; González, A. G. A.; Miranda, M. R. A. and Ayala, Z. J. F. 2013. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 86:321-328. [ Links ]
Montero, P. P.; Rodríguez, L. A. and Nerin, C. 2011. Active label-based packaging to extend the shelf-life of “Calanda” peach fruit: changes in fruit quality and enzymatic activity. Postharvest Biol. Technol. 60:211-219. [ Links ]
Nielsen, P. V. and Rios, R. 2000. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 60:219-229. [ Links ]
Paran, B.; Sharma, R. K.; Singh, R. S.; Ghosh, A. C. and Baruah, P. 1996. Fungicidal activity of some naturally occurring essential oils against Fusarium moniliformae. J. Essential Oil Res. 8:411-412. [ Links ]
Paranagama, P. A.; Abeysekera, K. H. T.; Abeywickrama, K. and Nugaliyadd, L. 2003. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Letters Appl. Microbiol. 37:86-90. [ Links ]
Ranasinghe, L.; jayawardena, B. and Abeywickrama, K. 2002. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L. M. Perry against crown rot and anthracnose pathogens isolated from banana. Letters Appl. Microbiol. (35):208-211. [ Links ]
Ranasinghe, L.; ayawardena, B. and Abeywickrama, K. 2003. Use of waste generated from cinnamon bark oil (Cinnamomum zeylanicum Blume) extraction as a post harvest treatment for Embul banana. J. Food Agric. Environ. 1:340-344. [ Links ]
Rosenberger, D. A. 1990. Blue mold. In: Jones, A. L. and Aldwinkle, H. S. (Eds.). Compendium of apple and pear diseases. American Phytopathological Society, St. Paul, MN. 54-55 pp. [ Links ]
Sharma, R.; Singh, D. and Singh, R. 2009. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biological Control. 50:205-221. [ Links ]
Seifer, K.; Morgan, J. G.; Gams, W. and Bryce, K. 2011. The genera of Hyphomycetes. CBS-KNAW fungal biodiversity Centre Utrecht, The Netherlands. 997 p. [ Links ]
Sekiguchi, J. and Gaucher, G. M. 1977. Conidiogenesis and secondary metabolism in Penicillium urticae. Appl. Environ. Microbiol. 33:147-158. [ Links ]
Singh, G.; Maurya, S.; Lampasona, M. P. and Catalán, A. N. C., 2007. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food and Chemical Toxicology. 45:1650-1661. [ Links ]
Taiz, L. and Zeiguer, E. 2010. Plant Physiology. 5 ed. Sinauer Associates. United States. 623 p. [ Links ]
Tzortzakis, N. G. 2007. Maintaining postharvest quality of fresh produce with volatile compounds. Inn. Food Sci. Emer. Technol. 8:111-116. [ Links ]
Tzortzakis, N. G., 2009. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Inn. Food Sci. Emer. Technol. 10:97-110. [ Links ]
Received: March 2016; Accepted: June 2016











 text in
text in 


