Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of behavior, health & social issues (México)
Print version ISSN 2007-0780
J. behav. health soc. ISSUES vol.4 n.2 Cuernavaca Nov. 2012
https://doi.org/10.5460/jbhsi.v4.2.34105
Artículos empíricos
Quantitative spectral EEG assessments during affective states evoked by the presentation of the international affective pictures
Medidas espectrales del EEG durante estados emocionales inducidos con las imágenes afectivas internacionales
Erzsébet Marosi-Holczberger, Dulce María Belén Prieto-Corona, María Guillermina Yáñez-Téllez, Mario Arturo Rodríguez-Camacho, Héctor Rodríguez-Camacho, Vicente Guerrero-Juárez
National Autonomous University of Mexico, FES Iztacala, Neuroscience Department. Estado de México, México.
Received: May 31, 2012
Revised: October 16, 2012
Accepted: Octuber 17, 2012
Abstract
This study was carried out to find changes in EEG power during emotions of positive and negative valence evoked by the International Affective Pictures. The subjects were 36 young, healthy male university students. Power values were calculated from 21 referential derivations. Statistical analyses were carried out with ANOVA- RM having 3 conditions (positive, negative and neutral picture presentations), four bands and the above mentioned 21 leads). Fisher's LSD post hoc test revealed the topography of the significant differences. The objective of the study was to see whether right hemisphere is preferential for emotions or there is a hemispheric frontal alpha decrease according to the valence of the emotions. Our results show that most differences between emotional conditions occurred in the delta and theta bands, without preference for right hemisphere participation. In the alpha band few differences were observed, none at frontal areas. Alpha and beta activity revealed only negligible differences without relation with the valence. The results obtained for relative power (RP) values were significant only in the delta band. Based on our results we can conclude that data do not show a defined pattern of activity, but various areas and both hemispheres respond to emotional stimulation.
Key words: Emotions of positive and negative valence, IAPs; spectral measurements of EEG.
Resumen
Este estudio se llevó a cabo durante emociones positivas, negativas y neutras. 36 universitarios jóvenes y sanos participaron en el estudio. Los valores de potencia se calcularon en 21 derivaciones referenciales El análisis estadístico se hizo con ANOVA-RM de 3 niveles (condiciones emocionales=positivas, negativas y neutras; 4 bandas; y 21 derivaciones) usando el Fisher's LSD post hoc test. El objetivo del estudio era ver si el hemisferio derecho es preferente en codificar las emociones o si existe desincronización alfa frontal según la valencia del estímulo. Nuestros resultados revelan que los cambios más significativos ocurrieron en las bandas delta y theta, sin participación preferente del hemisferio derecho. En la banda alfa se observaron pocas diferencias significativas y ninguna de ellas era de los electrodos frontales. Las bandas alfa y beta mostraron muy pocas diferencias entre condiciones y que éstas no tuvieron relación con la valencia. Los resultados de la potencia relativa solamente eran significativos en la banda delta. Con base en nuestros hallazgos podemos resumir que el EEG durante las emociones no corresponde ni a la teoría del hemisferio derecho, ni a la desactivación frontal, sino varias regiones cerebrales en ambos hemisferios responden con actividad lenta a los estímulos emocionales.
Palabras clave: Emociones de valencia positiva y negativa, IAP, medidas espectrales del EEG.
Introduction
The investigation on emotional behavior and cerebral responses to it is a problematic issue. Nobody can provoke strong emotions in laboratory settings, no matter what kind of stimulation methods are used. Subjects know that they are only spectators and not actors in the experimental situation. Researchers use many diverse methods to investigate emotions in laboratory. These procedures range from imaginary emotion-induction to present film clips or static pictures. Emotions provoked in laboratories are not the same strong as those experimented in real life, nonetheless, various types of stimulus showed to be useful to find correlates of emotions in the brain. We have decided to use in this experiment the International Affective Pictures (lAPs), one of the most widely studied elicitors that the University of Florida kindly made available to us. It is a large set of standardized, emotionally evocative, internationally accessible color photographs that contains various pictures depicting mutilations, snakes, insects, attack scenes, accidents, illness, loss, pollution, puppies, babies, and landscape scenes, among others. It provides a set of normative stimuli for experimental investigation. Most publications on IAPs evaluate its behavioral utility rating arousal and valence of the pictures (we only mention those of Lang, 1995; Lang, et al., 1998). Its use in providing electrophysiological evidences has proved to be useful. Questions about the neural bases of emotion have recently taken on new importance. Current models posit that emotions are valenced responses to external stimuli and/or internal mental representations that involve changes across multiple response systems. Attention control is an important aspect of emotional behavior, as subjects have to make judgments on emotional versus non-emotional stimulus attributes (Pribram et al., 1979; Ochsner & Gross, 2005). The medial prefrontal cortex has been implicated in monitoring, and proposed to act as a bridge between attention and emotion (Mayberg et al.1997).
The amygdala has long been implicated in appraising emotional valence of the stimuli and plays an important role in understanding what other people are feeling. Recent findings regard amygdala's role in directing attention to affectively salient stimuli and issuing a call for further processing of stimuli that have major significance for the individual. It is critical for recruiting and coordinating cortical arousal and vigilant attention for optimizing sensory and perceptual processing of the emotion stimuli (Whalen, 1998). Recent neuroimaging studies also helped to identify cerebral structures participating in emotion codification. Hermann et al., (2007) have reported the results of a fMRI study in response to emotional as compared to neutral stimuli and found increased activation of the occipital cortex. Britton et al., (2006) reported that both faces and IAPs pictures activated similar structures, including the amygdala, posterior hippocampus, ventromedial prefrontal cortex, and visual cortex. For the most part, these regions were activated in response to all specific emotions; however, some regions responded only to a subset of stimuli.
While many theories subsist on the relationship of emotions and brain structures, till this moment there is no clear evidence about, whether the different emotions are codified at the same cerebral region and only valence and intensity help to distinguish between them or every emotion is located at different brain regions. Right hemisphere theory posits that right hemisphere is dominant over left one for all forms of emotional expression and perception irrespective of valence. Clinical studies have supported the idea that right hemisphere damage interferes with the perception of emotions more than equivalent left hemisphere lesions (Borod et al., 1992). On the other hand, the valence hypothesis states that hemispheric asymmetry for expression and perception depends on emotional valence: right hemisphere is dominant for negative emotions and left hemisphere is dominant for positive emotions (Davidson, 1993). Both hypotheses have received empirical support (Hellige, 1993). Recent empirical data strongly suggest that the right hemisphere might play a major role in the automatic, unconscious generation of emotions, whereas the left hemisphere could be mainly involved in the conscious analysis and intentional control of emotion processing (Gainotti, 2005). Nevertheless, several groups have failed to demonstrate valence lateralization in EEG studies (Gotlieb et al., 1998; Hageman et al., 1998; Reid et al., 1998). Wager et al., (2003) addressed this controversy and made a meta-analysis of fMRI studies of the last ten years, reporting regional brain activation as a function of valence. They found no support for the right hemisphere as more likely to process emotional material than the left hemisphere. Left lateralization of emotion was found in some brain structures, and right lateralization in others. They did not encounter left hemisphere specialization for positive emotions and right for negative ones either. The analysis of the reported data also made evident that earlier theories of anatomical specificity are too coarse, the pattern is more complex than previous theories have suggested. The question is still open and asks for clarification. This is one of the purposes of this investigation.
EEG studies carried out by evaluation of brain electric activity offer countless advantages due to their objective, non invasive character, low cost and high temporal resolution. For the aforementioned reasons, EEG is highly preferred for monitoring brain activity on line during the executions of simultaneous cognitive tasks.
Quantitative spectral EEG assessments provide frequency information on networks in the human brain, while processing emotional pictures. These measurements can reveal specific neurophysiological correlates, observed through amplitude or frequency changes caused by picture viewing and valence identification (in this present case).
Most papers on this topic study frontal alpha asymmetry in order to learn hemispheric specialization of emotions (Davidson et al., 1979 and 1999; Davidson, 1993; Harmon-Jones et al., 1998; Aftanas et al, 2002; Kline, et al, 2007). The study of frontal alpha desynchronization may be a useful tool for the evaluation of frontal cortex participation in emotional coding; however, it could be an oversimplification, as emotion is such a complex function that probably the entire cortex plays an important role in its codification.
In a previous experiment on brain electric activity during emotional imagination, we used sentences describing joyful or frustrating situations during EEG recording analyzed AP (absolute power) and RP (relative power) measurements in broad and narrow bands (Marosi et al., 2001, 2002). Higher delta frequencies at left fronto-polar, right frontal, parietal and temporal and higher beta frequencies at left frontotemporal and temporal were related to joy. Meanwhile higher delta frequencies especially at left frontopolar and temporal regions and lower beta frequencies at midline frontal (Fz) and central (Cz) leads were associated to frustration. Narrow band calculations revealed more activity during emotional states at 7.6 and 9.5 Hz frequencies. Joyful situation usually provoked lower amplitudes than frustration.
Olofsson et al., (2008) in a revision of ERP studies reported higher amplitudes of different components to negative stimuli than to positive or neutral once mostly at parietal and frontal sites. Liu & Tian (2007) made an analysis of brain electric generators during neutral and emotional stimuli with the LORETA program using the IAPs pictures. They found generators in visual, temporal, ven-tromedial and dorsomedial prefrontal cortices and in anterior cingulate gyrus. Pleasant pictures activated the left middle prefrontal cortex and the posterior precuneus, while the unpleasant pictures activated the right orbitofrontal cortex, posterior cingulate gyrus and somatosensory region.
Event related potentials showed participation of occipital and right parietal cortices during emotion discrimination, independently of properties like color, brightness and complexity of the pictures (Junghöfer et al., 2001, Schupp et al., 2003).
In spite of the great interest in studying this topic, the way how brain copes with emotions is still not understood. There exist contradictory data regarding cortical regions participating in emotion codification and valence dependent hemispheric activity.
Here, in this paper, we study cortical responses to the presentation of pleasant, unpleasant IAPs images as compared with neutral pictures, in normal male subjects, trying to asses those cortical areas and hemispheres that participate in responses to emotional images and those frequency ranges that oscillate according to the different type of stimuli. We pretend to elucidate whether right hemisphere theory or valence dependent theory explains more brain functioning during emotions.
According to previously reported findings, we expect to obtain the following results:
1) As previous studies of Marosi et al. (2001, 2002) had reported higher energy in low frequency bands during emotion, we expect more AP and RP in the delta and theta bands.
2) Based on the results reported by Marosi et al. (2001) we expect higher power values during the presentation of the negative stimuli.
3) We also suggest finding lateralization effect according to the valence in the alpha band (right hemisphere for negative and left one for positive stimuli presentation) (Davidson, 1993).
4) We expect important right parietal participation in emotion codification proposed by Marosi et al. (2001).
Method
Participants
Thirty six healthy young male university students participated in this study (age range of 20-25 and mean age of 22.31). We have selected only male subjects due to gender determined EEG differences reported by various authors (Volf & Raumnikova, 1999; Clarke et al., 2001; Briere et al., 2003). All participants were right-handed, with normal or corrected-to normal vision and were not under prescription medication. Subjects with head injury, history of nervous system diseases, convulsive disorders or abnormal EEG with paroxistic activity did not take part in this experiment. All subjects signed a letter giving their informed consent to participate in the study.
Stimuli
Three hundred images of the "International Affective Pictures" series were presented in a fully random order on the screen of a computer. Hundred of them were of positive valence (pleasant ones), the other hundred of negative valence (unpleasant ones) and 100 were neutral.
Because subject's judgment on emotional content of the stimuli is widely variable and the original categorization was carried out in subject of different cultural background, we made a second categorization of the picture series for our Mexican university population that took place with 40 young male subjects. We included only those pictures in this experiment that had at least 80 % of coincidence in judging the emotional valence of the images.
EEG recording and edition
Subjects sat at a distance of 80 cm in front of the monitor, where the pictures appeared. Each trial started with a fixation point (a cross) in the centre of the screen during 200 ms. Then the image was presented during 2000 ms. When it disappeared a question mark asked for response. The subjects' task was to determine the valence of the stimuli and to answer pressing "1" key on the number pad if the image was pleasant, the "2" if it was unpleasant and the "3" if it was neutral. The response interval lasted until the participant responded, upon which a new trial began. The participants were instructed that the speed of the responses was not important for this experiment and that they were welcome to take a small break, to blink or to adjust a more comfortable position, when the pictures were not present. They were asked to avoid eye movement, withhold blinking or movements while the pictures were on the screen.
Twenty-one surface electrodes were placed over the scalp according to the International 10-20 System, (Fp1, Fp2, Fpz, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, Oz, O2) with linked earlobes as reference. Eye movements were monitored by electrodes on the outer canthi and above the left eye. The impedance of electrodes was kept below 5 kΩ Data acquisition was continuous with a sampling rate of 256 Hz. The bandwidth was set on 1.5-30 Hz. EEG and EOG signals were amplified with a Neuroscan SynAmps System. The segmentation and averaging of EEG signals were performed using ScanTM 4.3 software. The continuous EEG recordings were segmented in epochs of 1275 ms beginning with the stimulus onset. Segments containing eye blinks and artifacts were on and off-line rejected. One to two minutes of clean EEG was subjected to the Fast Fourier Transform. Band limits were defined as follows: delta (1.75-3.91 Hz), theta (3.917.81 Hz), alpha (7.81-12.5 Hz) and beta (12.5-24.22 Hz). Absolute (area under the curve, indirectly amplitude) and relative (percentage data) power data were calculated (John, 1977; Harmony, 1984).
Subjects that judged erroneously the valence of the stimuli and those that had a small number of correct responses and artifact free samples were eliminated from this study.
Statistical analysis
After cleaning the EEG, the normality of the distribution was examined by the Shapiro-Wilk's test, getting a range of W=0.69-0.98 with an average of W=0.83. Logarithmic transformations (ln) for absolute power data and ln(x/1-x) for relative power were calculated in the 21 derivations, in order to bring data closer to the normal distribution (John et al. 1977). Then Repeated Measures Analysis of Variance was used with three factor levels, a) condition (1=pleasant, 2=unpleasant, 3=neutral); b) bands (1=delta, 2=theta, 3=alpha, 4=beta); c) electrodes (the above mentioned 21 derivations). Main effects and interactions were assessed using Greenhouse-Geisser correction, in order to compensate the heterogeneity of the covariance that facilitates the occurrence of error type I. The Fisher's Least Square Differences test was used in post-hoc analyses. Significance between means was considered when the probability was lower than 0.05.
Results
The repeated ANOVA measures with Greenhouse-Geisser correction showed the followings for absolute power (AP (Table 1):
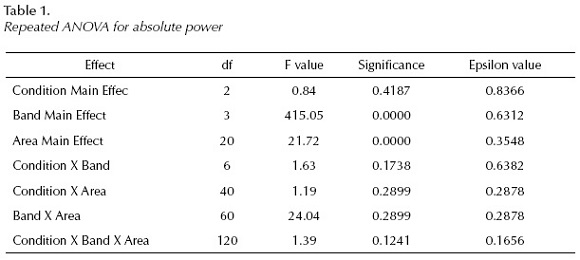

The Post Hoc (Fisher's LSD) Test revealed the following results:
When comparing AP during pleasant (1) and unpleasant (2) picture identification, we can appreciate significant differences with higher delta (Fp2) and theta (P3, Pz, P4, Oz) and beta power for negative than for positive pictures. Pleasant pictures show higher power values in delta (T4, O1), alpha (Pz) bands than unpleasant ones.
RP values reveal significant differences only in the delta band, having more delta activity for negative pictures at Fp2 and less at T4, O1, O2 than for positive ones.
Relative power values in the theta, alpha and beta bands did not show significant differences between pleasant and unpleasant picture presentations.
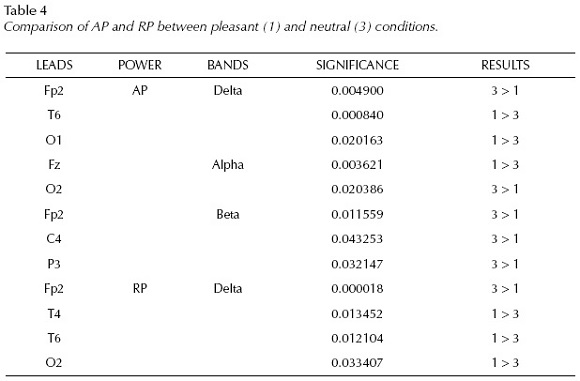
When comparing positive images (1) with neutral condition (3), AP values show higher delta power for pleasant pictures than for neutral ones at T6 and O1; and at Fz in the alpha band, meanwhile we have obtained higher power for neutral images at Fp2 in delta, at O2 in alpha and at Fp2, C4 and P3 in the beta band.
RP values show significant differences between pleasant and neutral conditions only in the delta band having more activity at T4, T6, O2 during pleasant image presentations and more delta activity for the neutral pictures at Fp2 than for pleasant ones.
Absolute power values in the theta band and relative power values in the theta, alpha and beta bands did not show significant differences between EEGs to pleasant and neutral picture presentations.

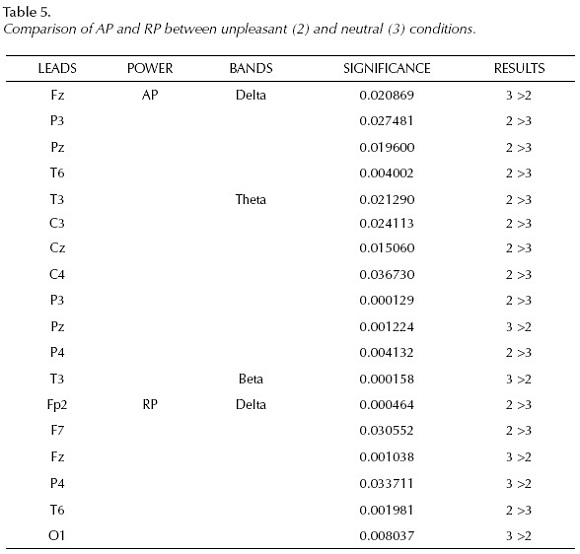
Significant differences can be observed between AP during unpleasant (2) and neutral (3) picture presentations with higher power values in response to unpleasant stimuli in delta (P3, Pz, T6), theta (T3, C3, Cz, C4, P3, P4) bands than to neutral ones, meanwhile higher. AP values in neutral condition at Fz and Pz in delta and at T3 in beta bands than in negative situation..
RP values show higher percentage of delta activity during unpleasant condition (Fp2, F7, T6) and during neutral situation (Fz, P4, O1).
Absolute power values in the alpha band and relative power values in the theta, alpha and beta bands did not show significant differences between unpleasant and neutral picture presentations.
Discussion
Emotions are one of the most important daily experiences, they guide our choices, help us to avoid danger, they are the base of our motivation and an important part of verbal and non-verbal communication. For these reasons the study of emotions is a highly important issue and justify why emotion assessment is a rapidly growing research field.
General understanding in EEG research is that cortical distribution of spectral power varies as a function of time, frequency, state and experimental condition (Knyazev, 2010).
In this experiment we have studied if quantitative spectral EEG assessments that provide information on oscillations of cortical networks in different frequency ranges can reveal specific neurophysiological correlates of differences in neural processing associated to negative and positive emotions.
Our results reveal higher power in the delta and theta bands with higher significance level. This increase of absolute power in slow bands is strongly supported by our percentage (RP) data showing strong relationship between slow activity and emotional response. These findings are in accordance with our first hypothesis and amplify the results of prior studies of Marosi et al., (2001, 2002) indicating that emotional pictures provoke higher power, more energy in low frequency bands. Especially the delta band is very sensitive to emotional situations having the most important role in its codification, all over the entire cortex. A large body of research has focused on the presence of delta activity during cognitive tasks: If we consider attention as the basic ingredient of emotion (Pribram, 1984) manifested in higher delta activity over the cortex, we have to refer to the paper of Harmony et al., (1996) who also observed that attention and internal concentration increased delta power of EEG. During attention, the corticofugal pathway gets activated inhibiting thalamo-cortical neurons. This produces a cortical disconnection to the irrelevant stimuli, in order to compute only the internal information, and provoking in this way delta activity (Vogel et al, 1968; Steriade et al, 1993). Delta activity depends on two sources according to experiments carried out with rats having intact hippocampus: a) hyper-polarization of pyramidal cells, GABAergic synchronic activity, and b) increase in inter-neuronal activity. According to these findings delta activity is related to processing in intracortical networks (Jazayeri et al. 2001). Lindsley (1951) based on the reticular activating core of the brain stem, had proposed an activation theory of emotion, in which emotion was conceived as an overall undifferentiated, conscious, attentive process. This hypothesis caused confusion because of the scientific understanding of the relation between emotion and the limbic system, provided that the functions of the amygdala proved to have more to do with attention and the formation of memory than with emotion (Anticevic et al., 2011; Plessen et al., 2006). Nowadays, it is difficult to find contradiction in this idea, as all cognitive functions are based on attention. Pribram (1984) also assures that emotional stimuli capture attention, receive increased perceptual processing resources and alter peripheral reflexes.
These ideas are supported by our results, as we observed that the most important manifestation during the presentation of emotional pictures was higher power (AP) and more activity (RP) in delta oscillation. The relation between concentration and slow waves is also supported by the observation that delta and theta activity is enhanced during deep meditation (Aftanas & Golocheikine, 2003).
Considering theta activity, Gevins at al., (1997) reported increased theta rhythms with increasing difficulty in cognitive tasks. Our subjects had to identify the valence of the IAPs images, a task not at all difficult, it did not occupy much cortical resources. Other idea is that theta activity is related to performance on attentional and working memory tasks (Böcker et al., 2010.) Sammer et al., (2007) posit that theta activity increases with mental workload and is associated with numerous processes including working memory, problem solving, encoding and self monitoring. Our data show higher theta power during the unpleasant condition at central and at parietal leads. This finding supports our second hypothesis that negative stimuli elicit higher power than positive and neutral ones. It is possible to imagine that unpleasant situations occupy more cortical resources than positive and neutral ones.
We have obtained negligible results in the alpha and beta bands. Higher amplitudes of the alpha band are associated with relaxation based on the observation that concentration on some tasks is accompanied with an alpha "desynchronization" (Niedermeyer & Lopes da Silva 1993). In our study, the scarce results in the alpha band may reflect this same mechanism; obviously relaxation did not take place during the picture categorization.
Our data can not support the theory on the preferential participation of the right hemisphere in emotional codification, as both right, left and midline leads showed significant differences between conditions. These data support the findings of Wager et al. (2003) that left lateralization of emotions occurs in some brain structures, and right lateralization in others.
When comparing pleasant and unpleasant conditions we tested the valence theory proposed by Davidson and colleagues (1999) who found higher participation of left frontal region in the alpha band during positive emotions (reinforcement) and right frontal alpha during negative emotions (withdrawal). The right frontopolar lead - in our study - showed higher amplitude and more activity during the negative situation, as compared with the positive one, but in the delta band, moreover left frontopolar leads never showed participation in none of the emotional situation. In the alpha band the frontal areas did not show significant hemisphere changes according to the emotional condition. Our results are in clear contraposition with the valence theory and with our third hypothesis.
The areas that showed higher energy during the emotional picture presentations were mostly frontopolar, temporal, parietal and occipital areas, having parietal cortex represented more frequently as reported previously by Marosi et al.(2001 and 2002), who reported enhanced parietal activity during emotional response. The role of occipital cortex shows that a part of the information is encoded in visual cortices. It seems logical as visual cortices respond when the stimulus is seen and it is in accordance with Hermann et al. (2007) who also found increased participation of the occipital cortex in their fMRI study.
Our data supports the results of a meta-analysis carried out by Wager et al. (2003) that it is useless to look for precisely located areas and simple rules, the way how brain works is much more complicated and for the moment we can only speculate which brain areas might be influenced by the emotional stimuli. The evidence drawn from our reported results suggests that during the emotional pictures viewing a complex patchwork type of activity occurs: simultaneous activation of different areas in defined frequency ranges, whose interpretation needs more understanding that we have for the moment. Probably years of investigation are necessary to shed light on the way how brain copes with emotions.
The main contribution of this paper is the evidence that EEG studies are efficient to unmask untrue theories and make possible to understand a little more about nature.
References
Aftanas, L. I., Varlamov, A. A., Pavlov. S. V., Makhev, V. P., & Reva, N. V. (2002). Time dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. International J. of Psycho-physiology, 44(1), 67-82, available via: http://dx.doi.org/10.1016/S0167-8760(01)00194-5 [ Links ]
Aftanas, L. I., & Golocheikine, S. A. (2003). Changes in cortical activity in altered state of consciousness: The study of meditation by high resolution EEG. Human Physiology, 29, 143-151, available via: http://dx.doi.org/10.1023/A:1022986308931 [ Links ]
Anticevic, A., Repovs, G., & Barch, D. M. (2011). Emotion effects on attention, amygdale activation, and functional connectivity in schizophrenia. Schizophr. Bull, 10, 1093. [ Links ]
Borod, J. C., Andelman, F., Obler, L. K., Tweedy, J. R., & Welkowitz, J. (1992). Right hemisphere specialization for the appreciation of emotional words and sentences: Evidence from stroke patients. Neuropsychologia, 30, 827-844, available via: http://dx.doi.org/10.1016/0028-3932(92)90086-2 [ Links ]
Böcker, K. B., Hunault, C. C., Gerritsen, J., Kruidenier, M., Mensinga, T. T., & Kenemans, J. L. (2010). Cannabinoid modulation of resting state EEG theta power and working memory are correlated in humans. J. Cogn. Neurosci, 22(9), 1906-16, available via: http://dx.doi.org/10.1162/jocn.2009.21355 [ Links ]
Briére, M. E., Forest, G., Chouinard, S., & Godbout, R. (2003). Evening and morning EEG differences between young men and women adults. Brain and Cognition, 53(2), 145-148, available via: http://dx.doi.org/10.1016/S0278-2626(03)00097-6 [ Links ]
Britton, J. C, Taylor, S. F, Sudheimer, K. D, & Liberzon, I. (2006). Facial expressions and complex IAPs pictures: common and differential networks. NeuroImage, 31(2), 906-919, available via: http://dx.doi.org/10.1016/j.neuroimage.2005.12.050 [ Links ]
Clarke, A. R., Barry, R. J., McCarthy, R., & Selikowicz, M. (2001). Age and sex effects in the EEG: development of the normal child. Clin. Neurophysiol, 112, 806-814, available via: http://dx.doi.org/10.1016/S1388-2457(01)00488-6 [ Links ]
Davidson, R., Schwartz, G., Saron, C., Bennett, J., Goleman, D. (1979). Frontal versus parietal EEG asymmetry during positive and negative affect. Psychophysiology, 16, 202-203. [ Links ]
Davidson, R. J. (1993). Cerebral Asymmetry and Emotion: Conceptual and Methodological Conundrums, Cognitive Emotion, 7, 115-138, available via: http://dx.doi.org/10.1080/02699939308409180 [ Links ]
Davidson, R. J, Ekman, P., Saron, C. D., Senulis, J. A., & Friesen, W. V. (1999). Approach-Withdrawal and Cerebral Asymmetry: Emotional Expression and Brain Physiology I. Journal of Personality and Social Psychology, 58(2), 330-341. [ Links ]
Gainotti, G. (2005). Emotions, unconscious processes and the right hemisphere. Neuropsy-choanalysis, 7, 71-81. [ Links ]
Gevins, A., Smith, M. E., McEvoy, L., & Yu, D. (1997). High resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing and practice. Cerebral Cortex, 7, 374-385, available via: http://dx.doi.org/10.1093/cercor/7.4.374 [ Links ]
Gotlieb, I. H., Ranganath, C., & Rosenfled, J. P. (1998) . Frontal EEG alpha asymmetry, depression and cognitive functioning. Cogn. Emot. 12 (3), 449-478, available via: http://dx.doi.org/10.1093/cercor/7.4.374 [ Links ]
Harmon-Jones, E., Allen, J., & John, J. B. (1998). Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology, 74(5), 361-387, available via : http://dx.doi.org/10.1037/0022-3514.74.5.1310 [ Links ]
Harmony, T. (1984). Functional Neuroscience. Vol.III. Neurometric Assessment of Brain Dysfunction in Neurological Patients. Lawrence Erlbaum. [ Links ]
Harmony, T., Fernández, T., Silva, J. Bernal, J., Díaz-Comas, L., Reyes, A., Marosi, E., Rodríguez, M. A., & Rodríguez, M. T. (1996). EEG delta activity: An indicator of attention to internal processing during the performance of mental tasks. International Journal of Psychophysiology, 24, 161 -171. [ Links ]
Hellige, J.B. (1993). Hemispheric asymmetry. Harvard University Press, Cambridge, MA. [ Links ]
Hageman, D., Naumann, E., Becker, G., Maier, W., & Bartussek, D. (1998). Frontal brain asymmetry and affective style: a conceptual replication. Psychophisiology, 35, 372-388, available via: http://dx.doi.org/10.1111/1469-8986.3540372 [ Links ]
Hermann, M. J., Huter, T. M., Puller, F., Muhlberger, A., Pauli, P., Reif, A., Renner, T., Canli, T., Fallgatter, A. J., & Lesch, K. P. (2007). Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on emotional processing. Cerebral Cortex, 17(5), 1160-1163. [ Links ]
Jazayeri, M., Zhang, L., & Skinner, F. K. (2001). A dynamical feedback mechanism for the generation of hippocampal field rhytms. Neurocomputing, 38-40, 683-689, available via: http://dx.doi.org/10.1016/S0925-2312(01)00439-8 [ Links ]
John, E. R., Karmel, B. Z., Corning, W. C., Easton, P., Brown, D .J., Ahn, H., John, M., Harmony, T., Prichep, L. S., Toro, A., Gerson, I., Bartlett, F., Thatcher, R., Kaye, H., Valdés, P., & Schwartz, E. (1977). Neurometrics, Science, 196, 1393-1410, available via: http://dx.doi.org/10.1126/science.867036 [ Links ]
Junghöfer, M., Bradley, M. M., Elbert, T. R., & Lang, P .J. (2001). Fleeting images: A new look at early emotional discrimination. Psycho-physiology, 38, 175-178, available via: http://dx.doi.org/10.1111/1469-8986.3820175 [ Links ]
Kline, J. P., Blackhad, G., & Williams, W. C. (2007). Anterior EEG asymmetries and opponent process theory. Int. J. of Psychophysiology, 63(3), 302-307 available via: http://dx.doi.org/10.1016/j.ijpsycho.2006.12.003 [ Links ]
Knyazev, G. G. (2010). Antero-Posterior EEG Spectral Power Gradient as a Correlate of Extroversion and Behavioral Inhibition. The Open Neuroimaging Journal, 4, 114-120, available via: http://dx.doi.org/10.2174/1874440001004010114 [ Links ]
Lang, P. J. (1995). The emotion probe: studies of motivation and attention. American Psychology, 50, 372-385, available via: http://dx.doi. org/10.1037/0003-066X.50.5.372 [ Links ]
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1998). Emotion, motivation and anxiety: Brain mechanism and psychophysiology. Biological Psychiatry, 44, 1248-1263, available via: http://dx.doi.org/10.1016/S0006-3223(98)00275-3 [ Links ]
Lindsley, D.B. (1951). Emotion: In Handbook of Experimental Psychology. Edited by Stevens SS. New York: John Wiley, 473-516. [ Links ]
Liu, J., Tian, J. (2007). Spatiotemporal analysis of single trial EEG of emotional pictures based on independent component analysis and source location. Proc. SPIE, 6511, 121, available via: http://dx.doi.org/10.1117/12.709288 [ Links ]
Marosi, E., Rodríguez, H., Yañez, G., Bernal, J., Rodríguez, M., Fernández, T., Silva, J., Reyes, A., & Guerrero, V. (2001). Broad band spectral measurements of EEG during emotional tasks. Intern. J. Neuroscience, 108(3), 251-279, available via: http://dx.doi.org/10.3109/00207450108986517 [ Links ]
Marosi, E., Bazán, O., Yañez, G., Bernal, J., Fernández, T., Rodríguez, M., Silva, J., & Reyes, A. (2002). Narrow band spectral measurements of EEG during emotional task. Intern J. Neurscience, 112, 879-899, available via: http://dx.doi.org/10.1080/00207450290025897 [ Links ]
Mayberg, H. S., Brannan, S. K., Mahurin, R. K., Jerabek, P.A., Brickman, J. S., Tekell, J. L., et al. (1997). Cingulate function in depression: A potential predictor of treatment response. Neuroreport, 8, 1057-1061. [ Links ]
Niedermeyer, E., & Lopes da Silva, F. (1993). Electroencephalography: Basic principles, Clinical applications and related fields. Lippincott Williams and Wilkins. [ Links ]
Ochsner, K., & Gross, J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9 (5), 242-249, available via: http://dx.doi.org/10.1016/j.tics.2005.03.010 [ Links ]
Olofsson, J., Nordin, S., Sequeira, H., & Polich, J. (2008). Affective picture processing: An integrative review of ERP findings, Biological Psychology, 77(3), 247-265, [ Links ]
Plessen, K. J., Bansal, R., Zhu, H., Whiteman, R., Amat, J., Quackenbush, G.A., Martin L., Durkin, K., Blair, C., Royal, J., Hugdahl, K., & Peterson, B.S. (2006). Hippocampus and amygdale morphology in attention deficit/ hyperactivity disorder. Arch. Gen. Psychiatry, 637(7), 796-807, available via: http://dx.doi.org/10.1001/archpsyc.63.7.795 [ Links ]
Pribram, K. H., Reitz, S., McNeil, M., & Spevack, A. A. (1979). The effect of amydalectomy on orienting and classical conditioning in monkeys. Pavlov J Biol. Scie, 14, 203-217. [ Links ]
Pribram, K. H. (1984). Emotion: A neurobiological Analysis. In Approaches to Emotion K Scherer and P. Ekman (Eds). Hillsdale: Lawrence Erlbaum Ass. [ Links ]
Reid, S. A., Duke, L. M., & Allen, J. J. B. (1998). Resting frontal EEG asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophyisiology, 35:389-404. [ Links ]
Sammer, G., Blecker, C., Gebhardt, H., Bischoff, M., Stark, R., Morgen K., & Vaitl, D. (2007). Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Human Brain Mapping, 28(8), 793-803, available via: http://dx.doi.org/10.1002/hbm.20309 [ Links ]
Schupp, H. T., Junghófer, M., Weike, A. I., & Hamm, A. O. (2003). Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport, 14, 1107-1110, available via: http://dx.doi.org/10.1097/00001756-200306110-00002 [ Links ]
Steriade, M., Contreras, D., Curro Dossi, R., & Nuñez, A. (1993). The show oscillation in reticular thalamic and thalamocortical neurons: Scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J. Neuroscie., 13, 3284-3299. [ Links ]
Vogel, W., Broverman, D. M., & Klaiber, E. L. (1968). EEG and mental abilities. EEG and clin Neurophysiol., 24, 166-175, available via: http://dx.doi.org/10.1016/0013-4694(68)90122-3 [ Links ]
Volf, N. V., & Razumnikova, O. M. (1999). Sex differences in EEG coherence during a verbal memory task in normal adults. International J. of Psychophysiology, 34(2), 113-122, available via: http://dx.doi.org/10.1016/S0167-8760(99)00067-7 [ Links ]
Wager, T. D., Luan Phan, K., Liberzon, I., & Taylor, S. F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage, 19: 513-531, available via: http://dx.doi.org/10.1016/S1053-8119(03)00078-8 [ Links ]
Whalen, P. J. (1998). Fear, vigilance and ambiguity: Initial neuroimaging studies of the human amygdale. Current Directions in Psychological Science, 7, 177-188, available via: http://dx.doi.org/10.1111/1467-8721.ep10836912 [ Links ]
Notes
Author's contribution to this paper was as follows: BPC: assessing children, experimental design, statistical analysis of the data, writing and reviewing of manuscript; MRC: experimental design, statistical analysis, writing and reviewing manuscript, GYT: assessing children and reviewing the manuscript; JBH: experimental design and reviewing manuscript. JSP: statistical analysis, LL: assessing children; EM: reviewing final version of the manuscript; VG: neurological evaluations.
Self-reference for authors: 3
Self-references for journal: 0
 Correspondencia:
Correspondencia:
Mario Rodríguez-Camacho, PhD.,
Laboratorio de Neurometría,
FES Iztacala, Universidad Nacional Autónoma de México,
Av. de los Barrios No. 1, Los Reyes Iztacala,
Tlalnepantla, México, C. P. 054090,
correo: marcizta0gmail.com
Information about authors:
Nombre: Erzsébet Marosi Holczberger. Grado: Doctorado en Ciencias Fisiológicas, obtenido en el Instituto de Biomédicas, UNAM. Adscripción: FES Iztacala, Universidad Nacional Autónoma de México, UNAM. UIICSE, Proyecto de Neurociencias. Nombramiento: Titular "C" T.C. definitivo. Línea de investigación: Electrofisiología de la conducta emocional tratando de ver que las emociones de valencia positiva y negativa en cuáles áreas se codifican en el cerebro de humanos sanos. Dirección Postal: Laboratorio de Neurometría. Edificio UIICSE, FES Iztacala, UNAM, Ave. de los Barrios No. 1, Col. Los Reyes Iztacala, Tlalnepantla, Estado de México, C.P. 54090, México. Correo: marosie@gmail.com y marosi@unam.mx
Nombre: Dulce María Belén Prieto Corona. Grado: Doctor en Psicología. Adscripción: Universidad Nacional Autónoma de México, Facultad de Estudios Superiores, FES Iztacala, Proyecto de Neurociencias, Laboratorio de Neurometría. Línea de investigación: Psicofisiología y neuropsicología de los procesos cognoscitivos (lenguaje y cálculo) en adultos y en niños normales y con trastornos de aprendizaje. Dirección postal: Unidad de Investigación Interdisciplinaria en Ciencias de la Salud y Educación (UIICSE), cub. 16. FES Iztacala, Universidad Nacional Autónoma de México, Ave de los Barrios No. 1, Los Reyes Iztacala, Tlalnepantla Estado de México, México C.P. 54090. Correo: bemapado@gmail.com
Nombre: Ma. Guillermina Yáñez Téllez. Grado: Dra. En Psicología, Universidad Nacional Autónoma de México. Adscripción: Universidad Nacional Autónoma de México. Proyecto en Neurociencias, Facultad de Estudios Superiores Iztacala, Profesor Titular "C" TC. Línea de investigación: Evaluación neuropsicológica del TDAH y de los trastornos del aprendizaje de la lectura. Otros cargos: Responsable académico de la Residencia en Neuropsicología Clínica en la FESI Distinciones: Distinción Universidad Nacional Para Jóvenes Académicos Dirección postal: Laboratorio de Neurometría. Edificio UIICSE, FES Iztacala, UNAM, Ave. de los Barrios No. 1, Colonia Los Reyes Iztacala, Tlalnepantla, Estado de México, C.P. 54090, México. Correo: axel.enrique@gmail.com
Nombre: Mario Arturo Rodríguez Camacho. Grado: Doctor en Ciencias Fisiológicas. Adscripción: Universidad Nacional Autónoma de México, Facultad de Estudios Superiores (FES) Iztacala, Proyecto de Neurociencias, Laboratorio de Neurometría. Línea de investigación: Psicofisiología de los procesos cognoscitivos (lenguaje y memoria) en niños y en pacientes adultos con patología cognoscitiva. Dirección postal: Unidad de Investigación Interdisciplinaria en Ciencias de la Salud y Educación (UIICSE), cub. 16. FES Iztacala, Universidad Nacional Autónoma de México, Ave de los Barrios No. 1, Los Reyes Iztacala, Tlalnepantla Estado de México, México CP 54090. Correo: marcizta@gmail.com
Nombre: Héctor Rodríguez Camacho. Grado: Maestría en Ciencias de la Computación. Obtenido en la Fundación Arturo Rosenblueth. Adscripción: Universidad Nacional Autónoma de México, UNAM, FES Iztacala, Posgrado. Profesor ordinario de Asignatura A. Línea de investigación: Programas computacionales de estimulación como apoyo a la investigación. Dirección postal: Laboratorio de Neurometría. Edificio UIICSE, FES Iztacala, UNAM, Ave. de los Barrios No. 1, Colonia Los Reyes Iztacala, Tlalnepantla, Estado de México, C.P. 54090, México. Correo: hectorrc61@gmail.com.mx
Nombre: Vicente Guerrero Juárez. Grado: Especialidad en Neurología en el Instituto Nacional de Neurología y Neurocirugía (Dr. Manuel Velasco Suárez). Adscripción: Instituto Nacional de Neurología y Neurocirugía (Dr. Manuel Velasco Suárez) adscrito al departamento de Urgencias y Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, adscrito a la carrera de medicina. Líneas de Investigación: Trastornos del desarrollo. Dirección Postal: Av. De los Barrios No. 1, Los Reyes Iztacala, Tlalnepantla, Edo. Méx. C.P. 54090, Tel. +52-555-6231333 ext 39726 ó 39796. Correo: vic.gue.j@gmail.com














