Introduction
Information on avian life-history traits such as clutch size, incubation period, and nest-type is important to assess the vulnerability of populations to habitat degradation (Martin 1993). Nest survival in birds is considered an index of the influence of habitat on reproductive success, while the modeling of daily survival rates reflects factors that influence nesting success (Armstrong et al. 2002). One of the most important causes of the decline of Snowy Plover (Charadrius nivosus) populations is low nest survival (Morrison et al. 2006, USFWS 2007, Andres et al. 2012), resulting from a variety of factors, including human disturbance, high tides, inclement weather, and predation of eggs and chicks by mammals and other bird species (Hardy and Colwell 2008, Saalfeld et al. 2011, Galindo-Espinosa 2015). However, the primary threat at the present is degradation and loss of suitable nesting habitat (Page et al. 2009, Colwell 2010).
Nest survival rate is known to be variable; its variation might show patterns of changes annually, seasonally or even within a single reproductive season. In shorebirds, it has been suggested that nest survival decreases with nest initiation date (Ackerman et al. 2014), a pattern that has been found for the Snowy Plover, in which nest survival rate is higher at the beginning of the breeding season and declines with time (Saalfeld et al. 2011). Some underlying factors that have been suggested might be involved are the selection of the best nest sites (Ellis et al. 2015), lower number of potential predators (Galindo-Espinosa 2015), and stability of surface water availability in saline lakes (Saalfeld et al. 2011). Some other events might increase variability, such as extreme weather conditions (strong winds, floods by tides and excessive rain during the reproductive season) (Colwell 2010, Saalfeld et al. 2011, Plaschke et al. 2019), as well as human disturbance (Colwell et al. 2005, Page et al. 2009, Webber et al. 2013).
Approximately 9% of the breeding population of the Snowy Plover occurs in Mexico (Thomas et al. 2012), where the species is considered threatened (SEMARNAT 2019), although there is not enough information to substantiate the status of breeding populations at a local and regional levels. Mellink and Riojas-López (2005) first registered nesting of the Snowy Plover in the Marismas Nacionales protected area on the Pacific coast of Mexico. However, it was not until 2010 that studies were conducted to characterize nesting sites and evaluate reproductive success of the Snowy Plover at this site (Martínez 2012, Vargas 2012, Bustamante 2013, Martínez et al. 2019). These studies used the traditional Mayfield method to evaluate nest success, with estimates of 42% to 58% nest success. However, the Mayfield method has fallen into disuse as it requires that a series of restrictive assumptions are met (Rotella et al. 2004, Shaffer 2004). As a result, a new approach of analyses has been suggested, and detailed studies to generate specific information related to the influence of various biological factors on the likelihood of nest success are considered timely, suitable, and necessary. In order to evaluate the influence of habitat on nest survival of the Snowy plover at Marismas Nacionales, and to provide basic information that would enable us to support management and conservation strategies for this species, we analyze changes in Daily Survival Rate (DSR) throughout the breading season, and the influence of nest age, distance to the nearest vegetation patch and body of water, type of habitat, and nesting substrate on annual nest survival.
Methods
Study area
The study was conducted in Las Garzas-Chahuin Chihua wetland (22°29’26.1” N -105°35’ 51” W, 22°25’20.9” N -105° 35’59.1”W), a large coastal wetland complex of more than 350,000 ha located within Marismas Nacionales area in northwestern Nayarit and southern Sinaloa. Marismas Nacionales is a site of international importance as it harbors a considerable number of migratory and resident birds (Ortega-Solis 2011); part of this area (133,854 ha) has been designated as Biosphere Reserve (CONANP 2005, DOF 2010). The area includes deltaic plains, marshes with coastal lagoons, and coastal beds (González et al. 2009). The general climate for the region is semi-warm subhumid Aw1(h’), with annual rainfall over 1500 millimeters and influence of humid monsoon-type winds from the sea. The average annual temperature is 26ºC to 28ºC, with an average annual maximum temperature of 30ºC to 34ºC. Total annual precipitation is 800 to 1200 mm with an annual relative humidity greater than 75% and a total annual evaporation of 1800 to 2000 mm3 (SEMARNAT-CONANP 2013). Dominant vegetation is represented by halophyte species, being among the most common plants Salicornia sp. (glasswort), Batis maritima (turtleweed), Sesuvium portulacastrum (shorline sea purslane), and Suaeda nigra (bush seepweed). Mangroves are present on the shores of estuaries, lagoons, and other coastal water bodies with four species: Laguncularia racemosa (white mangrove), Rhizophora mangle (red mangrove), Hilairanthus germinans (black mangrove), and Conocarpus erectus (button-tree) (SEMARNAT-CONANP 2013).
The study site was selected based on earlier studies on Snowy Plover at Marismas Nacionales (Ortega-Solis 2011, Martínez 2012, Vargas 2012, Bustamante 2013). Las Garzas-Chahuin Chihua is important as a feeding, wintering, and nesting area for shorebirds (Ortega-Solis 2011); it encompasses an area of 85 ha located 12 km from the shoreline, exposed to mixed semi-diurnal tides with no apparent effect on shorebirds (Carmona et al. 2011, Bustamante 2013, Martínez et al. 2019). The lagoon is less than 20 m above sea level, with a tidal range of ±50 cm (Ortega-Solis 2011; Figure 1), and includes a mosaic of habitats such as saline waters, shell sites, muddy beaches, dry sandy plains, and shallow water-bodies. On the lagoon periphery there are 14 artificially created dredge-spoil islands (known locally as “tarquinas”) that are used as nesting sites by Snowy Plovers (Bustamante 2013). These islands have shell mounds of 1.5 to 2 m high, and although they are deprived of vegetation cover, there are a few small patches of halophytic vegetation and very scarce mangroves (Ortega-Solis 2011, Martínez 2012, Bustamante 2013).
Nest Surveys
We visited nesting areas once a week during the 2014 and 2015 breeding seasons (late March-early July) to locate and monitor Snowy Plover nests (Ralph et al. 1996). We geolocated each nest using a handheld Global Positioning System (GPS) device. We gathered data on habitat type (dredge-spoil islands, saline waters, road-edge, or sandy plains) and substrate (bare soil, vegetation, shell knoll, or any other object such as cow pat, canvas, or plastic) where nests were located. We also recorded clutch initiation date, clutch size, incubation stage, and the number of hatchlings. Incubation stage was defined according to the egg flotation technique of Westerkov (1950) with six categories (A to F: A = egg on bottom approximate embryo age 0-3 days; F = egg on top of water approximate embryo age 24-27 days). Clutch initiation date was estimated retrospectively assuming a period of four laying days and a total incubation period of 27 days (Page et al. 2009, Székely et al. 2011). We defined a nest as being successful when: a) at least one of the eggs hatched, b) we observed the presence of adults with young near the nest, and c) there were small eggshell fragments <5 mm in size (Mabbe and Estelle 2000, Ellis et al. 2015). On the other hand, a nest was classified as failed if: 1) it was lost to predation, with evidence of large eggshell pieces (>8 mm), yolk stains, or animal tracks in or around the nest; 2) flooding occurred, when there was evidence of a recent heavy rain or high tide events that might have caused the eggs to disappear; and 3) lost to unknown cause, when no evidence of hatching or predation was found. In addition, we included predictors of nest age (Nage = estimated from date of clutch initiation), distance to the water (Dwater = Distance to the nearest body water to the nest), distance to the nearest patch of vegetation (Dvege = Distance to the closest vegetation patch to the nest), habitat used for the nest settlement (Habit: dredgespoil islands, saline fields, borders of the road, sandy plains) and substrate used by the species to place the nest (Subst: on bare soil, on vegetation, on shell knoll, on any other object). Nest age was estimated from date of clutch initiation to the date of hatching or nest failure. Distance to water and the nearest vegetation patch was measured using a flexometer.
Data analysis
We used the program MARK v7.2 (White and Burnham 1999) to model nest survival and estimate daily survival rate (Dinsmore et al. 2002, Rotella et al. 2004, Shafer 2004), based on the last day the nest was active, and the fate of the nest (successful vs. failed). The construction and analysis of models was carried out using the application RMark (Laake 2013) within the statistical program R (R Development Core Team 2015). We used the Akaike’s Information Criterion (AIC) adjusted for small samples (AICc) to evaluate relative model support (Akaike, 1998; Burnham and Anderson 2002). We selected models with ΔAICc < 2 and the highest value of Akaike weight (wi), using a 95% confidence interval to evaluate support in favor of each competing model (Burnham and Anderson 2002).
Model construction was divided into two hierarchical stages based on a combination of predictors. In the first stage, we built models to evaluate the effect of a) clutch initiation date, b) habitat, c) temporary linear effect (T) to evaluate whether daily survival rate increased or decreased as the season progressed, d) a temporary quadratic effect (TT) to evaluate whether daily survival rate was higher at some point in the season, e) nest age in days, and f) daily survival rate during the breeding season, as well as all possible combinations of these factors. In the second stage, we used the best model from the first stage and added all the possible predictor combinations of a) distance to water, b) distance to vegetation, and c) type of nest substrate. We estimated annual nest success using an exponential finite daily survival rate of 27 days (Page et al. 2009). The 95% confidence intervals of the three covariates included in the best model were not overlapped with zero, with both the upper and lower confidence intervals showing a clear (+) (+) or (-) (-) trend, suggesting that these covariates were biologically relevant. We also used a chi-square test to determine whether the number of nests was associated with habitat type and nest substrate in the two reproductive seasons.
Results
Nest Locations
The start date of the reproductive season was 29 March in 2015 and 12 April in 2014, with a breeding season duration of 99 days. Most nests were initiated between 27 April and 28 May in 2014, while in 2015 nests were initiated earlier (from 29 March to 12 April). The last clutches for both seasons were recorded in early June, with the end of the breeding season estimated on 5 July in 2015, when the last active nest was located.
We located a total of 84 nests, 27 in 2014 and 57 in 2015. For both breeding seasons combined, 52% of the nests were successful (n = 44 nests) and 48% failed (n = 40 nests). The main cause of nest failure was predation (75% of failed nests), followed by flooding (22.5% of failed nests); we were unable to establish the cause failure in one nest (Table 1). We observed raccoon (Procyon lotor) and coyotes (Canis latrans) footprints around failed nests.
Table 1 Number of successful and failed Snowy Plover nests found at Marismas Nacionales, Nayarit (2014-2015)
| 2014 | 2015 | Total | |
| Successful | 14 | 30 | 44 |
| Failed | 13 | 27 | 40 |
| Predation | 10 | 20 | 30 |
| Flooded | 3 | 6 | 9 |
| Unknown | 0 | 1 | 1 |
We found a significant association of Snowy Plover nests with habitat (X 2 3 = 13.2, P < 0.05), where most nests were located on dredge-spoil islands (n = 46 nests; 55% of all nests), 19 nests on road-side edges (23%), 12 nests on sandy plains (14%), and only 7 nests in saline habitats (8%) over both breeding seasons combined. However, the number of nests on dredge-spoil islands varied significantly between the two study seasons (2014: 13 nests, 2015: 33 nests; X 2 1= 8.7, P < 0.05).
The substrate of most Snowy Plover nests was bare ground (n = 39 nests; 47% of all nests), followed by shell sites (n = 27 nests; 32%), other objects (n =12 nests; 14%), and 6 nests were located over vegetation (7% of nests). However, we found no significant association of nests with substrate. When comparing between breeding seasons, only the substrates of shell sites (2014: 5 nests, 2015: 22 nests; X 2 1 = 10.7, P < 0.05) and objects (2014: 2 nests, 2015: 10 nests; X 2 1 = 5.33, P < 0.05) were significantly different.
Factors influencing nest survival
We built valid encounter histories for 83 of the 84 nests found over the two breeding seasons. The combined daily survival rate for both breeding sea sons was 0.969 (95% CI = 0.95 0.97) producing a 42% finite nesting success; nesting success was lower (36%) in 2014 compared with 2015 (45%). Nest survival rate varied among habitats (Figure 2); nests in saline habitats had the lowest 0.92 daily survival rate (95% CI = 0.78 0.97), while on sandy plains and road-side edges daily survival rates (0.97) were higher (95% CI: sandy plains = 0.94 0.99; road-side edges = 0.94 0.98), followed by dredge-spoil islands with 0.96 (95% CI = 0.95 -0.97) daily survival rate.
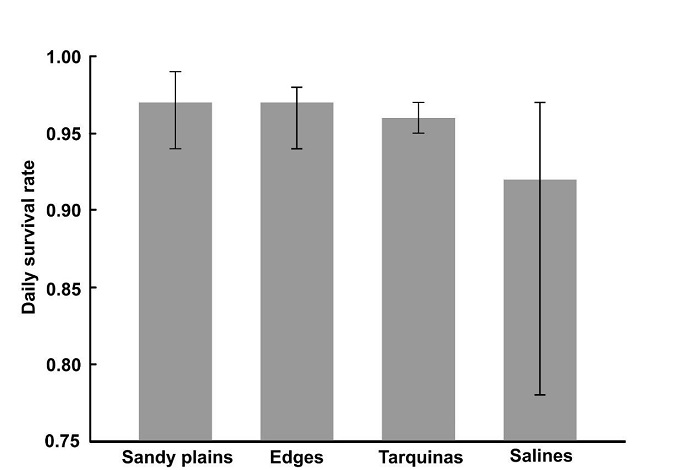
Figure 2 Daily survival rate of Snowy Plover nests with 95% confidence intervals by habitat type in Marismas Nacionales, Nayarit (2014-2015 combined).
From 29 candidate models in two stages, there were 22 candidate models in the first stage and seven models in the second stage. In both breeding seasons, daily survival rate of Snowy Plover nests declined as the season progressed (Figure 3). The model with best fit to Snowy Plover nest survival had four parameters, an Akaike weight (w i ) of 0.51, and included the variables of nest age, a temporary linear effect, and distance to the nearest vegetation patch (Table 2). There was one competing model (ΔAICc = 0.87, w i = 0.33) that included the same variables as the best model, but with the addition of distance to the nearest water-body (Table 2). Daily survival rate increased with nest age (β = 0.06, 95% CI = 0.02 - 0.11), and distance from the nearest vegetation patch (β = 0.23, 95% CI = 0.01 - 0.44), but decreased with day of nesting season (β = -0.02, 95% CI = -0.04 to -0.00).
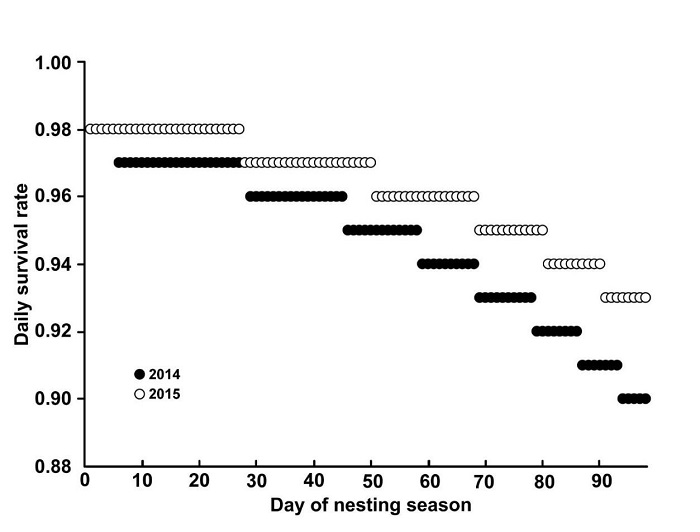
Figure 3 Daily survival rate of Snowy Plover nests over 99 days of the breeding season in 2014 and 2015 at Marismas Nacionales, Nayarit. Day 1 corresponds to 29 March and day 99 to 05 July.
Table 2 Models of daily nest survival (S) for Snowy Plover in Marismas Nacionales, Nayarit (2014-2015) Variables are Nage = Nest age, T = linear temporal effect, Dvege = Distance to nearest vegetation, Dwater = Distance to water, Subst = Substrate. ΔAICc = the differrence between each model and the best model, k = the number of parameters in each model, w i the best
| Stage | Model | k | ΔAIC | w | Deviance |
| 2 | S(Nage + T + Dvege) | 4 | 0.00 | 0.51 | 169.38 |
| 2 | S(Nage + T + Dwater + Dvege) | 5 | 0.87 | 0.33 | 168.24 |
| 2 | S(Nage + T + Dwater) | 4 | 4.43 | 0.05 | 173.82 |
| 2 | S(Nage + T + Dvege + Subst) | 7 | 4.74 | 0.04 | 168.06 |
| 1 | S(Nage + T) | 3 | 0.00 | 0.51 | 173.83 |
Discussion
Daily survival rate of Snowy Plover nests for both 2014 and 2015 combined was 0.97, which coincides with previous figures estimated by Vargas (2012) and Bustamante (2013) at Marismas Nancionales; and does not differ greatly from estimates gathered for other areas, such as San Quintin, Baja California, Mexico (0.95, n = 3 years; Galindo-Espinosa 2015), and Great Salt Lake, Utah (0.96, n = 8 years; Ellis et al. 2015). Our estimate of 42% finite nest success at Marismas Nacionales was similar but lower to previous reports in the same area, which were 48% (Vargas 2012) and 58% (Martinez 2012) nest success in 2010, and 50% nest success in 2011 (Bustamante 2013). Nest success for Snowy Plovers in Marismas Nacionales, Mexico, was also similar to that for breeding populations from other locations, such as the Ceuta Bay in Sinaloa, Mexico (46%; Küpper 2006), Florida (57%; Himes et al. 2006), San Diego, California (50%; Powell 2001), and northern California (48%; Colwell et al. 2011). Predation was the main cause of nest failure for Snowy Plovers at Marismas Nacionales. This was previously reported by Bustamante (2013; 70% of failed nests) for the same area, and several studies have shown that in shorebird breeding areas the primary cause of nest failure is predation (Paton 1995, Smith et al. 2007, Colwell et al. 2012). In our study, we found raccoon (P. lotor) and coyote (C. latrans) tracks, indicating that these are predators of Snowy Plover nests at Marismas Nacionales, with raccoons being the primary predator within these wetlands (Bustamante 2013).
The second cause of nest failure in our study was flooding. Floods have been found to contribute significantly to nest failure at other sites (Warnock et al. 2001, Sexson and Farley 2012, Vargas 2012, Bustamante 2013, Galindo-Espinosa 2015). Plaschke et al. (2019) suggested that nest flooding was more likely later in the breeding season, and is a pattern that may vary among years as a result of weather conditions and the intensity of rain at the end of spring or the beginning of summer. Furthermore, Marismas Nacionales is an important area for shorebirds that has been strongly impacted by habitat loss and degradation, producing changes in water dynamics. This makes it imperative to implement habitat restauration as part of management plans for the recovery of this large wetland.
Our results suggest that early nests are more successful than those initiated later in the breeding season. The best model showed a negative linear temporal trend, which indicates that nests initiated at the start of the breeding season had a higher probability of success than those initiated at the end, as found by Galindo-Espinosa (2015) for San Quintin, Baja California, Mexico. This pattern could be related to changes in levels of tides and the start of the rainy season (Vega-Picos 2008, Vargas 2012), as is the case with other shorebirds where climatic factors may reduce nest success during the breeding season (Smith et al. 2007)
According to the best model, nest survival increased with nest age, which is likely due to the fact that nests are more vulnerable in early developmental stages (Dinsmore et al. 2002, Hood and Dinsmore 2007, Smith and Wilson 2010, Ellis et al. 2015). Distance to the nearest vegetation patch also positively affected survival rates of Snowy Plover nests at Marismas Nacionales, as found by other studies (Prindville and Ryan 1988, Mabee and Estelle 2000, Bustamante 2013). This may be because nearby vegetation reduces visibility for shorebirds to detect approaching predators, and allows predators to make surprise attacks (Cresswell 1996, Galindo-Espinosa 2003).
In our study, we found a strong association of Snowy Plover nests with dredge-spoil island habitats that typically lack vegetation, and most nests occurred on bare soil substrate. Other studies have also found that Snowy Plovers tend to use bare substrates for nesting (Page et al. 1985, Paton 1995, Hood and Dinsmore 2007, Martínez 2012). This may be a reflection of the likelihood of decreased nest success closer to vegetation. Further research could evaluate the risks of predation for Snowy Plover nests at Marismas Nacionales in relation to characteristics of the habitat (Mabee and Estelle 2000). In terms of management, although enclosures may be considered as a strategy to increase nest survival, they do not protect nidifugous chicks from predation (Hardy and Colwell 2008). The potential effects of enclosures on other aspects of Snowy Plover reproductive biology also needs to be seriously considered before being applied at Marismas Nacionales.











 text new page (beta)
text new page (beta)