Introduction
Hemipterans classified within Cercopidae Leach, 1815 are commonly called spittlebugs, because their nymphal stages produce a frothy covering that provides it a microhabitat and protection from predators (Rakitov, 2002; Tonelli et al., 2018). Like other Cercopoidea Leach, 1815 (superfamily that also includes Aphrophoridae Amyot & Serville, 1843, Clastopteridae Dohrn, 1859, Epipygidae Hamilton, 2001 and Machaerotidae Stål, 1866), cercopid spittlebug adult are characterized by their powerful jumping ability (Burrows, 2006; Gorb, 2004). Cercopidae are strict plant feeders, integrating the largest xylem-sap sucking insect group in the world (Carvalho & Webb, 2005). They are adapted to feed from a great diversity of plant hosts, such as herbaceous monocots, herbaceous dicots, flowering trees, shrubs, and conifers (Castro et al., 2005; Fagan & Kuitert, 1969; Notario et al., 1981; Peck, 1998). The spectrum of feeding structures is also wide, including leaves, branches, crowns, and exposed roots. Among hosts, spittlebugs show preferences for nitrogenfixing plants (Thompson, 1994). This feature, combined with their high reproductive potential and short life cycles, makes numerous Cercopidae species significant pests, particularly of forage grasses and sugar cane in Latin America (Cardona et al., 2004; Carvalho, 1990; Fagan & Kuitert, 1969; Foieri et al., 2016; Orozco-Restrepo et al., 2017; Paladini & Cryan, 2012; Peck, 2001).
Cercopidae are classified within the monophyletic superfamily Cercopoidea, which, together with Cicadoidea Latreille, 1802 and Membracoidea Rafinesque, 1815, integrate the infraorder Cicadomorpha Evans, 1946 (Hemiptera: Auchenorrhyncha) (Johnson et al., 2018).
Cercopoidea encompass approximately 3,000 species, classified in 320 genera, 34 tribes and 5 families (Cryan, 2005; Cryan & Svenson, 2010; Evans, 1963; Li et al., 2017; Paladini et al., 2015). Cercopidae species make up half of Cercopoidea diversity, with more than 1,540 species worldwide included in 176 genera (Le Cesne & Soulier-Perkins, 2021; Paladini & Cavichioli, 2015; Roskov et al., 2020). Seventy percent of spittlebug species have a tropical distribution (Carvalho & Webb, 2005; Fennah, 1953; Hamilton, 1977), with the highest number in the Indo-Malayan, Afrotropical, and Neotropical regions. The family Cercopidae is divided into 2 subfamilies:
Ischnorhininae Schmidt, 1920 (= Tomaspidinae) (Carvalho & Webb, 2005; Schmidt, 1920) and Cercopinae Leach, 1815. The New World Ischnorhininae are characterized by 2 lateral spines on the posterior tibia, with the subgenital plates fused to the pygofer as a putative synapomorphy and the Old World Cercopinae are characterized by a single lateral spine on the posterior tibia and subgenital plates free or partially fused (Carvalho & Webb, 2005; Cryan & Svenson, 2010; Fennah, 1968, 1979; Paladini et al., 2018). Molecular phylogenetic analyses and morphological data support that Cercopinae is a paraphyletic group (Carvalho & Webb, 2005; Cryan & Svenson, 2010; Fennah, 1968), while Ischnorhininae are a monophyletic taxon that arose from Cercopinae (Carvalho & Webb, 2005; Cryan & Svenson, 2010; Fennah, 1968; Paladini et al., 2018). In Ischnorhininae there are 460 species and 65 subspecies, classified in 60 genera (Carvalho et al., 2016; Carvalho & Paladini, 2017; Carvalho & Webb, 2005; Castro-Valderrama, Carvalho et al., 2018; Castro-Valderrama, Peck et al., 2018; 2020; Hamilton, 2016; Meneghetti et al., 2019; Paladini, 2011; Paladini & Carvalho, 2007, 2008, 2013; Paladini & Cavichioli, 2015; Paladini & Cryan, 2012; Paladini et al., 2016). Fennah (1968) recognized 4 tribes for the New World Ischnorhininae: Hyboscartini Lallemand, 1949, Ischnorhinini Schmidt, 1920, Neaenini Fennah, 1968 and Tomaspidini Schmidt, 1922 (Carvalho, 1992; Fennah, 1968). Phylogenetic analyses using both morphological and molecular data, however, have revealed definitional inconsistences, with only Neaenini supported as monophyletic, and a significant redefinition of these tribes has been established as follows: Ischnorhinini, Neaenini and Tomaspidini (Paladini et al., 2015, 2018), but “results strongly suggest that a significant redefinition of these tribes is necessary for a natural classification” (Paladini et al., 2018).
Knowledge of cercopids in Mexico is based mainly on species with negative economic impact on crops (De la Cruz-Llanas et al., 2005; López-Collado & Pérez-Aguilar, 2012; Martin et al., 1995; Morales-Pérez et al., 2014; Oomen, 1975). For instance, Aeneolamia albofasciata (Lallemand, 1939), A. contigua (Walker, 1851) and Prosapia simulans (Walker, 1858) are pests of pasture grasses and sugar cane (Clark et al., 1976; De la Cruz-Zapata et al., 2016; García et al., 2006, García-García et al., 2006; García-González et al., 2017; Morales-Pérez et al., 2014; Thompson, 2004). In addition, there is a scattered scientific literature documenting the presence of other species within the national territory (Amyot & Serville, 1843; Carvalho & Webb, 2005; Fowler, 1897; Guérin-Méneville, 1844; Hamilton, 2012; Jacobi, 1921; Lallemand, 1912, 1939; López-Collado & Pérez-Aguilar, 2012; Nast, 1950; Stål, 1864; Walker, 1851, 1858). Among these, the compilation by Carvalho and Webb (2005) stands out. They recorded 8 genera and 30 species and 4 subspecies Cercopidae in Mexico. Also important is the work of Hamilton (2016), who described 4 new species in the genera Microsargane Fowler, 1897 and Neaenus Fowler, 1897 for Mexico. Finally, during the last 3 years, records and description have been published of the first reported species of the genus Mahanarva Distant 1909 reported in Mexico, as well as new species of the genera Ocoaxo Fennah, 1968 and Prosapia Fennah, 1949 (Castro-Valderrama, Carvalho et al., 2018; Castro-Valderrama, Peck et al., 2018; Castro-Valderrama et al., 2020). In the case of Ocoaxo, the new records have been of great interest, since the adults of 3 species and the nymphs of 1 displayed feeding preferences for pine tree (Castro-Valderrama et al., 2017; Castro-Valderrama, Carvalho et al., 2018; Cid-Muñoz et al., 2020). This habit is only known to occur in 3 other species of the family worldwide in the pine pest Haematoloma dorsatum (Ahrens, 1812) in the Mediterranean region (Hernández Alonzo et al., 1992; Notario et al., 1981), Sphenorhina conspicua Distant, 1879, which has been reported as a pest of non-native Pinus caribaea Morelet in Costa Rica (Arguedas, 2007; CATIE, 1991), and an undetermined species of Eoscarta Breddin, 1902 that is abundant on Pinus kesiya Royle ex Gordon in Vietnam (VT, pers. observ.).
Despite the importance and diversity of the family Cercopidae in Mexico, there are not a consolidated inventory or comprehensive taxonomic keys of specimens of the country. Our objective is to provide comprehensive taxonomic keys and distribution of genera and species of Cercopidae in Mexico, based on a survey of specimens in Mexican foreign entomological collections and the study of male genitalic characters and external morphology.
Materials and methods
Specimens from the following collections were examined: AMNH: American Museum of Natural History, New York, NY, USA; BMNH: (British) Natural History Museum, London, UK; CACH: Colección Entomológica, Ciencias Agronómicas de Chiapas, Universidad Autónoma de Chiapas, Chiapas; CCFT: El Colegio de la Frontera Sur (ECOSUR)-Tapachula, Colección de Artrópodos asociados con cultivos de la región del Soconusco, Chiapas; CEAM: Colección de Insectos, Colegio de Posgraduados, Montecillo, Texcoco, Estado de México; CECR: Colección de Insectos del Centro de ReferenciaSENASICA, Tecámac, Estado de México; CECT Colección Entomológica, Colegio de Postgraduados-Tabasco, Tabasco; CEFS: Colección Entomológica ECOSURSan Cristóbal de las Casas, Chiapas; CLPV: Colección del Laboratorio de Parasitología Vegetal, Universidad Autónoma del Estado de Morelos, Morelos; CNIN: Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de Mexico, Mexico City; EBTLT: Colección Nacional de Insectos-Estación Biológica Trópical Los Tuxtlas-UNAM, Veracruz; IEXA: Colección Entomológica, Instituto de Ecología, Xalapa; NCSU: North Carolina State University Insect Collection, Raleigh, NC, USA; USNM: National Museum of Natural History, Washington, DC, USA.
For each species, individuals from several representative localities in Mexico were examined (see “Material examined” by species for the numbers in each case). The sex of specimens was determined by both genitalia and external morphology. Some characters included in the keys were carried over from previous keys and taxonomic studies (Castro-Valderrama, Carvalho et al., 2018; Castro-Valderrama, Peck et al., 2018; Clark et al., 1976; Fennah, 1949, 1953; Hamilton, 1977, 2016; Thompson & León, 2005). Male genitalia were dissected from selected specimens (1 to 10 per species), cleared, mounted, and examined following the protocol of Valdez-Carrasco (Castro-Valderrama, Carvalho et al., 2018). Morphological terminology is adapted from Fennah (1949, 1953), Hamilton (1977, 2012, 2016), and Paladini et al. (2015). The taxonomic keys follow, in part, those of Clark et al. (1976), Dietrich (2005) and Hamilton (1977, 2012, 2016), using external morphological attributes. Each species is briefly described in the Diagnosis section and modified to include more characters. In the case of species not examined (marked with ** next to the species title), diagnosis is based on published literature or figures. The Host (s) section lists the host plants or plant associations for each species of Cercopidae in Mexico and the scientific name was confirmed as a host plant in Worldfloraonline (2021). Geographic ranges of genera and species are summarized by state in the Distribution section for each taxon. Distribution maps of the genera are mapped.
Results
The superfamily Cercopoidea is represented in Mexico by 3 families: Clastopteridae, Aphrophoridae, and Cercopidae (Figs. 1-3). Cercopidae belong to 3 previously recognized tribes, Neaenini, Ischnorhinini, and Tomaspidini (Paladini et al., 2018), as well as the recently proposed tribe Microsarganini (Hamilton, 2016). Material examined included more than 1,200 specimens distributed in the 10 genera of Cercopidae (Carvalho & Webb, 2005; Castro-Valderrama, Carvalho et al., 2018; Castro-Valderrama, Peck et al., 2018, Castro-Valderrama et al., 2020; Hamilton, 2016). Studied Cercopidae include 39 described species, 1 subspecies, and 1 undescribed species. The most diverse genera are Ocoaxo with 16 species and Prosapia with 8. Aeneolamia Fennah, 1949 has 3 species and 1 subspecies; Iphirhina Fennah, 1968 and Neaenus have 3 species each one; Microsargane and Olcotomaspis Lallemand, 1949 have 2 species each one; and Mahanarva, Huaina Fennah, 1979 and Zulia Fennah, 1949 have only 1 species each one. Huaina is monotypic.
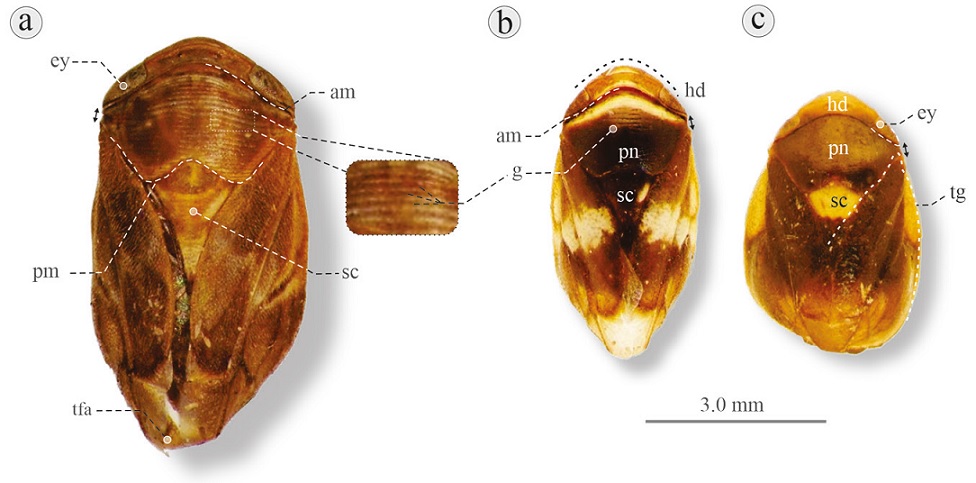
Figure 1 Dorsal habitus of 3 species of Clastopteridae: a) Clastoptera aff. globosa, San Cristóbal de las Casas, Chiapas; b) C. compta, Tepalcingo, Morelos; c) C. aff. funesta, El Encanto, Veracruz. am, Anterior margin (convex); ey, eye; g, grooves; hd, head; pmm posterior margin (“concave”); pn, pronotum; sc, scutellum; tg, tegmen; tfa, folded appendices overlapping at rest. Black arrows at the right or left, indicate distance between eye and base of tegmen.
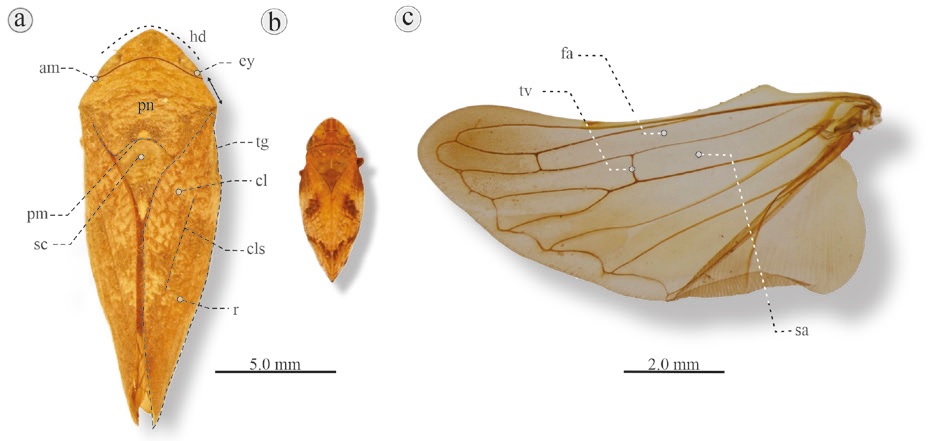
Figure 2 Dorsal habitus of 2 Aphrophoridae species and hind wing: a) Cephisus variolosus, Catemaco, Veracruz, Mexico. b) Lepyronia quadrangularis, Massachusetts, USA. am, Anterior margin, convex (2a) or slightly straight (2b); cl, clavus; cls, claval suture; ey, eye; hd, head; pm, posterior margins (concave); pn, pronotum; r, remigium; sc, scutellum; tg, tegmen. c) Hind wing of C. variolosus. fa, First anteapical cell; sa, second anteapical cell; tv, transverse vein. Black arrows at the right of C. variolosus indicate distance between eye and base of tegmen.
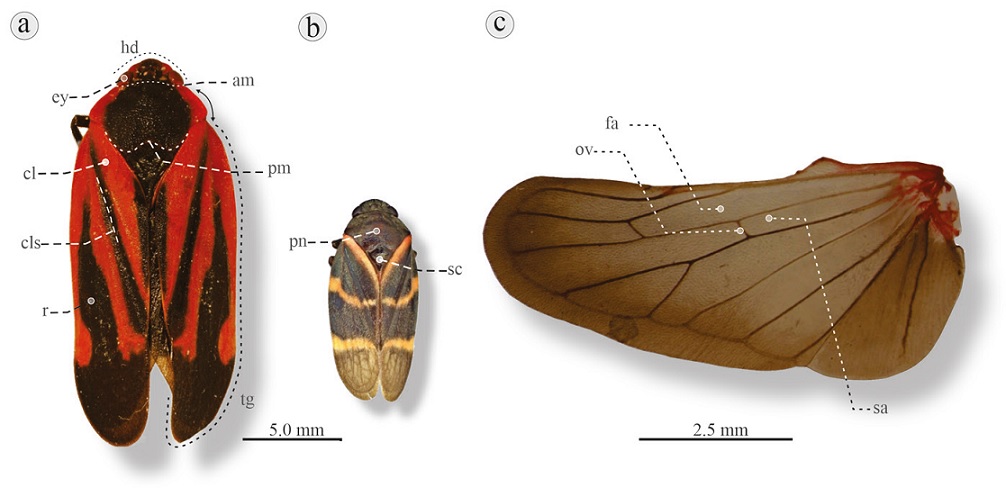
Figure 3 Dorsal habitus of 2 Cercopidae species and hind wing: a) Mahanarva jurael, Estación Biológica los Tuxtlas, Veracruz. b) Aeneolamia contigua, Cardel-Salmoral, Veracruz. am, Anterior margin (straight); cl, clavus; cls, claval suture; ey, eye; hd, head; pm, posterior margin (concave); pn, pronotum; r, remigium; sc, scutellum; tg, tegmen. c) Hind wing of Prosapia ignifera, Montecillos, Estado de México. Fa, First anteapical cell; sa, second anteapical cell; ov, oblique vein. Black arrows at the right of M. jurael indicate distance between eye and base of tegmen.
Key to the families of Cercopoidea (Hemiptera: Auchenorrhyncha) in Mexico
1. Tegmina with broad, folded appendix overlapping at rest, apparently inserted close to the eyes; pronotum with anterior margin convex and posterior margin concave, pronotal surface with transverse grooves; small adults, rounded (globose) or elliptical; length about 0.4 cm, width about 0.2 cm (Fig. 1a-c) ..........................................Clastopteridae Dohrn, 1859
1´. Tegmina lacking appendix or not folded and not overlapping at rest, well separated from eyes by sides of the pronotum; pronotum without transverse grooves (Figs. 2a, b, 3a, b) ................................................................................ 2
2. Boomerang-shaped head; pronotum with anterior margin convex or slightly straight, and posterior margin concave (Fig. 2a, b); vein of first anteapical cell of hind wing connected to the second anteapical cell by transverse vein; the apex of first anteapical cell usually narrower than base of second anteapical cell (Fig. 2c); small to large adults, elliptical or boat-shaped; length 0.7-2.0 cm, width 0.3-0.8 cm (Fig. 2a, b) ............................Aphrophoridae Amyot & Serville, 1843
2´. Triangular-shaped head; pronotum with anterior margin straight and posterior margin concave (Fig. 3a, b); vein of first anteapical cell of hind wing connected to second anteapical cell by oblique vein, apex of first anteapical cell usually wider than base of second anteapical cell (Fig. 3c); small to large adults, elliptical-shaped; length 0.6-2.0 cm, width 0.2-0.8 cm (Fig. 3a, b) ....................................................................................................................Cercopidae Leach, 1815
Key to the genera of Mexican Cercopidae (lateral view, except where mentioned)
1. Body shape like “Aphrophoridae” in dorsal view (Fig. 2a, b); head almost as wide as pronotum (Fig. 5)...................................................Microsargane Fowler, 1897 .................................................... See key to species.
1´. Body shape like “Cercopidae” in dorsal view; head narrower than pronotum (Fig. 3a, b) ......................................... 2
2. Small insects ≤ 7.6 mm (Fig. 6a, b); postclypeus without median carina and laterally inflated (wider than long), in frontal view (Fig. 4a)............................................Neaenus Fowler, 1897 ............................................. See key to species.
2´. Large insects > 7.6 mm; postclypeus with or without median carina and slightly inflated laterally (as wide as long) in frontal view (Fig. 4b) or laterally compressed (narrower than long) in frontal view (Fig. 4c) .................................... 3
3. Insects ≥ 15 mm .............................................................................................................................................................. 4
3´. Insects < 15 mm ............................................................................................................................................................. 5
4 Postclypeus rounded (Fig. 4d), with median carina and slightly inflated laterally in frontal view (Fig. 4b); black tegmina with a triangular red spot on claval base, and 2 red traverse lines on remigium (Fig. 7) ..................................................................................... Huaina Fennah, 1979 - H. inca (Guérin-Méneville, 1844)
4´. Postclypeus slightly angled (Fig. 4e) with median carina and slightly inflated laterally in frontal view (Fig. 4b); tegmina black with 3 red thick lines, one of them on clavus and 2 on remigium, the last 2 extend to 3 quarters of the tegmina, one parallel to claval suture and other on costal vein (Figs. 3a, 9) .....................................Mahanarva Distant, 1909 - M. jurael Castro-Valderrama, Carvalho and Peck, 2018
5. Postclypeus angled (Fig. 4f); tegmina black or brown with a complete or incomplete line on costal margin, red, yellow or orange .............................................................................................................................................................................. 6
5´. Postclypeus rounded or slightly angled (Fig. 4d, e); tegmina black or brown with or without line on costal margin .................................................................................................................................................................................. 7
6. Postclypeus angled and acute (Fig. 4f) with median carina and compressed laterally in frontal view (Fig. 4c); tegmina black (Fig. 8a) or costal margin with thick line that surrounds 3 quarters or entirely each tegmen (Fig. 8b) ........................................................................................................ Iphirhina Fennah, 1968 - See key to species.
6´. Postclypeus angled (Fig. 4e); postclypeus with median carina and slightly inflated laterally in frontal view (Fig. 4b); Tegmina black, costal vein with thin, incomplete red line, 2 incomplete transverse lines one at basal third (3 red spots) and another at distal third (2 red spots) (Carvalho & Webb, 2005: fig. 801) ....................................................................................................Zulia Fennah, 1949 - Z. obscura (Fowler, 1897)
7. Postclypeus rounded (Fig. 4d) without median carina and slightly inflated laterally in frontal view (Fig. 4a); tegmina without transverse lines, translucent (Carvalho & Webb, 2005: fig. 330) or tegmina light brown with elongated white marking on costal margin (Fig. 16a-c).............................Olcotomaspis Lallemand, 1949 ................... See key to species.
7´. Postclypeus rounded or slightly angled (Fig. 4d, e); tegmina with transverse and/or longitudinal lines, complete or incomplete ............................................................................................................................................................................ 8
8. Postclypeus slightly angled (Fig. 4e), with median carina and compressed laterally in frontal view (Fig. 4c); tegmina brown or black, with 1 or 2 longitudinal lines of variable color: red, orange, yellow, pale cream or white; the lines joined or not one to the other, with a spot at the base, this spot joined or not with the longitudinal lines (Figs. 10, 11)........................................................Ocoaxo Fennah, 1968 ................................................................ See key to species.
8´. Postclypeus rounded or slightly angled (Fig. 4d, e); tegmina black, yellow, brown or red with 1 or 2 complete or incomplete transverse lines .................................................................................................................................................. 9
9. Postclypeus rounded or slightly angled (Fig. 4d, e), with median carina and slightly inflated laterally in frontal view (Fig. 4b); tegmina brown, black or red with 1 or 2 complete or incomplete transverse lines, one at basal third, the other at distal third (if present); pronotum commonly crossed by transverse line (Fig. 12a-h)...............................................Prosapia Fennah, 1949 ........................................................ See key to species.
9´. Postclypeus rounded (Fig. 4d), with median carina and slightly inflated laterally, in frontal view (Fig. 4b); pronotum without line; tegmina brown or black with 1 or 2 complete or incomplete transverse lines, one at basal third, other at distal third, the basal or distal line sometimes absent; these lines displaying various colors: yellow orange, white or red; some species with “V” shaped spot on interior margin of claval portion of tegmina (Figs. 13-15)............................................Aeneolamia Fennah, 1949 ..................................................... See key to species.
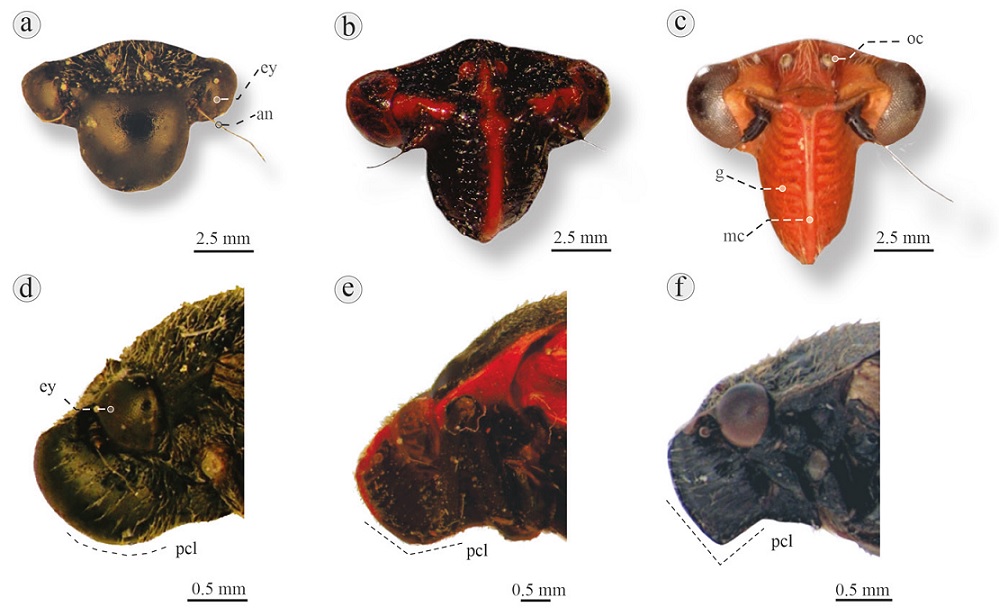
Figure 4 Frontal and lateral view of head of 5 Cercopidae species: a) Neaenus varius, Sierra de Huautla, Morelos, displaying postclypeus wider than long, without median carina, and laterally inflated; b) Prosapia ignifera, Texcoco, Mexico state, displaying postclypeus as wide as long, with median carina, and slightly inflated laterally; c) Ocoaxo varians, Tlaxcala, Tlaxcala, displaying postclypeus narrower than long, with median carina and laterally compressed; d) Neaenus varius, Almolonga, Guerrero, displaying rounded postclypeus; e) Mahanarva jurael, Los Tuxtlas, Veracruz, displaying slightly angled postclypeus; f) Iphirhina sepulchralis, Huatusco, Veracruz, displaying angled postclypeus. an, Antenna; ey, eye; g, grooves; mc, median carina; pcl, postclypeus; oc, ocellus.

Figure 5 Lateral view of Microsargane martialis, Pinotepa Nacional, Oaxaca, Mexico. hd, Head; oc, ocellus; pn, pronotum.
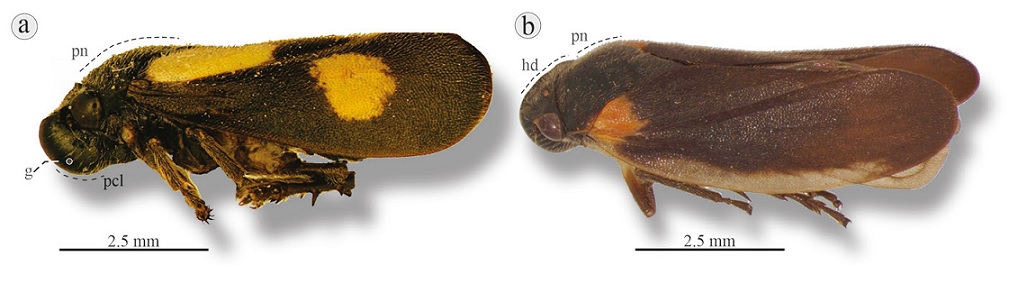
Figure 6 Lateral view of Neaenus species: a) N. varius, Almolonga, Guerrero; b) N. hystricosus, Cerro El Vidrío, Oaxaca. hd, Head; g, grooves; pcl, postclypeus rounded; pn, pronotum.
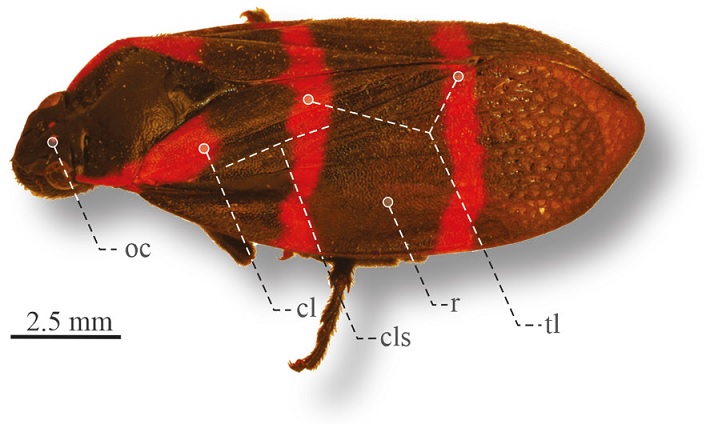
Figure 7 Lateral view of Huaina inca, road Tehuacán-Oaxaca. cl, Clavus; cls, claval suture; r, remigium; oc, ocellus; tl, transverses lines.

Figure 8 Lateral view of adults of Iphirhina species: a) I. sepulchralis, Huatusco, Veracruz; b) I. limbata, San Andres Tuxtlas, Veracruz. lc, Line surrounding the tegmen; pcl, postclypeus “angled and acute”.
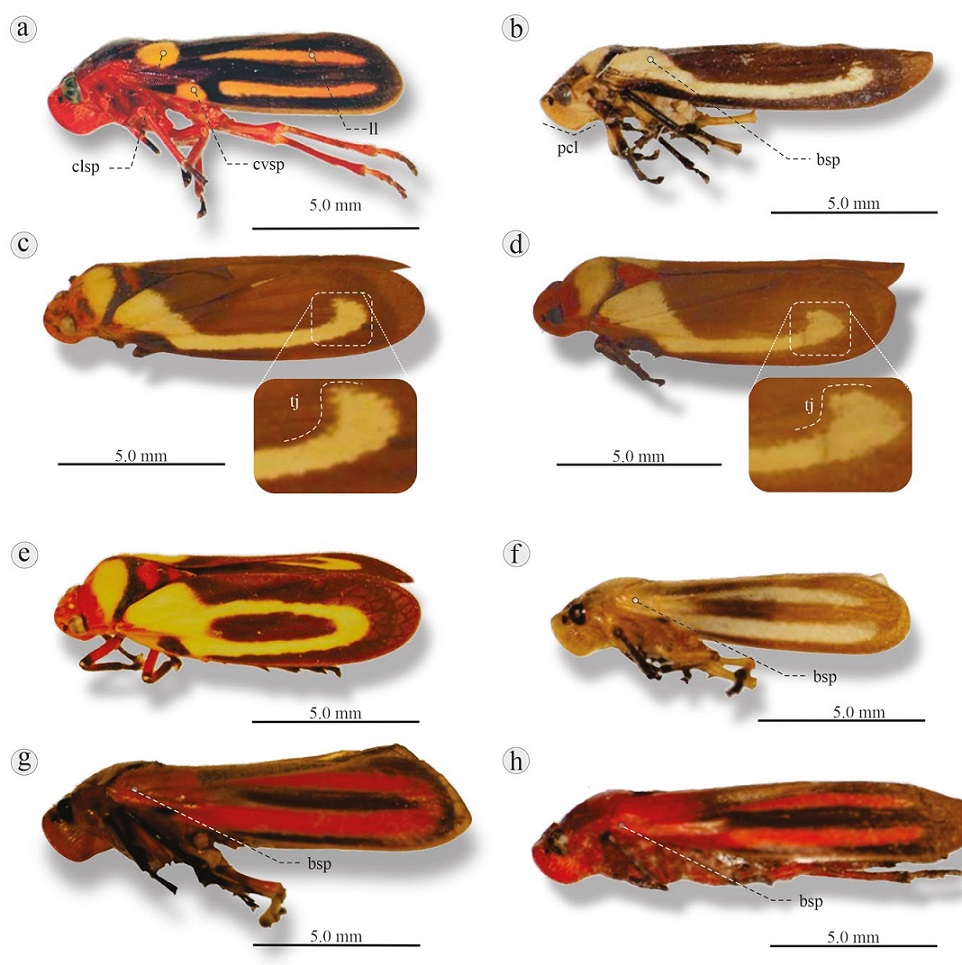
Figure 10 Lateral view of 8 Ocoaxo species: a) O. nuptialis, San Andrés Tuxtlas, Veracruz; b) O. fowleri, km 164.5 road Puerto Escondido-Oaxaca, Oaxaca; c) O. varians, km 190 road Miahutlán-Puerto Ángel, Oaxaca; d) O. assimilis, Nicolas Bravo, Puebla; e) O. cardonai, Tétela de Ocampo, Puebla; f) O. lineolatus, Valle de Bravo, Estado de México; g) O. punctus, Villa Unión Durango, Sinaloa; h) O. bivittus, Xalapa, Veracruz. pcl, Postclypeus slightly angled; bsp, basal spot; clsp, claval spot; cvsp, costal spot; ll, longitudinal line; tj, “tajamata” spot.
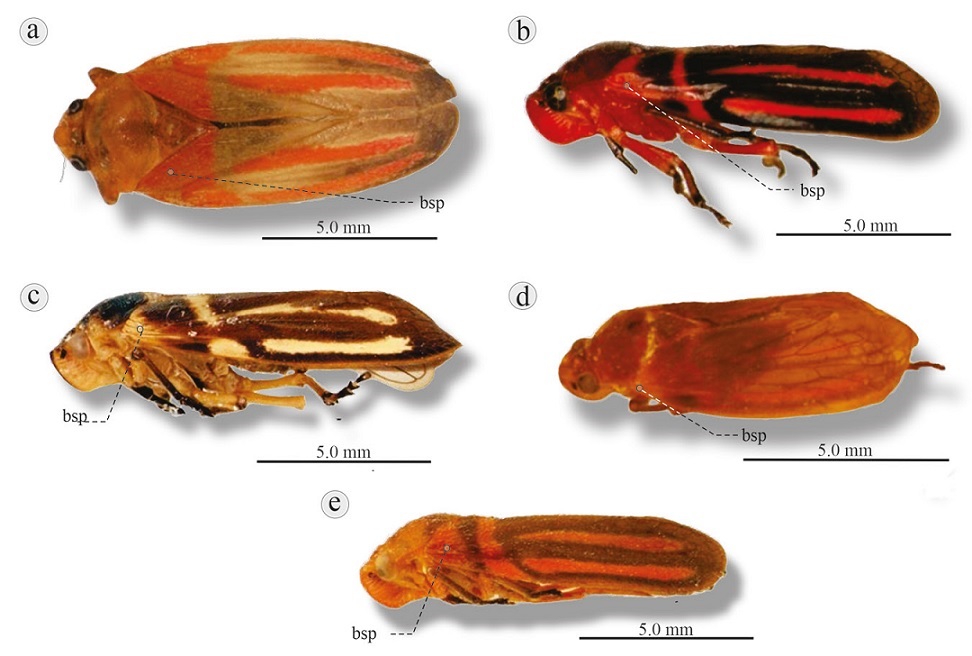
Figure 11 Lateral view of 5 Ocoaxo species: a) O. sinai, Valle Nacional, Oaxaca (left tegmen edited); b) O. cruciatus, Los Tuxtlas, Veracruz; c) O. confusus, Huixtán, Chiapas; d) O. inflexus, Tehuantepec, Oaxaca; e) O. lineatus, Los Tuxtlas, Veracruz. bsp, Basal spot.
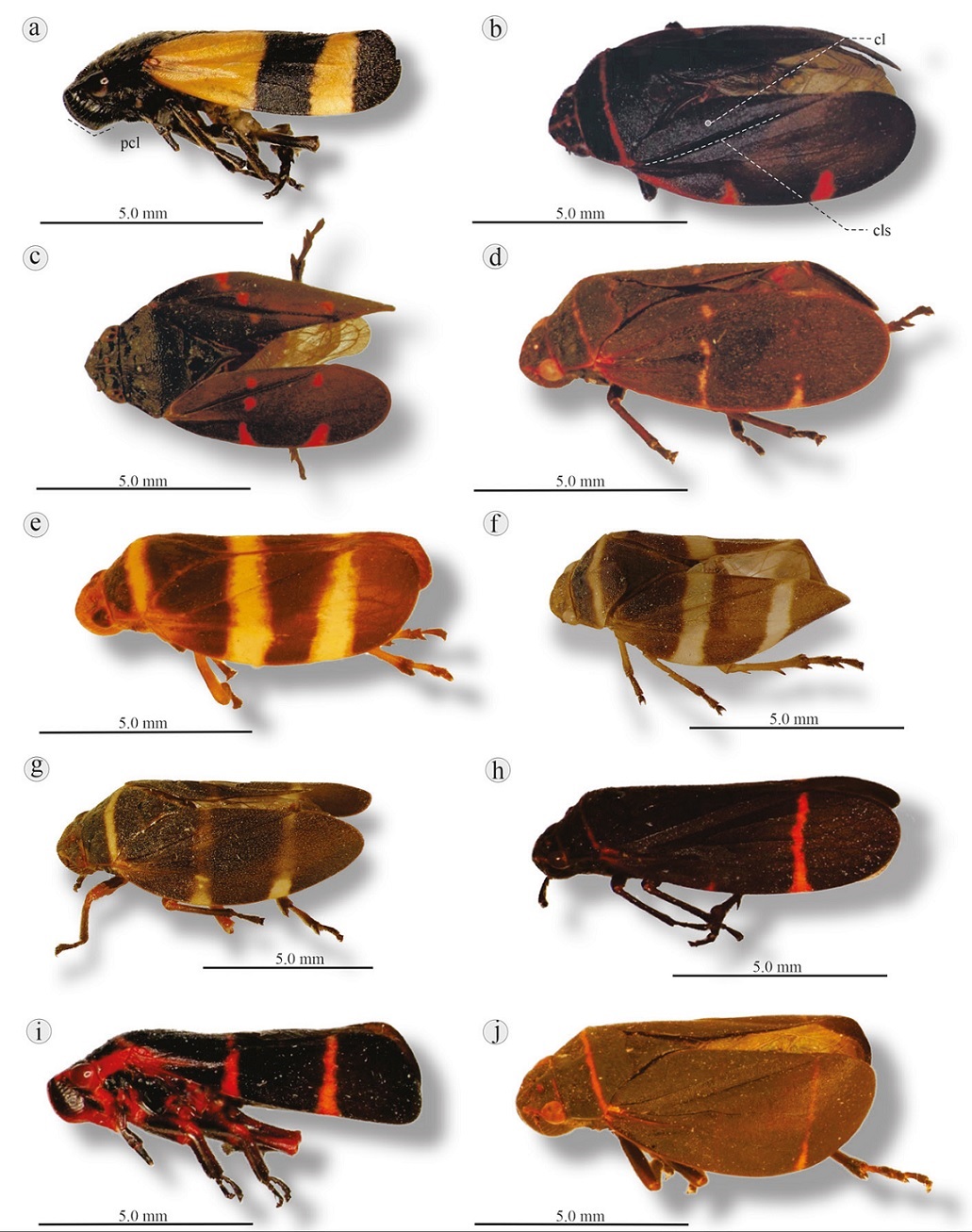
Figure 12 Lateral view of 7 Prosapia species: a) P. isobar, Km 77 Fed. road Temascaltepec-Tejupilco, Estado de México; b) P. ignifera, Tasquillo, Hidalgo; c) P. chiapana, Ocotal Grande, Veracruz (left tegmen edited); d) P. teapana, Tétela de Ocampo, Puebla. P. simulans: e) Putla, Oaxaca (♂); f) Zaachila, Oaxaca (♀); g) Isla, Veracruz (♀); h) P. inferens, Tepoztlán, Morelos. P. morelosi: i) Tangamandapio, Michoacán (with 2 lines on tegmina); j) free road Maravatio-Morelia, Michoacán (with 1 line on tegmina). cl, Clavus; cls, claval suture.
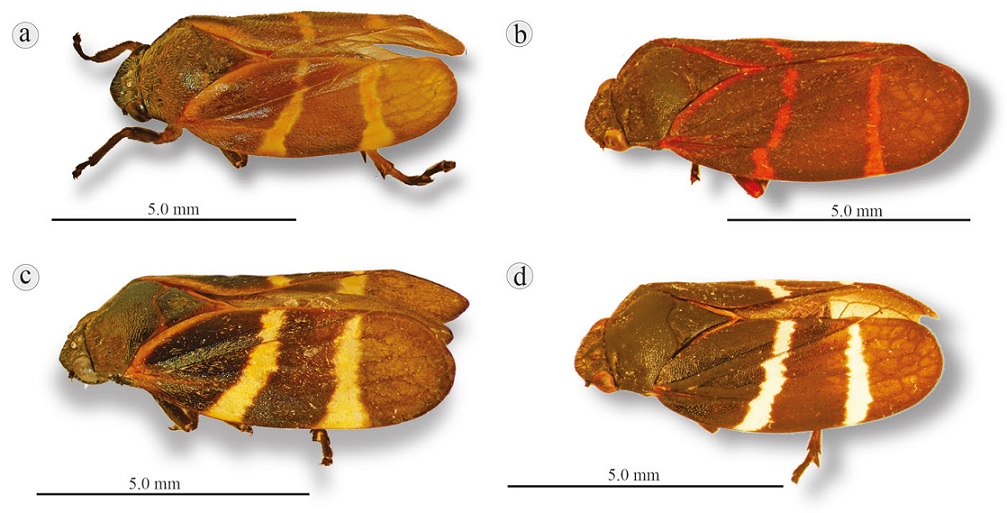
Figure 13 Lateral view of 2 Aeneolamia species and 1 subspecies: a) A. contigua, Isla, Veracruz (orange transverse lines); b) A. contigua campecheana, Chetumal, Quintana Roo (red transverse lines); c) A. albofasciata, San José de Tapia, Córdoba, Veracruz (yellow transverse lines); d) A. albofasciata, Tangamandapio, Michoacán (white transverse lines).

Figure 14 Lateral view of Aeneolamia aff. albofasciata, Sola de Vega, Oaxaca. pcl, Postclypeus rounded.
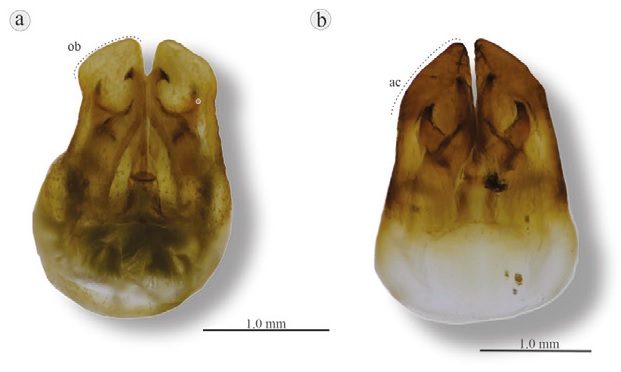
Figure 15 Dorsal view of subgenital plates of Aeneolamia genus: a) A. contigua displaying oblique and truncated; b) A. albofasciata displaying distally acute. ac, Acute; ob, oblique truncate.
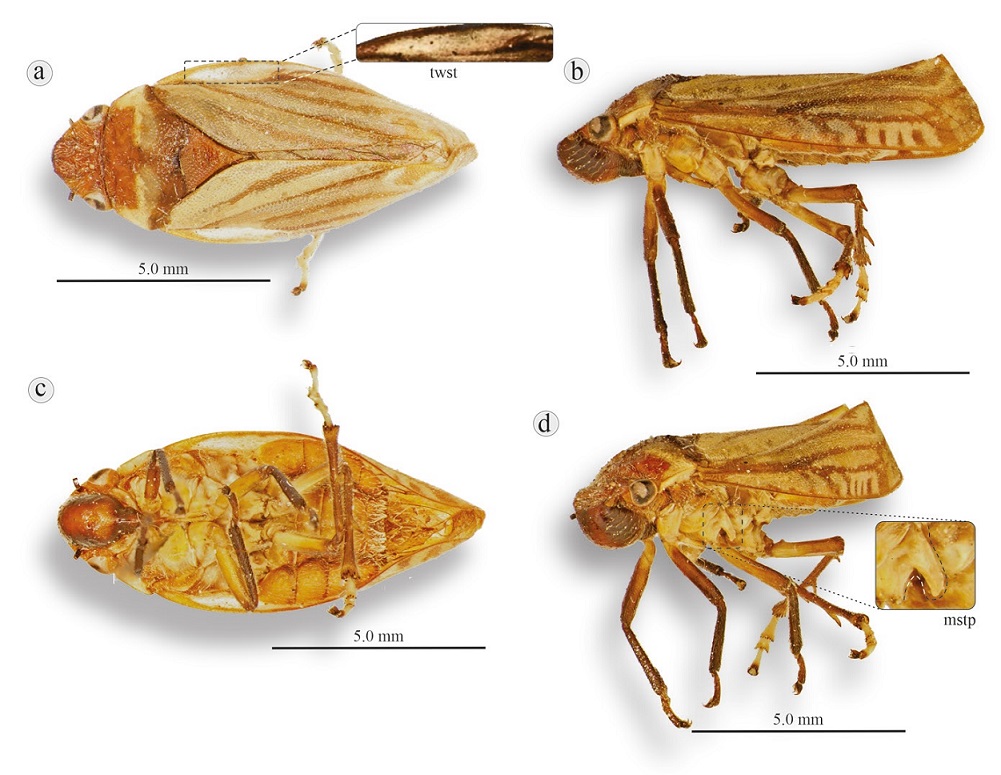
Figure 16 Olcotomaspis laterinotata, La Esperanza, Oaxaca: a) dorsal view, b) lateral view, c) ventral view, d) anterolateral view. mstp, Mesosternum with prominence; twsp, triangular white lateral spot (above the first third of the costal vein).
The Cercopidae of Mexico, by tribes and genera Tribe Microsarganini Hamilton, 2016: 208 Microsarganini; Paladini et al., 2018: 329. Microsargane Fowler, 1897: 196 (s. str. Cryan & Svenson, 2010: 408).
Microsargane (Microrhaphe) Hamilton, 2016: 214.
Microsargane (Microrhaphe) martialis Hamilton, 2016: 215, figs. 9H-K, 24H, 55A-E.
Diagnosis. Male length. 6.7-7.3 mm, female 6.9-8.0 mm (Hamilton, 2016). Body shape like “Aphrophoridae” (Fig. 5). Head crossed by longitudinal lines; 2 black lines cover ocelli, 3 yellow lines, 2 at the edges and one at the middle, these lines extending to pronotum and scutellum or only reaching anterior area of pronotum. Tegmina dark, light brown or black. Depending on presence and distribution of black and yellow spots, 3 patterns of tegminal color variation can be recognized: a) 2 lines (black and yellow) that extend and reach 2-thirds of each tegmen, followed by a yellow arched oblique line, continuing a semihyaline area, and then a distal black “E”-shaped spot, a longitudinal pale marking on the anterior edge of the costa (Fig. 5); b) without conspicuous longitudinal black and yellow lines, with trace of arched oblique line and black “E”-shape spot, and anterior edge of costal vein with longitudinal pale marking (Hamilton, 2016: fig. K); c) without conspicuous longitudinal black and yellow lines or oblique line, with black “E”-shape spot, and anterior edge of costa with longitudinal pale marking (Hamilton, 2016: fig. 9H).
Taxonomic summary
Distribution. Chiapas, Guerrero (Hamilton, 2016), Oaxaca (Fig. 17f).
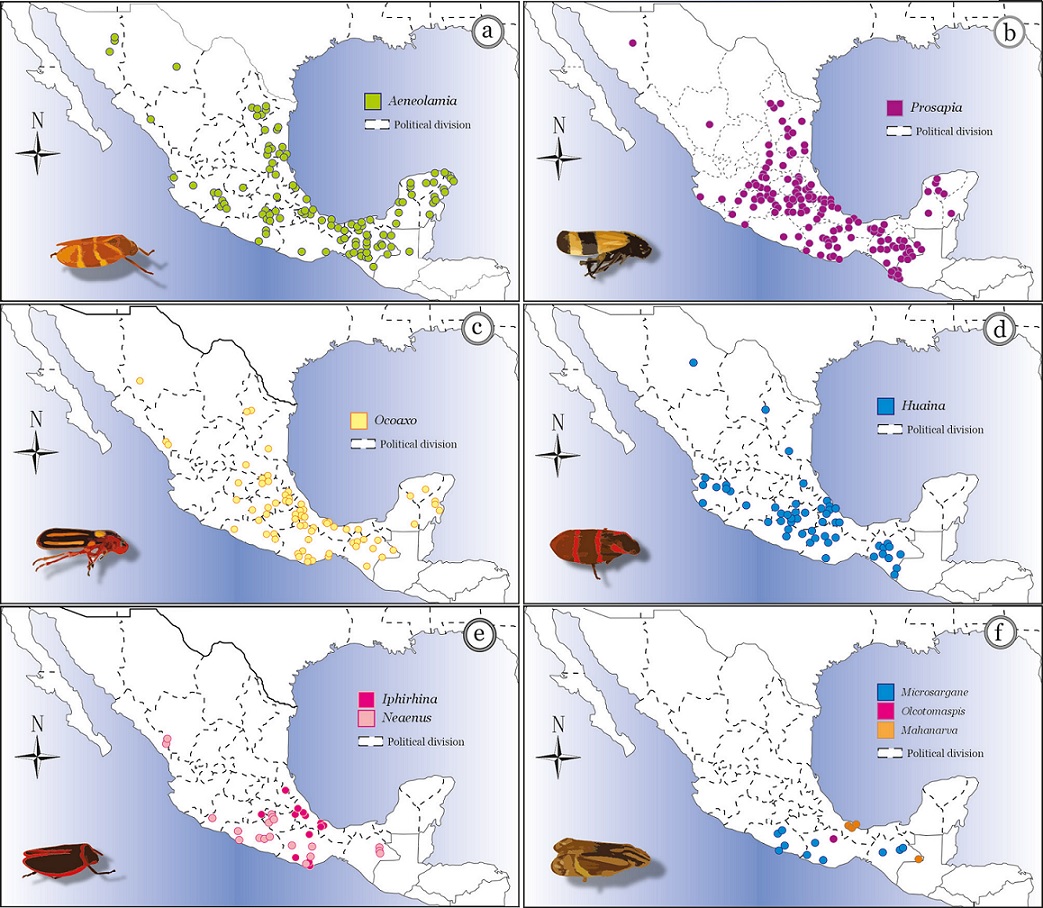
Figure 17 Distribution maps of 9 genera (species combined) of Cercopidae in Mexico: a) Aeneolamia, b) Prosapia, c) Ocoaxo, d) Huaina, e) Iphirhina and Neaenus, f) Microsargane, Olcotomaspis, and Mahanarva. Because Huaina has a single species, d) is also distribution map for H. inca.
Material examined. Guerrero. 11 km E Apoxtla de Castrejón, 14/IX/2005, E. Barrera, Selva baja con ecotonia de encinar, 18°03’52’’ N, 99°58’12’’ W, 1♂; Tierra Colorada km 2 a la Venta, 20/IX/2005, L. Cervantes y R. Carranza, 1♀; Tlalnipatlan, 14/IX/2005, 956 m, E. Barrera, 18°09’45’’ N, 99°44’33’’ W, 1♂; Oaxaca. 20 km W Cerro del Vidrio, 13/VII/2004, 1,857 m, L. Cervantes, A. Delgado, C. Mayorga y G. Gámez, 16°11’21.48’’ N, 97°05’51.47’’ W, 1♂; Cacahuatepec, Km 45 de Pinotepa Nacional a Pie de la Cuesta, 15/VII/2004, 178 m, L. Cervantes, A. Delgado y C. Mayorga, 16°37’35.82’’ N, 98°07’27.6’’ W, 1♀, 1♂; km 16 Carr. Tlaxiaco-Huajuapan de León, 16/VII/2004, 2,292 m, L. Cervantes, A. Delgado, C. Mayorga y G. Gámez, 17°23’27.9’’ N, 97°36’23.46’’ W, 1♂. All at CNIN.
Hosts. Unknown, but a collector reported the habitat as low forest with Quercus “ecotonia”.
Remarks
Body shape like “Aphrophoridae” and head with dark lines traversing the ocelli are diagnostic characters.
Microsargane (Microtholia) sobria Hamilton, 2016: 217, figs. 9E, 56A-E**
Diagnosis. Male length 6.6 mm, female 6.6-6.7 mm (Hamilton, 2016). Body shape like “Aphrophoridae”. Head dark brown without lines. Pronotum and scutellum dark brown. Ornamentation pattern of tegmina as in variation “c” of M. martialis, but with dark brown background and thinner pale longitudinal mark on anterior costa (see Hamilton, 2016: fig. 9E).
Taxonomic summary
Distribution. Chiapas (Hamilton, 2016).
Type material. Holotype ♂, Costa Rica. Punt. Monte Verde Res., 16 Aug. 1986, L. Masner. Paratypes: 4♀♀, same data as holotype. All No. 24231 (CNCI). Not examined in this research.
Other material. Chiapas. Chiapas, Municipio de Ocosingo, 70-75 km SW Palenque on road to Ocosingo, 31/VIII/1981, 762 m, D. E. and P. M. Breedlove, 1 ♂ (CAAS). Not examined in this research.
Hosts. Unknown.
Remarks
Body shape, ornamentation pattern of tegmina and the presence of a thinner and paler longitudinal mark on the anterior costa than in M. martialis are diagnostic characters.
Key to Mexican species of Microsargane Fowler, 1897
1. Head crossed by yellow and black longitudinal lines that extend to pronotum or scutellum (Fig. 5) .....................................................................................................................M. (Microrhaphe) martialis Hamilton, 2016
1´. Head dark brown; pronotum and scutellum dark brown (see Hamilton, 2016: fig. 9E) .........................................................................................................................M. (Microtholia) sobria Hamilton, 2016
Tribe Neaenini Fennah, 1968: 188
Neaenini; Hamilton, 2016: 201; Paladini et al., 2015: 93; Paladini et al., 2018: 327.
Neaenus Fowler, 1897: 191
Neaenus Carvalho & Webb, 2005: 79; Paladini et al., 2015: 93.
Neaenus (Neaenus) Hamilton, 2016: 220.
Diagnosis. Small ≤ 7.6 mm. Head black; postclypeus rounded in lateral view (Fig. 4d), without median carina, inflated in frontal view (Fig. 4a). Lateral margins of pronotum smaller and shorter than compound eyes, black or dark brown body, except spots at base or on costal margin of tegmina.
Taxonomic summary
Distribution. Sinaloa, Michoacán, Morelos, Guerrero Oaxaca and Chiapas (Fig. 17e).
Neaenus (Neaenus) varius Fowler, 1897: 191, plate XII, figs. 1, 2.
Neaenus varius Fowler, 1897; Metcalf, 1962: 9-10; Carvalho & Webb, 2005: 78; Paladini, 2015: 103. Neaenus (Neaenus) varius: Hamilton, 2016: 220.
Diagnosis. Length 6.1-7.6 mm. Head black; postclypeus rounded in lateral view. Pronotum and scutellum black. Tegmina black with yellow-orange spot that reaches middle of clavus, yellow-orange irregular spot at distal third (Fig. 6a), some specimens without spots.
Taxonomic summary
Distribution. Guerrero, Michoacán, Morelos, Oaxaca.
Material examined. Guerrero. 10 km al W de Teloloapan, 23/VI/1990, H. Brailovsky, E. Barrera, 1♂ (CNIN); 6 km E Chilpancingo, 29/VII/1969, F. Pacheco M., 1♂ (CEAM); Almolonga, 30/VII/1962, F. Pacheco M., 2♀♀, 3♂♂ (CEAM); Chilpancingo, 28/VIII/1993, E. Barrera, H. Brailovsky, 1♀ (CNIN); km 130 CoyucaZiguatanejo, 26/VII/1985, F. Arias, R. Barba, 1♂ (CNIN); km 95 Coyuca de Catalán-Zihuatanejo, 24/VII/1985, R. Barba, 1♂ (CNIN); Omeapa Municipio de Tixtla, 10/VIII/2005, 1,518 m, E. Barrera, 17°35’00’’ N, 99°21’11’’ W, 2♀♀, 1♂ (CNIN); Tetipac km 2 a Chontalcuatlan, 29/VIII/2005, 1,427 m, L. Cervantes, R. Carranza, 18°37’34’’ N, 99°32’54’’ W, 2♀♀ (CNIN); Tuxtla La Estacada Microondas, 26/VIII/2005, 1,571 m, L. Cervantes, R. Carranza, 17°35’11’’ N, 99°17’00’’ W, 1♀ (CNIN). Michoacán, Charando, 22/IX/1987, R. Barba, E. Barrera, 1♀ (CNIN). Morelos. 1.5 km N Est. Microondas, EL Salto, P. de Ixtla Res. de la Bio, Sierra de Huautla, 3/VIII/2000, 1,645 m, J. Romero Nápoles, 18°27’54’’ N, 99°16’30’’ W, 2♀♀ (CEAM); Cañón de Lobos, 7/IX/1976, C. Mendieta, 1♀ (CNIN); Cañón de Lobos, 7/IX/1976, C. Mendieta, 1♀ (CNIN); Cañón de Lobos, 7/IX/1976, G. Mena, 1♀ (CNIN); Cañón de Lobos, 9/IX/1976, P. Rodríguez, 1♀ (CNIN); Tepoztlán, 19/VIII/1995, Alma R. Valle, Maleza, 1♀ (CEAM); Tepoztlán, 19/VIII/1995, J. Cisneros H., 1♀ (CEAM); Tepoztlán, 19/VIII/1995, Mabel Martínez, Maleza, 1♀ (CEAM). Oaxaca. Concepción Carrrizal Copala 2 km desviación Juxtlahuaca, 15/VII/2004, 1,851 m, L. Cervantes, A. Delgado, C. Mayorga, G. Gámez, 17°09’21.6’’ N, 97°52’51.18’’ W, 5♀♀, 5♂♂ (CNIN); Oaxaca. Km 79 Carr. Oaxaca-Pto. Ángel, 10/IX/1979, E. Mariño, 1♂ (CNIN); La Galera, 16/VII/1986, L. Torres, 1♂ (CNIN).
Hosts. Unknown, but some collectors reported weeds.
Remarks
The small body and the color pattern of tegmina are diagnostic characters.
Neaenus (Neaenus) hystricosus Hamilton, 2016: 220, figs. 10B, 47A-C
Diagnosis. Length 5.7-6.9 mm. Head black; postclypeus rounded. Pronotum and scutellum black. Tegmina dark brown with an orange or light brown spot at base of clavus and a light brown spot, almost translucent, that extends along costal margin (Fig. 6b).
Taxonomic summary
Distribution. Sinaloa, Oaxaca.
Type material. Sinaloa. Potrerillos, 15 mi W El Palmito, 04/VIII/1964, 1500 m, J. F. McAlpine, 1♂, paratype No.24222 (CNCI); Potrerillos, 15 mi W El Palmito, 09/VIII/1964, 1,500 m, L. A. Kelton, 1♀, paratype No.24222 (CNCI); Sta. Lucia, 04/VIII/1964, 1524 m, L. A. Kelton, 1♂, holotype No.24222 (CNCI); Sta. Lucia, 05/VIII/1964, 1,524 m, L. A. Kelton, 2♂♂, paratypes No.24222 (CNCI). Not examined in this research.
Material examined. Oaxaca. 20 km W Cerro El Vidrío, Oaxaca, 13/VII/2004, 1,309 m, L. Cervantes, A. Delgado, C. Mayorga, G. Gamez, 16°11’21’’ N, 97°5’51.42’’ W, 1♀, 3♂♂ (CNIN).
Hosts. Unknown.
Remarks
The small body and color pattern of the tegmina are diagnostic characters.
Neaenus (Neaenus) natrix Hamilton, 2016: 222, figs. 10D, 46A-C**
Diagnosis. Length 5.2-6.5 mm. Head black and rounded postclypeus. Pronotum and scutellum black. Tegmina black and ornamented with 2 elongated white spots on costal vein, frequently connected with a thin line (Hamilton, 2016: fig. 10D).
Taxonomic summary Distribution. Chiapas.
Type material. Chiapas. Mt. Tzontehuitz, 17/V/1969, 3,000 m, J. M. Campbell, 1♀, paratype No. 24223 (CNCI); Zontehuitz, nr. S[an] Crist[obal], 27/V/1969, 2,850 m, W. R. M. Mason, 1♂, holotype No. 24223 (CNCI); Zontehuitz, nr. San Cristobal, 27/V/1969, 2,850 m, W. R. M. Mason, 4♀♀, 1♂, paratypes No. 24223 (CNCI). Not examined in this research.
Hosts. Unknown.
Remarks
Color pattern of tegmina is diagnostic character.
Key to Mexican species of Neaenus Fowler, 1897 (sensu Hamilton, 2016)
1. Conspicuous wing venation, tegmina mottled; length > 8 mm .........................................................................subgenus Neaniskus, species from Guatemala, see Hamilton (2016)
1´. Inconspicuous and obscure wing venation; tegmina black and ornamented spots evenly colored at the base, clavus or costal margin; length < 7.6 mm...............................................subgenus Neaenus ......................................................... 2
2. Tegmina black with 2 spots, one yellow-orange covering 2 thirds of proximal clavus, the other irregular yellow-orange at distal third. Some individuals without the spots ...........................................................N. varius Fowler, 1897 (Fig. 6a)
2´. Tegmina dark brown with orange or light brown spot at base of clavus and light brown almost translucent spot that extends on costal margin .......................................................................................N. hystricosus Hamilton, 2016 (Fig. 6b)
2´´. Tegmina black without spot on clavus or distal third and 2 elongated white spots on costal margin, frequently connected with a thin line ............................................................... N. natrix Hamilton, 2016 (Hamilton, 2016: fig. 10D)
Tribe Ischnorhinini Schmidt, 1920: 66
Ischnorhinini; Paladini et al., 2015: 93; Paladini et al., 2018: 326, 327.
Huaina Fennah, 1979: 270; Carvalho & Webb, 2005: 62; Paladini et al., 2015: 93.
Huaina inca (Guérin-Méneville, 1844: 368)
Cercopis inca Guérin-Méneville, 1844: 368.
Tomaspis inca: Fowler, 1897: 176; Metcalf, 1961: 99
Huaina inca: Fennah, 1979: 271; Carvalho & Webb, 2005: 62; Paladini et al., 2015: 84, 103; Paladini et al., 2018: 323.
Diagnosis. Length 14-15.5 cm. Head black with postclypeus rounded in lateral view. Pronotum completely black or with red anterolateral margins; scutellum black. Tegmina with triangular red spot on base of clavus, 2 red transverse lines, one on basal third, one on distal third (Fig. 7).
Taxonomic summary
Distribution. From Guatemala probably up to the south of Sonora on the Pacific coast (Naturalista, 2020) and from the border with Belize to Tamaulipas on the Gulf of Mexico. Found in Chihuahua, Coahuila, Tamaulipas, San Luis Potosí, Hidalgo, Nayarit, Jalisco, Michoacán, Guerrero, Edo de Mexico, Morelos, Puebla, Oaxaca, Veracruz and Chiapas.
Material examined. Chiapas. Villaflores, 02/III/1987, Leonel Flor M., 1♂ (CACH); Finca Belén, Motozintla, 27/VI/2000, A. Mazariegos, 1♂ (CCFT); Tapachula, 10/IV/2014, K. Serrano, 1♂ (CCFT); Arriaga, 7/VI/1966, Alberto Ortiz, 2♂♂ (CEAM); Carr. 190, km 1,190 Chiapas, 6/VIII/1965, Alberto Ortiz, 8♀♀, 2♂♂ (CEAM); Chiapa de Corzo, 25/IX/1961, Hiram Bravo M., 1♀ (CEAM); El Chorreadero, Tuxtla Gutiérrez, 26/IX/1961, F. Pacheco M., 1♂ (CEAM); Santiago, Tuxtla, 25/VI/1966, Alberto Ortiz, 1♀, 8♂♂ (CEAM); Selva El Ocote, Las Palmas, 24334 ECO-SC-E, Mpio Ocozocoautla, 13/VIII/1994, B. Gómez, 1♀ (CEFS); Cañón del Sumidero, 25/VII/1978, E. Barrera y A. Cadena, 1♂ (CNIN). Chihuahua. Milpillas, 15/VIII/1976, J. Mathieu, 1♀ (IEXA). Coahulia. Saltillo, 22/VI/1966, S. Southern, 2♂♂ (NCSU). Estado de México. Ixtapan de la Sal, 13/VIII/1961, F. Pacheco M., 3♂♂ (CEAM); Tonatico, 12/VIII/1961, F. Pacheco M., 17♂♂ (CEAM); Tonatico, 13/VIII/1961, F. Pacheco M., 1♂ (CEAM); Valle de Bravo, 12/VIII/1969, 1,600 m, J. Ramos Elorduy de Conconi, 1♀ (CNIN). Guerrero. Cd. Altamirano, 8/VII/2001, Carlos Torres G., 1♂ (CEAM); km 165 Carr Zihuatanejo, Limite Esd. de Mexico, 16/ VII/1962, R. Balderas, 1♀ (CEAM); San Agustín Oapan, 10/VIII/2009, 505 m, J. Amith, Zae mays L., 17°57’14’’ N, 99°26’21’’ W, 1♂ (CEAM); Telolapan, 18/IX/1957, D. Douglas, 1♀ (CEAM); Capilintla, Taxco, 23/VII/1989, S. Ortega G., 1♂ (CLPV); Tlapa km 4 Tlapa-Santa Cruz, 27/VIII/2005, 1424 m, L. Cervantes y R. Carranza, 17°33’42’’ N, 98°29’45’’ W, 1♂ (CNIN). Hidalgo. Km 25 Carr. Atlapezco-Calnali, 5/VIII/1999, E. Barrera, 1♂ (CNIN). Jalisco. Ajijic, 18/VII/1966, Alberto Ortiz, 6♀♀, 16♂♂ (CEAM); Ameca, 22/VII/2000, A. Miranda A., 1♂ (CEAM); Chalapa, 17/VII/1966, Alberto Ortiz, 3♀♀, 4♂♂ (CEAM); La Huerta, Estación Biológica Chamela, 26/VIII1980, J. A. Solis, 1♀ (CEAM); San Agustín, 15/VIII1988, G. Moya, 1♀ (CEAM); San Sebastián del Oeste, 20/X/2017, Santiago Niño, 1♀ (CEAM); Santa Anita, 8/IX/1988, G. Moya, 1♀ (CEAM). Michoacán. Uruapán, VII71998, G. Ascencio B., Maleza-aguacate, 1♂ (CEAM). Morelos. 1.5 km N Est. Microondas, Reserva Huautla, 3/VII/2000, 1,695 m, J. Romero Nápoles, 18°27’51’’ N, 18°27’51’’ N, 1♀ (CEAM); Amado Salazar, Yautepec, 30/VIII/1980, J. Romero Nápoles, Posada sobre una compuesta, 1♂ (CEAM); Amatlán, 13/VIII/1994, F. Santos G., Arbusto, 1♂ (CEAM); Cañón de Lobos, 1/X/1979, Carlos Solorio, 1♀ (CEAM); Cerro la Tortuga, Zacatepec, 3/VI/2015,928 m, U. Castro-Valderrama, Maleza trepadora, 18°39’28.43’’ N, 99°13’1.93’’ W, 2♂♂ (CEAM); Cuautla, 4/V/1996, E. Mariscal M., Sauce, 1♀ (CEAM); Cuautlixco, 24/VIII/1990, 1291 m, G. Sánchez R., 1♂ (CEAM); Cuernavaca, 20/VIII/1983, T. H. Atkinson, Croton sp., 1♀, 1♂ (CEAM); km 49 Xochimilco-Oaxtepec, 11/X/1979, Ana Guzmán, 1♂ (CEAM); Las Estacas, 8/X/1995, Alma R. Valle, Maleza, 1♂ (CEAM); Oaxtepec, 6/VIII/1971, Ceballos, Maleza, 4♂♂ (CEAM); Tenextepango, 10/IX/1995, 1,210 m, Susana Yépez, Maleza, 2♂♂ (CEAM); Tepalcingo, 12/VII/1997, Pastos, 1♀ (CEAM); Tepoztlán, 19/VIII/1995, Mabel Martínez, Maleza, 1♀ (CEAM); Tlayacapan, 12/IX/1981, 1,080 m, C. R. Bernal, Pastos, 1♀ (CEAM); Xochicalco, 24/IX/1988, 1,300 m, C. Romero, 1♂ (CEAM); Xochicalco, 7/X/1995, J. Romero Nápoles, Maleza, 2♀♀, 1♂ (CEAM); Yecapixtla, 8/VIII/1990, 1260 m, G. Sánchez R., 1♂ (CEAM); Zona arqueológica de Xochicalco, 3/IX/2015, 1,303 m, Susana Eva Rodríguez Rodríguez, 18°48’31.5’’ N, 99°17’43.8’’ W, 2♂♂ (CEAM); Col. Vista Hermosa, Cuernavaca ,10/IXI/1989, A. Trejo L., vegetación secundaria, 1♂ (CLPV); UAEM, Chamilpa, Cuernavaca, 6/IX/1988, 1,850 m, S. Ortega y A. Burgos, veg., gramínea, 1♀, 1♂ (CLPV); Temixco, 5/IX/2004, A. Bitar, Vegetación arbustiva, 1♂ (IEXA); Xochitlan, 5/IX/ 2004, A. Bitar, Selva baja, 1♂ (IEXA). Nayarit. Compostela, G 1208, 30/VIII/1980, 680 m, Jorge Valdez, Papaya silvestre, 2♂♂ (CEAM). Oaxaca. El Camarón, 28/IX/1961, Jorge Navarro T., 2♂♂ (CEAM); Huajuapan de León, 30/IX/1961, F. Pacheco M., 3♀♀, 1♂ (CEAM); Ixtlán de Juárez, 14/VIII/1967, Alberto Ortiz, B., 1♂ (CEAM); km 201 Aut. Tehuacán-Oaxaca, 27/VIII/2018, 1886 m, J. Romero Nápoles, Arbusto, 17°21’09’’ N, 97°03’14.1’’ W, 9♀♀, 6♂♂ (CEAM); Putla, 1/XI/1994, 1♂ (CEAM); Ruinas de Monte Albán, 29/IX/1961, Hiram Bravo M., 2♀♀, 2♂♂ (CEAM); Simojovel, 28/IX/1961, F. Pacheco M., 1♀ (CEAM); Atlixco, 14/IX/1994, S. Villa E., Maleza, 1♂ (CEAM); km 114 Huajuapan-Putla, 4/IX/1990, E. Barrera y A. Cadena, 1♀ (CNIN); Puebla. Km 9.5 carretera a Zoquitlan, 22/X/2005, 2840 m, J. Romero Nápoles, 18°16’26’’ N, 97°06’47’’ W, 1♀, 2♂♂ (CEAM). Tamaulipas. Ocampo de Allende, 16/IX/2003, L. Cervantes y Q. Santiago, 1♂ (IEXA). Veracruz. Córdoba, 10/VI/2000, Adriana Briseño G., Saccharum officinarum L., 1♂ (CEAM); Presidio, 28/VIII/1965, Alberto Ortiz, 1♀ (CEAM); Salmoral, 15/IX/1994, S. Villa E., Maleza, 1♂ (CEAM); Tepetate, 14/IXI/1988, A. Rodríguez L., Pasto, 1♂ (CEAM); Tres Valles, 15/VIII/1981, J. Cuevas G., Cynodon sp., 1♂ (CEAM); Córdoba, Miguel Aguilar, 20-26/VII/1996, 1,000 m, E. Saitos y L. Delgado, 1♀ (IEXA); Naranjal, P. Nacional, 20/VIII/1988, A. Grajales Z., 1♀ (IEXA); Palmarejo, 13/VIII/1988, Alejandra P., 1♀ (IEXA); Rt. 140 15 km SE Jalapa, 23/VII/1984, H. H. y K. M. Neunzing, 1♀, 3♂♂ (NCSU).
Hosts. Poaceae: Cynodon sp., Croton sp., Zea mays L., Saccharum officinarum L.
Remarks
This is the second largest Mexican cercopid. The color pattern of the tegmina is diagnostic. This species may have additional host plants, as some collectors have reported it on weeds, shrubs and secondary vegetation (see additional comments in Discussion below).
Iphirhina Fennah, 1968: 175
Iphirhina Carvalho & Webb, 2005: 62; Paladini et al., 2015: 93; Paladini et al., 2018: 327.
Diagnosis: Length 8.6-14 mm. Head black to dark brown, postclypeus angled and acute in lateral view (Fig. 8a). Tegmina unornamented or ornamented with a red, orange, or yellow line that partially or completely surrounds each tegmen.
Taxonomic summary
Distribution. Morelos, Puebla, Veracruz and Oaxaca (Fig. 17e).
Iphirhina sepulchralis (Stål, 1864: 65)
Tomaspis sepulchralis Stål, 1864: 65; Fowler, 1897: 181; Metcalf, 1961: 111.
Iphirhina sepulchralis: Carvalho & Webb, 2005: 63.
Diagnosis. Length 8.6-8.8 mm. Head black to dark brown, postclypeus angled and acute. Pronotum and scutellum black. Tegmina black to dark brown (Fig. 8a).
Taxonomic summary Distribution. Veracruz.
Material examined. Veracruz. Huatusco, 12/VIII/1974, 1♀ (CNIN); Huatusco, 23/VII/1965, Alberto Ortiz, 1♀ (CEAM); Rio Jamapa, 12/VIII/1974, 1♀, 2♂♂ (CNIN).
Hosts. Unknown.
Remarks
Body size, shape of the postclypeus, and color of the body are diagnostic.
Iphirhina limbata (Stål, 1864: 65)
Tomaspis limbata Stål, 1864: 65; Fowler, 1897: 179; Metcalf, 1961: 102.
Iphirhina limbata: Fennah, 1968: 176; Carvalho & Webb, 2005: 62; Paladini et al., 2018: 323, 328.
Diagnosis. Length 13.4-14 mm. Head black to dark brown; postclypeus acute angled. Pronotum black with red margin, scutellum black. Tegmina unornamented or with a red line surrounding edge of each tegmen (Fig. 8b).
Taxonomic summary
Distribution. Morelos, Puebla, Oaxaca, Veracruz.
Material examined. Morelos. Zona arqueológica de Xochicalco, 22/V/2015, 1,303 m, Susana Eva Rodríguez Rodríguez, 18°48’31.5’’ N, 99°17’43.8’’ W, 2♀♀ (CEAM); Oaxaca. Pluma Hidalgo, 1/II/1988, E. Barrera, A. Cadena, E. Ramírez, 1♂ (CNIN); Puebla. Patla, Municipio de Zihuatehuatla, Barranca del rio Necaxa, 24-25/II/2017, 600 m, R. Turrent, 1♂ (CNIN); Veracruz. Cañada Bastonal, Estación Biológica Los Tuxtlas, San Andres Tuxtlas, 4/ VIII/1985, 720 m, S. Sinaca, 1♀ (EBTLT); Los Tuxtlas, I/1986, 1♀ (CNIN).
Hosts. Unknown.
Remarks
The body size and color pattern of the tegmina differentiate this species from other Mexican cercopids.
Iphirhina discontinua (Fowler, 1897: 179, plate XII, fig. 10a)
Tomaspis discontinua Fowler, 1897: 179; Metcalf, 1961: 92.
Iphirhina discontinua: Fennah, 1968: 176; Carvalho & Webb, 2005: 62.
Diagnosis. Length 9-9.7 mm. Head black to dark brown; postclypeus acute angled. Pronotum black ornamented with orange or yellow margin, scutellum black. Tegmina ornamented with a red or yellow line surrounding each tegmen (see Carvalho & Webb, 2005: fig. 786).
Taxonomic summary Distribution. Oaxaca, Veracruz.
Type material. Syntypes, 2♀♀, 2♂♂, Teapa [Tabasco State], H. H. Smith (BMNH), 1♀ (NMW), Mexico. Not examined in this research.
Material examined. Oaxaca. La Esperanza, 17/IV/1983, 1,642 m, A. Ibarra, 17°37’42.54’’ N, 96°22’03’’ W, 2 undetermined sex (CNIN); Veracruz. Cerro El Vigía, Santiago Tuxtla, 7/IV/1966, J. Ramos Elorduy de Conconi, 3 undetermined sex (CNIN); Estación Biológica Los Tuxtlas, San Andres Tuxtlas, 4/III/1985, A. Ibarra, 1 undetermined sex (CNIN); Los Tuxtlas, carretera a Balzapote10/III/1977, E. Barrera, 1 undetermined sex (CNIN); Los Tuxtlas, road to Balzapote, 4/III/1990, G. Ortega y C. Mayorga, 2 undetermined sex (CNIN). Note: The COVID-19 pandemic interrupted photography and sex determination for this species.
Hosts. Unknown.
Remarks
Color pattern of tegmina is diagnostic.
Key to Mexican species of Iphirhina Fennah, 1968
1. Length > 7.6 mm. Tegmina ornamented with red, orange or yellow line partially or completely surrounding each
tegmen .................................................................................................................................................................................. 2
1´. Length ≤ 7.6 mm. Tegmina unornamented (Fig. 8a) ..............................................................I. sepulchralis Stål, 1864
2. Tegmina unornamented or with red line that completely surrounds each tegmen (Fig. 8b) ..........I. limbata Stål, 1864
2´. Tegmina with red or yellow line on costal margin, that partially surrounds each tegmen (see Carvalho & Webb, 2005: fig. 786) ..................................................................................................................................... I. discontinua Fowler, 1897
Tribe Tomaspidini Schmidt, 1922: 175
Tomaspidini; Paladini et al., 2015: 93; Paladini et al., 2018: 323.
Zulia Fennah, 1949: 616
Zulia (Zulia) Fennah, 1949: 616
Zulia (Zulia) obscura (Fowler, 1897: 181)**
Tomaspis obscura Fowler, 1897: 181; Metcalf, 1961: 184.
Zulia (Zulia) obscura: Carvalho & Webb, 2005: 105, stat. nov.
Diagnosis. Length 9.4 mm; Head black head, postclypeus angled, reddish-black eyes. Pronotum and scutellum black. Tegmina black, each ornamented with a thin, incomplete red line on costal vein and with 2 incomplete transverse lines, one at basal third (with 3 red spots), and the other at distal third (with 2 red spots) (see Carvalho & Webb, 2005: fig. 801).
Taxonomic summary
Distribution. Unknown.
Type material. The female holotype deposited at Naturhistorisches Museum Wien, Austria. Not examined in this research.
Host. Unknown.
Mahanarva Distant, 1909: 210
Mahanarva Carvalho & Webb, 2005: 68; Paladini et al., 2015: 84; Paladini et al., 2018: 327; Schöbel & Carvalho, 2021: 301.
Mahanarva jurael Castro-Valderrama, Carvalho & Peck, 2018: 3, Figs. 1A-J, 2A-C
Mahanarva jurael: Schöbel & Carvalho, 2021: 301. Table 1.
Diagnosis. Large insects, 17.5-20.5 cm. Head black with red margins; postclypeus slightly angled. Pronotum black with red lateral margins, black scutellum. Tegmina black, ornamented with 3 thick red lines, the first on the clavus and remigium, the last 2 extending to 3 quarters of the tegmina, one parallel to claval suture, the other on costal margin (Figs. 3a, 9).
Taxonomic summary
Distribution. Found at Estación Biológica Los Tuxtlas (UNAM), Reserva de la Biosfera Los Tuxtlas, Veracruz, and Chajul, Reserva de los Montes Azules, Chiapas (Fig. 17f).
Material examined. Veracruz. Los Tuxtlas, 13/V/1985, L. Cervantes, 1♂, holotype (HOM- TIP92, CNIN). Paratypes: 14/VI/1969, C. Beutelspacher B., on “platanillo”, 1♀ (HOM-TIP-93, CNIN); 1968, J. Ramos Elorduy de Conconi, 1♂ (HOM-TIP-93, CNIN); 14/VI/1969, C. Beutelspacher B., on “platanillo”, 1♀ (HOM-TIP-93, CNIN); Est. Biol. Ver., 22/VI/1964, C. Beutelspacher B., 2♀♀ (HOM-TIP-93, CNIN); Estación Biológica Los Tuxtlas, 11/VI/1986, 170 m, A. Ibarra, 1♀ (EBTLT); Estación Biológica Los Tuxtlas, 20/VII/1986, 160 m, A. Ibarra, 1♀ (EBTLT); Estación Biológica Los Tuxtlas, El Vigía, TM, 25/V/1986, 480 m, P. Sinaca, 1♂ (EBTLT); Tecolapa, 27/V/1951, A. Barrera, 1♀ (CECR). Chiapas. Tecolapa, 27/V/1951, I. Bassols, 1♀ (CECR). Chiapas. Chajul, 2017, Julio Medin, 5♀♀ (AMNH).
Other material examined: Bio. Station Los Tuxtlas, 31 km NE Catemaco, 4-15 May 1981, C.M & G.S. Flint, 1♂, 1♀ (USNM).
Host. Unknown, but a collector reported “platanillo”. In Veracruz, platanillo is the common name of Heliconia spp., the known host group of 2 Costa Rican Mahanarva species (Thompson, 1997) (see below).
Remarks
This is the largest cercopid in Mexico. The color pattern of the tegmina is diagnostic. Schöbel and Carvalho (2021) included this species within the 53 species of the genus.
Ocoaxo Fennah 1968: 181
Sphenorhina Nast, 1950: 114; Metcalf, 1961: 205. Ocoaxo Fennah, 1968: 181; Carvalho & Webb, 2005: 82; Paladini et al., 2015: 114.
Diagnosis. Length 8.6-14 mm. Head displays different colors among the species: dark brown, light brown, pale yellow, orange and red; postclypeus slightly angled.
Pronotum and scutellum have the same color as the head, but in different patterns. Tegmina dark brown, light brown or black with 1 or 2 spots on base, 1 or 2 transverse lines close to the base, and generally 2 longitudinal lines. Some species display longitudinal line joining the basal spots. Color of spots and lines variable: brown, pale yellow, orange or red (Figs. 10, 11).
Taxonomic summary
Distribution. Chihuahua, Durango, Nuevo León, Sinaloa, Tamaulipas, San Luis Potosí, Guanajuato, Queretaro, Michoacán, Estado de México, Puebla, Guerrero, Oaxaca, Tabasco, Chiapas, Campeche, Yucatán and Quintana Roo (Fig. 17c).
Ocoaxo nuptialis (Stål, 1864: 64)
Tomaspis nuptialis Stål, 1864: 63; Metcalf, 1961: 198.
Sphenorhina nuptialis: Nast, 1950: 131.
Ocoaxo nuptialis: Fennah, 1968: 183; Carvalho & Webb, 2005: 81.
Diagnosis. Length 11.8-12.6 mm. Head red; postclypeus slightly angled. Pronotum completely brown or half brown and half black; scutellum dark brown or black. Tegmina black or dark brown, displaying 2 longitudinal salmonorange lines, and 2 spots of same color, one close to the base of clavus and other on costal margin (Fig. 10a).
Taxonomic summary
Distribution. Veracruz.
Material examined. Veracruz. Estación Biológica Los Tuxtlas, San Andrés Tuxtlas, 18/VI/1985, E. Ramírez, 2 ♂♂ (CNIN); Estación Biológica Los Tuxtlas, San Andrés Tuxtlas, 18/VI/1985, P. Sinaca, 1♂ (CNIN).
Hosts. Unknown.
Remarks
This is the only species in genus with 2 spots near the base of the tegmina.
Ocoaxo fowleri (Lallemand, 1912: 91)
Sphenorhina assimilis var. fowleri Lallemand, 1912: 91; Metcalf, 1961: 192.
Sphenorhina fowleri: Nast, 1950: 134. Ocoaxo fowleri: Fennah, 1968: 182; Carvalho & Webb, 2005: 81.
Diagnosis. Length 12.0 mm. Head orange; postclypeus slightly angled. Pronotum with orange base, most of surface yellow, posterior edges black. Scutellum orange. Tegmina brown, each ornamented with a pale yellow basal spot joined with longitudinal line of same color (Fig. 10b).
Taxonomic summary
Distribution. Estado de México, Oaxaca.
Material examined. Estado de México. km 42 carretera federal Texcoco-Tlaxcala, 21/IX/2004, 2,840 m, J. Romero N., 19°33’16’’ N, 98°43’01’’ W, 1♀ (CEAM); Oaxaca. Km 164.5 Oaxaca-Puerto Escondido, 24-25/VI/1995, 1,800 m, J. Blackaller, A. Pérez y A. Sorio, 1♂ (CNIN).
Hosts. Unknown.
Remarks
According to Nast (1950) some specimens of this species show a trace of a longitudinal superior line. This species does not display the “tajamata” spot on the tegmina, characteristic of O. assimilis and O. varians (see below). Tajamata is a cutting tool used in gardening.
Ocoaxo varians (Stål, 1864: 65)
Tomaspis varians Stål, 1864: 65.
Sphenorhina varians: Nast, 1950: 133; Metcalf, 1961: 208. Ocoaxo varians: Fennah, 1968: 183; Carvalho & Webb, 2005: 8.
Diagnosis. Length 11.9-12.0 mm. Head orange; postclypeus slightly angled. Pronotum with orange base, most of surface yellow with black band at posterior margin. Scutellum displays variable coloration patterns: a) completely orange, b) orange base followed by dark brown to black area and orange tip, c) orange base, rest black. Tegmina dark brown, each ornamented with yellow basal spot joined with longitudinal line of same color ending distally in a “tajamata” shape (see remarks) with welldefined distal edge (Fig. 10c).
Taxonomic summary
Distribution. Guerrero, Oaxaca, Tlaxcala.
Material examined. Guerrero. El Tejocote, Torre de Microondas, 18/VIII/1984, 2,389 m, A. Ibarra, 18°10’23’’ N, 96°50’44’’ W, 1♀ (CNIN); Oaxaca. Carretera 131 km 164.5 Oaxaca-Puerto Escondido, 24-28/VI/1995, 1800 m, J. Blackaller, A. Pérez y A. Sorio, Bosque de encino y mesófilo, 1♀ (CNIN); Tlaxcala. Km 65 W ApizacoTlaxcala, 1/IX/2006, 1835 m, J. Romero N., 20°57’22’’ N, 99°12’51’’ W, 1♂ (CEAM).
Hosts. Pinaceae: Pinus spp. and some herbaceous plants.
Remarks
This species has a larger and more defined “tajamata” shape on the tegmina than O. assimilis. Tajamata is a cutting tool used in gardening.
Ocoaxo assimilis (Walker, 1858: 182) Sphenorhina assimilis Walker, 1858: 182; Nast, 1950: 121; Metcalf, 1961: 187.
Tomaspis assimilis: Fowler, 1897: 188, Plate 11, fig. 27a. Ocoaxo assimilis: Fennah, 1968: 182; Carvalho & Webb, 2005: 80.
Diagnosis. 11.5 mm in length. Head orange; postclypeus slightly angled. Color pattern of pronotum as in O. varians. Scutellum color variable: a) completely orange; b) orange base followed by dark brown or black band and orange tip; c) black base, larger orange area, followed by black band and orange band, with a black tip. Tegmina dark brown, each ornamented with yellow basal spot joined with longitudinal line of same color, line ending distally in a “tajamata” shape (see remarks) with diffuse distal edge (Fig. 10d).
Taxonomic summary
Distribution. Trans-Mexican Volcanic Belt in Puebla and Veracruz (Cid-Muñoz et al., 2020). In addition, in “Sierra Madre del Sur”, Oaxaca.
Material examined. Oaxaca. Carr. Puente de FierroMpio Santa María Chichotla, 29/VI/2010, A. Ibarra y H. García, 18°10’23’’ N, 96°50’44’’ W, 1♂ (CNIN); km 40 Oaxaca-Guelatao, 19/IX/1989, E. Barrera, 18°10’23” N, 96°50’44’’ W, 1♂ (CNIN); Puebla. Azumbilla, Nicolas Bravo, 17/IX/2016, 2,368 m, D. Cibrián Tovar, Uriel Barrera y Sergio Quiñonez, Pinus oaxacana Mirov, 18°38’56.65” N, 97°20’7.62’’ W, 10♀♀, 3♂♂ (CEAM); Veracruz. Cd. Mendoza, 15/IX/1994, F. Santos G., Malezas, 1♂ (CEAM).
Hosts. Nymphal hosts include Poaceae: Stipa ichu (Ruíz López et Pavón) Kunth (=Jarava ichu Ruiz and Pav); Asteraceae: Bidens pilosa L. (=Bidens odorata Cav.),
Tagetes lucida Cav.; Plantaginaceae: Penstemon barbatus (Cav.) Roth; Rubiaceae: Bouvardia ternifolia (Cav.) Schltdl; Caprifoliaceae: Symphoricarpos microphyllus (Humb. and Bonpl. ex Schult.) Kunth; Pinaceae: Pinus pseudostrobus var. apulcensis (Lindl.) Shaw; Fagaceae: Quercus rugosa Née (Cid-Muñoz et al., 2020). Adult hosts include Pinaceae: Pinus pseudostrobus var. apulcensis (Lindl.) Shaw (= Pinus oaxacana Mirov.) (Cid-Muñoz et al., 2020).
Remarks
Like O. varians, this species has tegmina ornamented with a “tajamata” shape, but not as sharply defined (Figs 10c, d). In addition, O. assimilis is distinguished by a black line on the mandibular plate that arises close to the antenna (Castro Valderrama, 2018).
Ocoaxo cardonai Castro-Valderrama, Carvalho, Valdez-Carrasco, 2018: 263, figs. 2A-C, 3A-G
Diagnosis. Length of 10.0-12.1 mm. Head orange; postclypeus slightly angled. Pronotum with orange base, creamy yellow central area, and black lateral-posterior and posterior edges. Scutellum completely orange or black or adorned with orange, yellow and black horizontal bands. Tegmina each with a creamy yellow basal spot, the basal spot joined by 2 longitudinal lines of the same color, both reaching the final third and joining distally to form an ellipsis that surrounds a darker area. (Fig. 10e).
Taxonomic summary
Distribution. Sierra Madre Oriental and Trans-Mexican Volcanic Belt.
Material examined. Ciudad de Mexico. Pedregal de San Ángel, 26/IX/1972, T. Bravo, 16°34’5.48’’ N, 96°55’8.54’’ W, 1♂ (CNIN); Reserva del Pedregal de San Ángel, Brecha 1, 2/IX/2006, 2,250 m, M. Torres, 1♀, 1♂ (CNIN); Coahuila. Arteaga km 7 W, La Carbonera, 23/VII/2014, 2,010 m, L. Cervantes, 25°27.49’ N, 100°42.87’ W, 4♀♀, 2♂♂ (CNIN); Estado de México. San Miguel Presa Necaxa, 1/VIII/1963, A. Ibarra, 1♂ (CNIN); Michoacán. Km 126 carr. Maravatio/Morelia, 16/XI/1987, R. Barba, 1♂ (CNIN); Nuevo León. Chipinque, 23/VI/1972, E. Barrera, 1♂ (CNIN). Oaxaca. 11 km W de San Martín Lochila, 12/VII/2004, 1,814 m, A. Delgado, C. Mayorga y S. Gámez, 16°34’5.48’’ N, 96°55’8.54’’ W, 1♀, 1♂ (CNIN); Mpio. Pluma Hidalgo, 17/VI/1982, L. Torres, 1♂ (CNIN); Puebla. Acatlán, Tétela de Ocampo, 20/VII/2015, 1,765 m, U. Castro-Valderrama, Pinus pseudostrobus Lil., 19°51’29.71’’ N, 97°50’01.87’’ W, 1♂, holotype (HOM-TIP-94, CNIN); Acatlán, Tétela de Ocampo, 20/VII/2015, 1,765 m, U. Castro-Valderrama, Pinus pseudostrobus Lil, 19°51’29.71’’ N, 97°50’01.87’’ W, 1♀ allotype (HOM-TIP-95, CNIN); Acatlán, Tétela de Ocampo, 20/VII/2015, 1,765 m, U. Castro-Valderrama, on Pinus pseudostrobus Lil, 19°51’29.71’’ N, 97°50’01.87’’ W, 7♀♀, 13♂♂ (CEAM); Acatlán, Tétela de Ocampo, 20/VII/2015, 1,765 m, U. Castro-Valderrama, Pinus sp., 19°51’29.71’’ N, 97°50’01.87’’ W, 3♂♂ (CEAM); Acatlán, Tétela de Ocampo, 20/VII/2015, 1,765 m, U. CastroValderrama, sotobosque, 19°51’29.71’’ N, 97°50’01.87’’ W, 5♀♀, 11♂♂ (CEAM); km 68 carr. fed. ZacatlánApizaco, Zacatlán, 10/VIII/2015, 2,112 m, U. CastroValderrama, Pinus sp., 19°54’06.81’’ N, 97°57’31’’ W, 7♀♀, 5♂♂ (CEAM); Rancho Alegre, Tétela de Ocampo, 10/VIII/2015, 2,270 m, U. Castro-Valderrama, P. patula Schiede Ex Schltdl et Cham, 19°50’34’’ N, 97°51’22’’ W, 8♀♀, 24♂♂ (CEAM); Queretaro. El Lobo Tres Lagunas km 2, 20/VIII/1998, H. Brailovsky y E. Barrera, 1♂ (CNIN); San Joaquín, Las Ranas, 16/X/1997, 2,450 m, E. Barrera y H. Brailovsky, 20°55’28’’ N, 99°33’53’’ W, 1♀ (CNIN); San Joaquín de Las Ranas, 23/XII/1991, H. Brailovsky y Ka. Brailovsky, 1♀ (CNIN); Toluquilla, 2/VII/1999, H. Brailovsky, 16°34’5.48’’ N, 96°55’8.54’’ W, 1♂ (CNIN); San Luis Potosí. Valle de los Fantasmas, 20/VIII/2008, 2,283 m, H. Brailovsky y E. Barrera, 22°03’32’’ N, 100°37’16’’ W, 1♀ (CNIN). RN El Potosí, sitio 1, 1/ IX/2017, E. Chamé, Trampa de Luz, 2♂♂ (CCFT).
Hosts. Pinaceae: Pinus pseudostrobus Lindl, Pinus patula Schiede Ex. Schltdl and Cham and Pinus sp. Also; Rosaceae: Rubus sp. (Castro-Valderrama et al., 2017).
Remarks
Color pattern and ornamentation of tegmina are diagnostic. Tajamata is a cutting tool used in gardening.
Ocoaxo lineolatus (Amyot & Serville, 1843: 563, Plate 10, fig. 7)
Sphenorhina lineolata Amyot & Serville, 1843: 563; Distant, 1909: 192; Nast, 1950: 114; Metcalf, 1961:195. Ocoaxo lineolatus: Fennah, 1968: 182; Carvalho & Webb, 2005: 81.
Diagnosis. Length 11.0-12.0 mm. Head yellow-beige; postclypeus slightly angled. Pronotum yellow-beige, scutellum light brown. Tegmina light brown and almost transparent, each ornamented with large white basal spot and 2 white longitudinal lines, one joined to the basal spot (Fig. 10f). According to Amyot & Serville (1843) their “inferior line joins a slightly transverse line close to the base”.
Taxonomic summary Distribution. Estado de México.
Material examined. Estado de México. Valle de Bravo, 21/VIII/1968, J. Ramos Elorduy de Conconi, 8♀♀, 2♂♂ (CNIN).
Hosts. Unknown.
Remarks
Color pattern and ornamentation of tegmina are diagnostic.
Ocoaxo punctus (Nast, 1950: 123, figs 2c, 6a-d) Sphenorhina punctum Nast, 1950: 120.
Sphenorhina puncta: Metcalf, 1961: 200. Ocoaxo punctus: Fennah, 1968: 183; Carvalho & Webb, 2005: 82.
Diagnosis. Length 10.0-12.5 mm. Head Orange; postclypeus slightly angled. Pronotum orange or orange ornamented with black posterior edges; scutellum orange. Tegmina brown, almost translucent, each adorned with orange basal spot that extends to one third of the tegmen and 2 longitudinal orange lines fused with basal spot, the lines joined distally or not. Small black or dark brown spot close to the costal margin (Fig. 10g).
Taxonomic summary
Distribution. Guerrero, Sinaloa.
Material examined. Guerrero. Chilapa, 29/VII/1962, Alberto Ortiz, 1♂ (CEAM); Sinaloa. Concordia km 72 Villa Unión Durango, 26/IX/2010, 1,620 m, L. Cervantes y C. Mayorga, 23°27’29’’ N, 105°49’51’’ W, 1♀ (CNIN).
Hosts. Unknown, but one specimen was collected at a place with Pinus spp. (km 72 Concordia Villa Unión Durango) and those are probably its hosts.
Remarks
Color pattern and ornamentation of tegmina are diagnostic.
Ocoaxo bivittus (Walker, 1858: 181)
Sphenorhina bivitta Walker, 1858: 181; Nast, 1950: 120; Metcalf, 1961: 188.
Tomaspis vittatipennis: Stål, 1864: 64.
Ocoaxo bivitta: Fennah, 1968: 182.
Ocoaxo bivittus: Carvalho & Webb, 2005: 80.
Diagnosis. Length 13.0 mm. Head orange; postclypeus angled. Pronotum light to reddish orange, scutellum orange. Tegmina light brown, each ornamented with light to reddish orange basal spot extending a quarter of the tegmina and 2 thick longitudinal orange lines fused with the basal spot (Fig. 10h).
Taxonomic summary
Distribution. Veracruz. In addition, Walker reported this species around the Orizaba volcano (Carvalho & Webb, 2005: 111).
Material examined. Veracruz. Xalapa, 6/XI/1984, J. Peña, 8♂♂ (CNIN).
Hosts. Unknown.
Remarks
This is the largest of all Ocoaxo species. Color pattern and ornamentation of tegmina are diagnostic.
Ocoaxo sinai Castro-Valderrama, Peck, Romero Nápoles 2018: 266, figs. 4A-C, 5A-G
Diagnosis. Length 11.8-12.2 mm. Head orange; postclypeus slightly angled. Pronotum and scutellum orange. Each tegmen light brown and almost transparent adorned with large reddish orange basal spot directed obliquely towards the costal margin, extending one third of tegmen, followed by 2 reddish orange longitudinal lines separated from the basal spot and extending almost to the tip (Fig. 11a).
Taxonomic summary Distribution. Oaxaca.
Material examined. Oaxaca. Valle Nacional, 31/V/1962, Alberto Ortiz, 1♂, holotype (HOM-TIP-124, CNIN).
Hosts. Unknown. The holotype was collected in a place surrounded by Pinus forest (Puente National).
Remarks
This species is known only by the holotype (left tegmen missing).
O. sinai has the same pattern of spots and lines as O. femoratus, but O. sinai has an orange head, pronotum and scutellum, while those of O. femoratus are red.
Ocoaxo femoratus (Nast, 1950: 124, figs 2d, 7a-e)** Sphenorhina femorata Nast, 1950: 120; Metcalf, 1961: 191.
Ocoaxo femoratus: Fennah, 1968: 182; Carvalho & Webb, 2005: 81.
Diagnosis. Length 12-13.0 mm. Head red; postclypeus slightly angled. Pronotum and scutellum red. Tegmina light brown, each ornamented with large red basal spot directed obliquely towards costal margin, extending one third of tegmen, followed by 2 red longitudinal lines separated from the basal spot (Carvalho & Webb, 2005: figure 737).
Taxonomic summary
Distribution. Estado de México (Nast 1950).
Material type. Holotype ♂, MEXICO, D. F, Real de Arriba, VII 1932, 6,000 ft.; Distrito Federal, Temascaltetec, 1931, G. B. Hinton, paratype 2♀♀, All types at BMNH (Nast 1950: 125). Not examined in this research.
Hosts. Unknown. Specimens were collected around Pinus forest.
Remarks
This species is very similar to O. sinai, but differs in body color (see remarks on preceding species). Temascaltetec is a typographical error for Temascaltepec. Today Temascaltepec is a municipality and Real de Arriba, a locality in the municipality of Temascaltepec.
Ocoaxo cruciatus (Walker, 1858: 183)
Sphenorhina cruciata Walker, 1858: 183; Nast, 1950: 122; Metcalf, 1961: 190.
Ocoaxo cruciatus: Carvalho & Webb (2005): 81.
Diagnosis. Length 10.8-11.8 mm. Head red; postclypeus slightly angled. Pronotum red ornamented with black or dark brown posterior edges. Scutellum black ornamented with red posterior base. Tegmina light brown to black, each ornamented with small basal red spot connected by a thin red band (in lateral view, forming a “J”), followed distally by 2 separate thick longitudinal red lines (Fig. 11b).
Taxonomic summary
Distribution. Veracruz. In addition, Walker reported this species around the Orizaba volcano (Carvalho & Webb, 2005).
Material examined. Veracruz. Los Tuxtlas, 20-26/ II/1985, A. Ibarra, 2♂♂; Los Tuxtlas, 24-28/III/1984, A. Ibarra, 1♂; Cerro El Vigía, Santiago Tuxtla, 11/III/1967, J. Ramos Elorduy de Conconi, 3♂♂. All at CNIN.
Hosts. Unknown.
Remarks
Tegminal ornamentation is diagnostic.
Ocoaxo ornatipennis (Stål, 1864: 64)**
Tomaspis ornatipennis Stål, 1864: 64; Nast, 1950: 115.
Sphenorhina ornatipennis: Nast, 1950:126; Metcalf, 1961: 199.
Ocoaxo ornatipennis: Carvalho & Webb, 2005: 82.
Diagnosis. Length 12.5 mm. Head red; postclypeus slightly angled. Pronotum and scutellum red. Tegmina dark brown, each ornamented with basal red spot, followed by distally by transverse red line, and then 2 red longitudinal lines separated from transverse line (see Carvalho & Webb, 2005: figure 459).
Taxonomic summary Distribution. Unknown.
Type material. Syntype ♀ deposited at (NRS) (Naturhistoriska Riksmusset, Stockholm, Sweden). Not examined in this research.
Hosts. Unknown.
Remarks
Color pattern of body and tegminal ornamentation are diagnostic. Carvalho & Webb (2005) reviewed a male from Mexico at BMNH and reported that the aedeagus was similar to that of O. varians.
Ocoaxo confusus (Nast, 1950: 142, figs 29a-e) Sphenorhina confusa Nast, 1950:142; Metcalf, 1961: 189. Ocoaxo confusus: Fennah, 1968: 182; Carvalho & Webb, 2005: 81.
Diagnosis. Length 12-13.0 mm. Head dark brown at the middle with light brown on the sides; postclypeus slightly angled. Color of pronotum similar to head; scutellum dark brown. Tegmina dark brown, each ornamented with pale yellow basal spot, followed by interrupted pale yellow transverse line and then 2 yellowish longitudinal lines (Fig. 11c).
Taxonomic summary Distribution. Chiapas.
Material examined. Chiapas. Mpio Huixtán, km 2 NO Chilil, 23/V/1995, R. Jones, 1♀ (No. 24919 CEFS).
Hosts. Unknown. The specimen was collected in a place surrounded by Pinus forest (Mpio Huixtán).
Remarks
Ornamentation pattern of tegmina is diagnostic.
Ocoaxo similis (Walker, 1858: 182)**
Sphenorhina similis Walker, 1858: 182; Nast, 1950: 120; Metcalf, 1961: 206.
Ocoaxo similis: Fennah, 1968: 183; Carvalho & Webb, 2005: 82.
Diagnosis. Length de 10.8-11.4 mm. Head orange with black tip; postclypeus slightly angled. Pronotum with orange anterior area, black posterior area, orange area with black spot as wide as distance between eyes. Base of scutellum orange, middle black, tip orange. Tegmina black, each ornamented with small yellowish orange basal spot, followed by black transverse line close to the base and a second, yellowish orange line, then by 2 yellowishorange longitudinal lines (see Carvalho & Webb, 2005: figure 666).
Taxonomic summary
Distribution. Walker reported the species around the Orizaba volcano (Carvalho & Webb, 2005: 111).
Type material. 2 male syntypes from Mexico were reported by Nast (1950: 127) at BMNH. Not examined in this research.
Hosts. Unknown.
Ocoaxo inflexus (Nast, 1950: 128, figs 2g, 10a-e)
Tomaspis lineata var. similis Fowler, 1897: fig. 30 Sphenorhina inflexa: Nast, 1950: 120; Metcalf, 1961: 193. Ocoaxo inflexus: Fennah, 1968: 182; Carvalho & Webb, 2005: 81.
Diagnosis. Length 8.8-9.0 mm. Head orange; postclypeus slightly angled. Pronotum orange. Scutellum with 2 patterns of variation: a) orange base, brown middle area and orange tip; b) brown base, remainder orange. Tegmina light brown, transparent, each adorned with small orange basal spot, followed by orange transverse line close to the base, then 2 orange longitudinal lines (Fig. 11d).
Taxonomic summary Distribution. Tabasco, Veracruz.
Material examined. Tabasco. CSAT, Cárdenas, 11/III/1979, J. Morales R., Trampa de luz negra, 2♂♂ (CECT); Veracruz. Playa Azul, 5/VIII/1994, S. Zaragoza, 1♂ (CNIN).
Hosts. Unknown.
Remarks
Ornamentation pattern of tegmina is diagnostic. This taxon also can be differentiated from O. lineatus by its transparent tegmina.
Ocoaxo lineatus (Walker, 1851)
Sphenorhina lineata Walker, 1851: 691; Nast, 1950: 120; Metcalf, 1961: 194-195; Fennah, 1968: 182.
Tomaspis lineata: Fowler, 1897: 189, fig. 29.
Ocoaxo lineatus: Carvalho & Webb, 2005: 81.
Diagnosis. Length 8.4-9.6 mm. Head orange; postclypeus slightly angled. Pronotum and scutellum similar to O. inflexus. Tegmina dark brown, each ornamented with small orange basal spot, followed by black transverse line close to the base, then a second, orange line, then 2 orange longitudinal lines (Fig. 11e).
Taxonomic summary
Distribution. Oaxaca, Veracruz, Yucatán.
Material examined. Oaxaca. San Mateo Yetla, Mpio. Valle Nacional, 13/X/1990, A. Cadena, E. Barrera, E. Ramírez, 1♀ (CNIN); Veracruz. Estación Biológica de Los Tuxtlas, 13/VI/1989, J. L. Colín, H. Rojas, 1♀ (CNIN); Estación Biológica de Los Tuxtlas, 2/IX/1989, F. Martínez, 1♀ (CNIN). Yucatán. Uxmal, 11/IX/1994, E. Barrera, 20º24’0.97’’ N, 89º47’6.74’’ W, 1♂ (CNIN).
Hosts. Unknown.
Remarks
This is the type species of genus (Fennah, 1968). Ornamentation pattern of tegmina is similar to O. inflexus, but differs in that O. lineatus does not display transparent tegmina.
Key to Mexican species of Ocoaxo Fennah, 1968
1. Tegmina black. Each ornamented with 2 nearly round salmon-orange spots at base and 2 parallel longitudinal lines the same color (Fig. 10a) ................................................................................................................O. nuptialis (Stål, 1864)
1´. Tegmina black or brown, ornamented with basal spot, a transverse line close to the basal spot and 1 or 2 longitudinal lines same color as basal spot ............................................................................................................................................. 2
2. Tegmina ornamented with basal spot, the spot joined 1 or 2 longitudinal lines, both basal spot and lines display same color lines ............................................................................................................................................................................. 3
2´. Tegmina ornamented with basal spot, a transverse line connected to or separated from the basal spot, and 2 longitudinal lines separated from the transverse line; basal spot and lines display the same color ................................ 11
3. Tegmina brown or dark brown ornamented with basal spot connected to 1 longitudinal line ..................................... 4
3´. Tegmina ornamented with basal spot connected to oval spot or longitudinal lines ..................................................... 6
4. Tegmina brown, ornamented with light yellow basal spot joined to longitudinal line of same color, sometimes there is a trace of superior line (Fig. 10b) ......................................................................................O. fowleri (Lallemand, 1912)
4´. Tegmina brown or dark brown ornamented with light yellow basal spot connected to 1 longitudinal line with “tajamata” shape, spot and longitudinal line of same color ............................................................................................... 5
5. Tegmina dark brown, “tajamata” shape with sharply defined distal edge (Fig. 10c).................O. varians (Stål, 1864)
5´. Tegmina brown wn “tajamata” shape with diffuse distal edge (Fig. 10d) ...................O. assimilis (Lallemand, 1939)
6. Tegmina with creamy yellow basal spot joined to 2 longitudinal lines of same color, both reaching final third of tegmina where they join forming an ellipsis that surrounds a darker area. (Fig. 10e) .......................................................................O. cardonai Castro-Valderrama, Carvalho and Valdez-Carrasco, 2018
6´. Tegmina ornamented with basal spot connected to 2 longitudinal lines, these not joining distally to form an ellipse ................................................................................................................................................................................... 7
7. Tegmina light brown, almost transparent, ornamented with large white basal spot and 2 white longitudinal lines, both longitunal lines joined to basal spot (Fig. 10f) or sometimes one joined to the basal spot ...........................................................................................................................O. lineolatus (Amyot & Seville, 1843)
7´. Tegmina ornamented with large basal spot connected to or separated from 2 longitudinal lines ............................... 8
8. Tegmina ornamented with large basal spot connected to 2 longitudinal lines .............................................................. 9
8´. Tegmina ornamented with large basal spot separated from 2 longitudinal lines ....................................................... 10
9. Tegmina brown, almost translucent, adorned with orange basal spot that extends to one third of tegmina, and 2 longitudinal orange lines fused with basal spot, these lines joined distally or not. Small black or dark brown spot close to the costal margin (Fig. 10g) ....................................................................................................... O. punctus (Nast, 1950)
9´. Tegmina light brown, ornamented with light orange to reddish orange basal spot extending a quarter of the tegmina and 2 thick longitudinal orange lines fused with basal spot (Fig. 10h) .....................................O. bivittus (Walker, 1858)
10. Tegmina light brown and almost transparent adorned with reddish orange basal spot directed obliquely towards the costal margin, extending one third of tegmina, and 2 reddish orange longitudinal lines separated from basal spot (Fig. 11a) ....................................................................................O. sinai Castro-Valderrama, Peck and Romero Nápoles, 2018
10´. Tegmina light brown, ornamented with a large red basal spot directed obliquely towards costal margin, extending one third of tegmina, and 2 red longitudinal lines separated from basal spot (see Carvalho & Webb, (2005): fig. 737) ........................................................................................................................................O. femoratus (Nast, 1950)
11. Tegmina light brown to black, ornamented with small basal red spot connected to thin red band (in lateral view forming a “J”), followed by 2 separate thick longitudinal red lines (Fig. 11b) ..................... O. cruciatus (Walker, 1858)
11´. Tegmina dark brown or light brown ornamented with basal spot, followed by a free transverse line, and 2 longitudinal lines .................................................................................................................................................................................... 12
12. Tegmina dark brown, ornamented with basal red spot, followed by transverse red line, and then 2 red longitudinal lines separated from transverse line (see Carvalho & Webb (2005): fig. 459) ......................O. ornatipennis (Stål, 1864)
12´. Tegmina ornamented with basal spot, followed by transverse line, and 2 longitudinal lines; tegmina ornamentations pale-yellow, yellow-brown, or orange .............................................................................................................................. 13
13. Tegmina dark brown, ornamented with pale yellow basal spot, followed by interrupted pale yellow transverse line and then 2 yellowish longitudinal lines (Fig. 11c) .......................................................................O. confusus (Nast, 1950)
13´. Tegmina with spot and lines yellowish-orange or orange ........................................................................................ 14
14. Tegmina dark brown, ornamented with small yellowish-orange basal spot, followed by black transverse line close to the base, then a yellowish-orange line, then 2 yellowish-orange longitudinal lines (see Carvalho & Webb (2005): fig. 666) ..................................................................................................................................................O. similis Walker, 1858
14´. Tegmina dark brown or light brown with spot and lines orange .............................................................................. 15
15. Tegmina light brown and transparent, adorned with small orange basal spot, followed by orange transverse line close to the base, then 2 orange longitudinal lines (Fig. 11d) ................................................................ O. inflexus (Nast, 1950)
15´. Tegmina dark brown and not transparent, ornamented with small orange basal spot, followed by black transverse line close to the base, another transverse, orange line, then 2 orange longitudinal lines (Fig. 11e) .....................................................................................................................................O. lineatus (Walker, 1851)
Prosapia Fennah, 1949: 606
Prosapia Metcalf, 1961: 208-209; Hamilton, 1977: 621; Carvalho & Webb, 2005: 84; Paladini et al., 2015: 89.
Diagnosis. Length 7.4-10.5 mm. In lateral view, postclypeus shape variable among species: angled, slightly angled or rounded (s. str. Hamilton, 1977). Head dark brown, light brown, pale yellow, orange, and/or red. Pronotum usually crossed by complete or incomplete line, but sometimes absent. Pronotum and scutellum exhibit same colors as head, but in different proportions. Tegmina with light or dark brown to black background, usually crossed by 2 complete or incomplete transverse lines; these light brown, pale yellow, yellow, orange or red. In ventral view, prothorax and mesothorax usually black or dark brown, but metathorax generally reddish. There is a huge amount of variation in both abdomen base color and in the color of the posterior edges, not to mention the pleaurites on either side of the ventral sternies. The abdomen base color ranges from black to brown to tan to red-tan to yellow -tan to grey. The edges range from red to tan to no distinct marking.
Taxonomic summary
Distribution. Campeche, Chiapas, Durango, Guanajuato, Guerrero, Hidalgo, Jalisco, Michoacán, Mexico, Morelos, Nuevo León, Oaxaca, Puebla, Quintana Roo, San Luis Potosí, Sonora, Tabasco, Tamaulipas, Tlaxcala, Yucatán and Veracruz (Fig. 17b).
Prosapia hemelytra Hamilton, 1977: 628, figs. 16, 29, 42, 48**
Prosapia hemelytra Hamilton, 1977: 628; Carvalho & Webb, 2005: 85.
Diagnosis. Length 8.8 mm. Head black with red edges; postclypeus slightly angled. Pronotum and scutellum black. Tegmina with basal third black; rest red, but tips display thin brown bands.
Taxonomic summary Distribution. Chiapas.
Type material. Holotype 1♂, MEXICO, Montebello, Chis., 14/VIII/1969, L. A Kelton (CNIC); paratype 1♀ same data as holotype (CNIC). Not examined in this research.
Hosts. Unknown.
Remarks
Color pattern of tegmina is diagnostic.
Prosapia isobar Hamilton, 1977: 629, figs. 22, 31, 35, 43, 44
Prosapia isobar Hamilton, 1977: 629; Carvalho & Webb, 2005: 85.
Diagnosis. Length 7.9-8.1 mm. Head black; postclypeus slightly angled. Pronotum black, but some specimens with basal half black and posterior half yellow. Scutellum black. Tegmina ornamented with alternating yellow and black bands almost the same width (Fig. 12a) or completely yellow.
Taxonomic summary
Distribution. Mexico, Michoacán, Morelos and Oaxaca states.
Material examined. Michoacán. Santa Mónica km 96 carr. Maravatio-Morelia, 16/IX/1987, E. Barrera V., 1♂ (CNIN); Oaxaca. Carr. 190 km 203 Huajuapan de León- Izucar de Matamoros, 20/IX/2003, H. Brailovsky, E Barrera, 1♂ (CNIN); San Baltazar Guelavila, 18/IX/2003, 1,670 m, H. Brailovsky, E Barrera, 1♂ (CNIN); Estado de México. Valle de Bravo, 2/X/1969, 1,600 m, J. Ramos E. de Conconi, 1♀ (CNIN); km 77 carr. Fed. TemascaltepecTejupilco, 3/IX/2015, 1,870 m, U. Castro V., 19°01’00’’ N, 100º03’3’’ W, 10♀♀, 10♂♂ (CEAM)
Hosts. Unknown.
Remarks
The ornamentation pattern of tegmina is diagnostic.
Prosapia ignifera Hamilton, 1977: 626, figs. 20, 30, 37, 46 Prosapia ignifera Hamilton, 1977: 626; Carvalho & Webb, 2005: 85.
Diagnosis. Length 9.5-10.3 mm. Head black with red edges; postclypeus rounded. Pronotum black crossed by a thin red line. Scutellum black. Tegmina black, each displaying in basal third thin red line that extends out the costa and ends in first costal triangular red spot, distal third of each tegmen with larger triangular costal red spot (Fig. 12b), or tegmina completely black.
Taxonomic summary
Distribution. Mexico City, Durango, Estado de México, Guanajuato, Hidalgo, Michoacán, Morelos, Puebla, Queretaro (Hamilton, 1977), San Luis Potosí, Tlaxcala.
Material examined. Ciudad de México. México D. F., Cantera Oriente, Reserva del Pedregal de San Ángel, 7/X/2006, M. Torres, C. Mayorga, 1♂ (CNIN); México D. F., Cantera Oriente UNAM, 30/X/2006, M. Torres, C. Mayorga, 1♀ (CNIN); Durango. Canatlán, 16/VIII/2011, 1,927 m, Huerto de manzano, 24º30’37’’ N, 104º43’47’’ W, 1♂. Estado de México. Condado de Sayavedra, 10/X/2009, J. Ramos Elorduy, 4♀♀, 15♂♂ (CNIN); Tepozotlán, 26/VIII/1995, H. Ka. Brailovsky, 1♂ (CNIN); Chapingo, VII-XII/1971, 1♀, 1♂ (CEFS). Guanajuato. Km 07 Dolores Hidalgo-Guanajuato, Los Hernández, 10/IX/2014, Harry Brailovsky, E. Barrera, 1♀ (CNIN); Km 18 Carr. Dolores Hgo-Guanajuato, 10/IX/2014, H. Brailovsky, E. Barrera, 2♀♀, 1♂ (CNIN). Hidalgo. 1 km desv. Hueyapan-Tlanchinol, 16/VI/2004, 850 m, J. Romero Nápoles, 1♂ (CEAM); Eloxochitlán, 18/VIII/2015, 1,936 m, Sergio Godínez-Cortés, Acacia farnesiana (L.) Willd, 20°43’56.19’’ N, 98°49’20.66’’ W, 1♂ (CEAM); km 151 Carr. Zimapan-Tamanzunchale, 25/X/2004, 2,220 m, Romero N.J., 20°52’16’’ N, 99°14’24’’ W, 1♂ (CEAM). Michoacán. Morelia, 1/VI/1963, F. Pacheco M., 3♂♂ (CEAM); Morelia, 13/VIII/1962, F. Pacheco M., 2♂♂ (CEAM). Morelos, Cuautla 6/VI/1963, S. López B., 1♀ (CEAM); Morelos, Tlayacapan, 27/IX/1980, 1,730 m, N. Vázquez, Gramínea, 1♂ (CEAM); Cuautla 6/VI/1963, S. López B., 1♀ (CEAM); Tlayacapan, 27/IX/1980, 1,730 m, N. Vázquez, Gramínea, 1♂ (CEAM). Puebla. Chopico, Tétela de Ocampo, 18/VIII/2016, 1,908 m, U. Castro-Valderrama, Pennisetum sp., 19°47’59.34’’ N, 97°48’49.49’’ W, 1♀, 2♂♂ (CEAM); Tehuacán, 12/X/1995, 1,750 m, Sergio Ramírez Rojas, 1♂ (CEAM). San Luis Potosí. Santa Catarina, Mpio San Nicolás Tolentino, 20/VIII/2006, 1,250 m, H. Brailovsky, E. Barrera, 22°03’07’’ N, 100°28’03’’ W, 1♂ (CNIN); Tierra Quemada, km 143 Carr. Querétaro-San Luís Potosí, 2/VIII/2006, 1,800 m, Harry Brailovsky, E. Barrera, 21°41’04’’ N, 100°41’49’’ W, 1♂ (CNIN). Tlaxcala. 8/VI/2000, J. I. Jacobo C., 1♂ (CEAM); Veracruz. 25/VII/1998, D. Robles B., 1♀ (CEAM).
Hosts. Poaceae: Pennisetum spp. and other grasses. Fabaceae: Acacia farnesiana (L.) Willd.
Remarks
The ornamentation pattern of the tegmina is different from Prosapia chiapana Hamilton (1977) and from other Prosapia species.
Prosapia chiapana Hamilton, 1977: 626, figs. 7, 18, 25 Prosapia chiapana Hamilton, 1977: 62; Carvalho & Webb, 2005: 85.
Diagnosis. Length 7.4-8.4 mm. Head dark brown to black; postclypeus angled or slightly angled. Pronotum black, unlined. Scutellum black. Tegmina with dark brown to black background; each with 3 red or white spots in basal third, 2 almost round, one triangular on the costa vein; in distal third of tegmen 2 red or white spots, one almost round, the other triangular on costa vein (Fig. 12c).
Taxonomic summary
Distribution. Chiapas, Veracruz.
Material examined. Chiapas, Mpio. Tenajapa de los Parajes, Yashanal camino al Ojo de Agua, 3/VIII/1991, C. Mayorga, 1♀ (CNIN); Veracruz, Ocotal Grande, 8/ XII/1985, L. Cervantes, 1♂ (CNIN).
Hosts. Unknown.
Remarks
Color of dark brown to black, the unlined pronotum, and the ornamentation pattern of the tegmina differentiate this species from Prosapia ignifera and other congenerics.
Prosapia teapana (Fennah, 1953: 343, fig. 7A-D) Prosapia simulans teapana Fennah, 1953: 343; Metcalf, 1961: 220.
Prosapia teapana: Hamilton, 1977: 626; Carvalho & Webb, 2005: 87.
Diagnosis. Length 9.0-10.5 mm. Head brown to black; postclypeus rounded or slightly angled. Pronotum crossed by complete or incomplete thin reddish line, background color variable: a) black; b) brown; c) basal half dark brown, distal half light brown. Scutellum brown or black. Tegmina with dark brown to black background, ornamented with 1 or 2 interrupted thin transverse lines, line in basal third red or pale yellow, line in distal third reddish or pale yellow (Fig. 12d), sometimes reduced to triangular spots on costa.
Taxonomic summary
Distribution. Chiapas (Hamilton, 1977), Morelos, Oaxaca, Puebla, Tabasco, Veracruz.
Material examined. Morelos, Miacatlán, V/1998, J. Pérez M., 1♂ (CNIN); Tetelcingo, 7/VII/1990, Sánchez R. G., 1♀ (CNIN). Oaxaca. S. de Juárez km 54 TuxtepecOax., 28/III/1984, M. García, 1♂ (CNIN). Puebla. Villa Juárez, 20/VI/1965, V. Navarro, 1♂ (CNIN). Predio San Vicente, Acatlán, Tétela de Ocampo, 10/VIII/2015, 1,706 m, U. Castro-Valderrama, Pennisetum sp., 19°51’28.09’’ N, 97°49’54’’ W, 6♀♀, 18♂♂ (CEAM). Tabasco. Km 21 CT-CP, 12/I/2013, Saccharum officinarum L., 1♂ (CECT). Veracruz. Córdoba, 23/X/2014, U. Castro-Valderrama, Saccharum officinarum L., 1♂ (CNIN); El Otate, Córdoba, 14/III/2015, F. Hernández-Rosas, Saccharum officinarum L., 1♂ (CEAM).
Hosts. Poaceae: Saccharum officinarum L., Pennisetum spp.
Remarks
Color pattern of pronotum and tegmina differentiate this species from other congenerics.
Prosapia simulans (Walker, 1858: 183)
Sphenorhina simulans Walker, 1858: 183.
Tomaspis fasciaticollis: Stål, 1864: 63; Hamilton, 1977: 229 (new syn.).
Tomaspis simulans: Fowler, 1897: 185; Fennah, 1949: 606.
Prosapia simulans: Fennah, 1953: 341; Metcalf, 1961: 218; Clark et al., 1976: 16; Hamilton, 1977: 629; Carvalho & Webb, 2005: 86.
Prosapia simulans unifasciata: Fennah, 1953: 345; Metcalf, 1961: 220; Carvalho & Webb, 2005: 87.
Diagnosis. Length 8.8-8.9 mm. Head brown-dark brown, lighter than basal portion of pronotum; postclypeus angled, slightly angled or rounded. Pronotum black in dark females or with the basal half dark brown and posterior half light brown, in all cases basal portion darker, and crossed by a yellow line thinner than tegminal lines (female and male) (Fig. 12e-g); in dark females this line visible (Fig. 12g), obscured or faint. Scutellum dark brown or black, always black in dark females. Tegmina with dark brown to black background (males, some females), 2 yellow transverse lines (Fig. 12e-g), yellow varying from pale almost white to intense, one in basal third and one in distal third, usually distal line thinner (Fig. 12e, f); in females both lines thinner than males (Fig. 12f), obscured or faint in dark specimens (Fig. 12g) (see more variations in Clark et al., 1976, fig. 4), rarely the 2 lines fusing to form a single broad yellow line.
Taxonomic summary
Distribution. Nuevo León, Tamaulipas, San Luis Potosí, Veracruz, Tabasco, Campeche, Yucatán, Quintana Roo, Puebla, Hidalgo, Sinaloa, Sonora, Colima, Nayarit, Guerrero, Oaxaca, Chiapas (López Collado and PérezAguilar, 2012), Morelos, Guanajuato.
Material examined. Holotype, 1♂ Mexico (BMNH). Chiapas. Rancho Mogritas, Villaflores ,22/X/2005, Julia Toledo, 1♀ (CACH); Villaflores, 14/V/1985, V. Nicolás, 1♂ (CACH); Cecilo del Valle, 26/V/1999, V. Castillejos, 1♂ (CCFT); El Edén, Tapachuala, 08/VIII/1997, P. Chiu, 1♂ (CCFT); Primera Sección de medio monte, Tuxtla Chico, 06/VI/1997, A. Dávila, 1♂ (CCFT); Sn Fco Tap., 04/VII/1997, M. Zenil V., 1♂ (CCFT); Tapachula, 05/VII/1995, C. Pérez, Delonix regia (Bojer ex Hook.) Raf., 1♀ (CCFT); Villa de Comatitlan, 11/VI/2013, J. López, Saccharum officinarum L., Zea mays L.1♂ (CCFT); Chapa de Corzo, Rancho San Miguel, Prop. Martin Castillo, 12/XI/2016, 589 m, U. Castro-Valderrama, Dichanthium aristatum (Poir.) C.E. Hubb., 16°43’42.11’’ N, 93°57’48.08’’ W, 7♀♀, 11♂♂ (CEAM); Reserva El Aguacero, Ocozocoautla, 25/IX/2007, 678 m, J. Romero Nápoles, 16°45’21.7’’ N, 93°31’28.6’’ W, 1♀ (CEAM); Loc. San Francisco en Terán, Tuxtla, 24536 ECO-SC-E, 29/X/1974, Antonio López, 1♂ (CECT); Carr. a Bonampak, 8/VII/2009, 573 m, L. F. De la Mora, 16°46’1.08’’ N, 91°06’1.95’’ W, 3♂♂ (CNIN); Cañón del Sumidero, 25/VII/1978, 1♂ (CNIN); Chiutul, 6/III/1986, R. Barba, E. Barrera y A. Cadena, 1♂ (CNIN); km 50 Palenque Ocosingo, 30/I/1985, F. Arias, 1♂ (CNIN); Mapastepec Rancho Puerta de Oro, 11/VIII/1991, C. Mayorga, 1♀, 7♂♂ (CNIN); Nututum, 20/VII/1978, E. Mariño, 2♀♀ (CNIN); Jalisco. Rio San Nicolás, 26/IX/1993, G. Ortega, C. Mayorga y S. Rodríguez, 2♀♀ (CNIN). Guerrero. Petaquillas 9 km W Chilpancingo, 6/VI/1963, S. López B., 1♂ (CEAM). Michoacán. Taretan, 19/VIII/2015, Paul Méndez, 1♂ (CEAM); km 95 Zihuatanejo Lázaro Cárdenas, 27/VII/1985, F. Arias, 1♀, 3♂♂ (CNIN); Oaxaca. Putla, 1/XI/1994, M. López T., 1♂ (CEAM); San Felipe Cihualtepec Cotzacan Mixe, VII/1978, Campaña Brigada Tuxtepe, 4♂♂ (CEAM); Zaachila, 20/V/2000, Rafael Pérez Pacheco, Pasto, 1♀ (CEAM); 6 km W San Pedro Uchatengo, 13/VII/2004, 1,145 m, L. Cervantes, A. Delgado, C. Mayorga y G. Gamez, 16°20’7.8’’ N, 97°06’46.92’’ W, 1♂ (CNIN); Carr. Oaxaca-Pinotepa Nacional Rio de Arena, 14/VII/2004, L. Cervantes, A. Delgado, C. Mayorga y G. Gamez,16°20’06.66’’ N, 98°00’29.81’’ W, 1♀ (CNIN); San Luis Potosí. Km 9 carr. San Felipe Agua de Gamotes, 21/VIII/2008, 970 m, H. Brailovsky y E. Barrera, 21°49’16’’ N, 99°33’19’’ W, 1♂ (CNIN); Sonora. Municipio de Magdalena de Kino, 10/VIII/2013, 747 m, T. R. VanDevender y J. D. Palting, 30°37’19.31’’ N, 110°59’16.69’’ W, 1♀ (CNIN); Tabasco. Cárdenas, 5-9/IX/1979, J. M. Lobougle, 1♂ (CEAM); Camp. Exp., CSAT, Cárdenas, 8/XI/1976, A. Córdova G., 1♂ (CECT); Comalcalco, 13/XI/1976, 1♂ (CECT); Laguna del Rosario, Humanguillo, G 012, 18/II/1982, R. Lamas, 1♂ (CECT); Tapijulapa, 13/III/1982, F. Rivera, 1♂ (CECT); Veracruz. Campus Córdoba, 18/IX/1993, J. Vivas R., Saccharum officinarum L., Zea mays L., 1♀ (CEAM); Córdoba, 28/VI/2002, Jorge Maltos B., Saccharum officinarum L., 1♂ (CEAM); Estación Biológica Los Tuxtlas, San Andrés Tuxtlas, 21/IX/2007, 152 m, J. Romero Nápoles, 18°35’06.3’’ N, 95°04’30.9’’ W, 1♀, 2♂♂ (CEAM); Isla, 13/VII/2002, Magali Odi L., 1♀ (CEAM); Laguna de Catemaco, 23/IX/1961, F. Pacheco M., 1♂ (CEAM); Paso de Ovejas, 21/VI/1990, F. Hdz Vela, 1♂ (CEAM); Úrsulo Galván, 10/IX/2015, 30 m, Youssef Utrera Vélez, Saccharum officinarum L., Zea mays L., 19°23’36.79’’ N, 96°21’37.81’’ W, 3♂♂ (CEAM); Puente Nacional, 18/IX/1993, L. Rodríguez R., 1♂ (CECT); Cerro El Vigía, Santiago Tuxtla, 12/XI/1966, J. Ramos Elorduy de Conconi, 1♂ (CNIN); S. de Eyipantla, 19/VIII/1982, J. Butze, 1♂ (CNIN); Xalapa, 26/IX/1984, J. Peña, 1♀ (CNIN); Palma Sola, 21/VIII/1972, G. Haffter y P. Reyes, Vegetación pionera en arena, 1♀ (IEXA).
Hosts. Poaceae: Brachiaria fasciculata (Sw.) Parodi, Rottboellia cochinchinensis (Lour.) Clayton, Pennisetum purpureum Schumach., Echinochloa colona (L.) Link, Panicum maximum Jacq., Paspalum arundinaceum Poir., Eleusine indica (L.) Gaertn., and Compositae Synedrella nodiflora (L.) Gaertn (De la Cruz-Zapata et al., 2016). Martin et al. (1995) reported Poaceae: Cenchrus ciliaris L., Zea mays L., Oryza sativa L., Sorghum bicolor (L.) Moench, Cynodon plectostachyus (K. Schum.) Pilg., Cynodon dactylon (L.) Pers., Chloris gayana Kunth, Sorghum halepense (L.) Pers., Chondrosum eriopodum Torr., Paspalum notatum Flüggé (De la Cruz et al., 2016). In addition, Oomen (1975) reported Poaceae: Digitaria eriantha subsp. pentzii (Stent) Kok. In this study; Poaceae: Saccharum officinarum L., Dichanthium aristatum (Poir.) C.E. Hubb. Peck (1998) reported adult males on trees such as Ilex haberi (Lundell) W. J. Hahn (Aquifoliaceae) in Costa Rica. In this research a specimen was taken from the tree Delonix regia (Bojer ex Hook.) Raf. (Fabaceae), but it may have been a resting stray from grasses.
Remarks
The complete and visible tegminal lines (Fig. 12e), obscured but present in dark females, differentiate this form from congenerics. Other species have incomplete lines (P. teapana, P. chiapana) or no lines (P. ignifera) or the lines are red or orange, and narrow (P morelosi, P. inferens).
Prosapia inferens (Walker, 1858: 176)
Monecphora inferens Walker, 1858: 176.
Tomaspis inferans: Fennah, 1949: 606. Prosapia simulans inferens: Fennah, 1953: 343; Metcalf, 1961: 219.
Prosapia inferens: Hamilton, 1977: 629; Carvalho & Webb, 2005: 85.
Diagnosis. Length 9.3-10.1 mm. Head black; postclypeus rounded or slightly angled. Pronotum black, crossed by thin orange or reddish line. Scutellum black. Tegmina background black with 3 alternative ornamentation patterns: a) 2 transverse lines, one in basal third, other in distal third, basal line reddish, distal line yellowish; b) each tegmen with small reddish spot on basal third on the costa, yellowish line on distal third (Fig. 12f); c) tegmina completely black.
Taxonomic summary
Distribution. Campeche, Chiapas, Estado de México, Hidalgo, Morelos, Puebla, Tlaxcala, Veracruz.
Material examined. Campeche. Km 7 Carr Escarcega-Campeche, 5/VIII/1982, M. Garua, 1♀ (CNIN); Chiapas. Balum Canal, 56041 ECO-SC-E, Tenajapa, 16/VIII/1997, J. M. Anzueta y M. Meza, Zae mays L., 1♀ (CEFS); Tejenapa, 57261 ECO-SC-E, Tenajapa, 26/VIII/1996, C. Alvarado F. y S. Gómez-L., Zae mays L., 1♀ (CEFS); Finca Irlanda, Mpio Tuzantán, 4/VI/1974, 1,200 m, J. Hendrichs, Cafetal, 1♂ (CNIN); Ladera este El Sicilar, 10/VII/1993, S. Zaragoza, 1♂ (CNIN); Larraizal km 22 Bochil-Larraizal, 26/VI/2007, 1,857 m, C. Mayorga, G. Ortega y L. Cervantes, 16°55’04’’ N, 92°45’36’’ W, 2♀♀, 1♂ (CNIN); Rayón, 2L5/VI/2007, 1,712 m, C. Mayorga, L. Cervantes y G. Ortega, 17°11’36’’ N, 92°59’19’’ W, 1♀ (CNIN); Yashanal camino al Ojo de Agua, Tenajapa, 3/VIII/1991, C. Mayorga, 2♀♀ (CNIN); Estado de México. Km 65 carr. Toluca-Temascaltepec, 3/IX/2015, 1,840 m, U. Castro-Valderrama, Pasto, 19°3’0.36’’ N, 100°1’53.29’’ W, 1♂ (CEAM); Hidalgo. Km 11 carr. Ixtlahuaco Calnali, 30/VIII/1999, 1500 m, E. Barrera, 20°52’64’’ N, 98°39’30’’ W, 1♂ (CNIN); Jalisco. Ciudad Guzmán, 17/VIII/1996, 1460 m, C. Sánchez y J. Vargas, 1♂ (CNIN); MICHOACÁN. Aguililla, 1/VIII/1985, R. Barba y F. Arias, 1♂ (CNIN); Morelos. Tepoztlán, 19/ VIII/1995, J. Cisneros H., Maleza, 1♂ (CEAM); Cañón de Lobos, 9/IX/1976, J. M. Pino, 1♂ (CNIN); Puebla. Atlixco, 17/VII/1963, F. Pacheco M., 1♂ (CEAM); Ciudad de Puebla, 11/IX/2009, A. Brailovsky y E. Barrera, 1♂ (CNIN); Hidroeléctrica Tlachichilco 1er Malacate, 11/VII/1984, G. Ortega y E. Barrera, 1♀ (CNIN); Huahuchinango, 3/VIII/1983, A. Ibarra, 1♀ (CNIN); San Miguel, Presa Necaxa, 1/VIII/1983, A. Ibarra, 3♀♀, 4♂♂ (CNIN); Tlaxcala. 1 km de Nativitas, 10/IX/1994, J. Vivas R., Medicago sativa L., 1♂ (CEAM); Veracruz. Ciudad Mendoza, 15/IX/1994, Luévano y Calderón, 1♀ (CEAM); Mendoza, 16/IX/1994, F. Santos G., Maleza, 1♂ (CEAM); Nogales, 17/IX/1994, H. Taddel M., Arbusto, 1♀ (CEAM); Tecolutla, 20/VI/1965, J. Kikushima, 1♂ (CEAM); Estación Biológica Los Tuxtlas, San Andrés Tuxtlas, 18/VI/1985, P. Sinaca, 1♂ (CNIN); Jalapa, 12/V/1985, J. Peña, 2♂♂ (CNIN).
Hosts. Poaceae: Pennisetum spp., Zea mays L. and probably other grasses. Fabaceae: Medicago sativa L.
Remarks
Color pattern of tegmina is diagnostic.
Prosapia morelosi Castro-Valderrama, Romero, Carvalho, 2020: 290, figs. 1A-I, 2A-F
Diagnosis. Length 9.7-10.6 mm. Head black; postclypeus slightly angled or rounded postclypeus. Pronotum black, ornamented by thin or wide, complete or incomplete coral red line. Scutellum black. Tegmina background black, ornamented with variable patterns: a) 2 reddish transverse lines (Fig. 12g); b) one of the 2 absent, usually the basal line (Fig. 12h), c) tegmina completely black.
Distribution Taxonomic summary Distribution. Michoacán, San Luís Potosí.
Material examined. Michoacán. Morelia, 13/VIII/1962, F. Pacheco M., 1♂ holotype (CNIN); Tangamandapio, Bordo Grande, Parcela Macedonio Campos, 14/IX/2017, 1,732 m, U. Castro y A. Manzo, Paspalum sp., 19°55’55.83’’ N, 102°23’34.41’’ W, 1♀ allotype (CNIN). Carr. Libre Maravario-Morelia, 1 km antes de Ucare, 13/IX/2017, 2,469 m, U. Castro y A. Manzo, Pennisetum clandestinum Hochst. ex Chiov., 19°52’45.02’’ N, 100°41’10.3’’ W, 8♀♀, 51♂♂, paratypes (CEAM); Carr. Libre MaravarioMorelia, km 85+400, entrada Huanimoro, 13/IX/2017, 2,149 m, U. Castro y A. Manzo, Ciperaceae, 19°54’48.31’’ N, 100°30’01.54’’ W, 16♀♀, 13♂♂, paratypes (CEAM); El Platanal, X/1992, C. O. Cabrera J., “Olleto”, 1♀ paratype (CEAM); Los Reyes, 11/X/1992, L. Arteaga, Maleza, 1♂, paratype (CEAM); Morelia, 13/VIII/1962, F. Pacheco M., 6♂♂, paratype (CEAM); Tangamandapio, Bordo Key to Mexican species of Prosapia Fennah, 1949 Grande, Parcela Macedonio Campos, 14/IX/2017, 1,732 m, U. Castro y A. Manzo, Paspalum sp., 19°55’55.83’’ N, 102°23’34.41’’ W, 40♀♀, 3♂♂, paratype (CEAM); km 126 carr. Maravatio-Morelia, 16/IX/1987, R. Barba, 1♂, paratype (CNIN); Santa Mónica km 96 carr. MaravatioMorelia, 16/IX/1987, E. Barrera V., 1 ♂. San Luis Potosí. Tamazunchale, 7/IV/1998, Y. Sánchez M., Fabaceae, 1♂, paratype (CEAM)
Hosts. Poaceae: Pennisetum clandestinum Hochst. ex Chiov., Paspalum spp. and Cyperaceae: Cyperus sp. One collector (Y. Sánchez M.) reported Fabaceae.
Remarks
The black variant of P. morelosi is similar to that of P. ignifera Hamilton, 1977. Since the species are sympatric, identification might be difficult. If so, the tips of the subgenital plates allow differentiation: the subgenital plates of P. morelosi are straight while those of P. ignifera are curved. Prosapia morelosi is also similar to P. inferens, but the aedeagus of P. morelosi is slightly c-shaped, while in P. inferens it is straight (Castro-Valderrama et al., 2020).
Key to Mexican species of Prosapia Fennah, 1949
Note. The key should be used on a series of specimens rather than a single specimen along with geographic information and photographs of the species. This is true of the completely black variants of P. ignifera, P. inferens, and P. morelosi.
1. Tegmen with brown or yellow basal third.......................................................................................................................2
1´. Tegmen basal third black or dark brown........................................................................................................................3
2. Basal third of tegmen brown, apical 2-thirds red...............................................................P. hemelytra Hamilton, 1977
2´. Tegmen with alternating yellow and black bands of almost the same width or the tegmen completely yellow (Fig. 12a)................................................................................................................................................P. isobar Hamilton, 1977
3. Tegmen with triangular spots on the costal vein.............................................................................................................4
3´. Tegmen, usually with 2 complete or interrupted transverse lines, sometimes one absent, or tegmen completely black......................................................................................................................................................................................5
4. Tegmen black, 2 red triangular spots on costal vein, one in basal third, one in distal third (Fig. 11b), or tegmen completely black.........................................................................................................................P. ignifera Hamilton, 1977
4´. Tegmen dark brown to black background; on basal third 1 triangular spot on costa vein and 2 almost round; on distal third 1 triangular spot on costa vein and one almost round. Triangular or rounded red or white spots (Fig. 12c)...........................................................................................................................................P. chiapana Hamilton, 1977
5. Tegmen background dark brown to black, 1 or 2 interrupted thin transverse lines, one red or pale yellow in the basal third, reddish or pale yellow on the distal third (Fig. 12d), or sometimes in distal third only a triangular spot on the costa vein...............................................................................................................................................P. teapana (Fennah, 1953)
5´. Tegmen background black, 1 or 2 complete transverse lines........................................................................................6
6. Tegmen with a dark brown to black background, 2 yellow or yellowish transverse lines, one in the basal third and one in the distal third, usually distal line thinner (Fig. 12e); in female, both lines thinner than males and obscured or faint in dark speciemens.............................................................................................................P. simulans (Walker, 1858)
6´. Tegmen black with 2 lines narrower than P. simulans, or one line absent, or tegmen completely black...................7
7. Tegmen background black with 3 alternative patterns: a) 2 transverse lines, one in basal third and other in the distal third, basal line reddish, distal line yellowish; b) small reddish spot in basal third on the costa vein and yellowish line on the distal third (Fig. 12f); c) tegmen completely black.........................................................P. inferens (Walker, 1858)
7´. Tegmen background black with alternative patterns: a) 2 reddish transverse lines (Fig. 12g); b) one line absent, usually the basal line (Fig. 12h), c) tegmen completely black....................................P. morelosi Castro-Valderrama et al., 2018
Aeneolamia Fennah, 1949: 608
Aeneolamia Metcalf, 1961: 133; Fennah, 1968: 178; Carvalho & Webb, 2005: 49; Paladini et al., 2015: 84.
Diagnosis. Length 6.5-8.7 mm. Head black to dark brown; postclypeus rounded. Pronotum and scutellum black. Tegmina background dark brown to black, ornamented with 2 yellowish, orange, white, brown or red transverse lines, one in basal third, other line in distal third, the basal line markedly oblique (Figs. 13, 14). If lines brown, then barely visible or faint (Fig. 14), some species and subspecies have lines on claval edges of tegmina that form a “v” bordering the pronotum and scutellum (Fig. 13a-c). Subgenital plates distally oblique and truncated (Fig. 15a) or distally acute (Fig. 15b)
Taxonomic summary
Distribution. Campeche, Chiapas, Chihuahua, Guanajuato, Guerrero, Hidalgo, Jalisco, Michoacán, Mexico, Morelos, Nayarit, Nuevo León, Oaxaca, Puebla, Quintana Roo, San Luis Potosí, Sonora, Tabasco, Tamaulipas, Yucatán, Veracruz (Fig. 17a).
Hosts: Poaceae: Saccharum officinarum L. and forage grasses, often pests (García-González et al., 2017; Martin et al., 1995; Morales-Pérez et al., 2014; Oomen, 1975). Adults are occasionally found on dicotyledonous plants, but they are not economically important on these hosts.
Aeneolamia contigua (Walker, 1851: 670) Triecphora contigua Walker, 1851: 670. Aeneolamia postica contigua: Fennah, 1953: 350; Metcalf, 1961: 139.
Aeneolamia postica santae-rosae: Fennah, 1953: 351; Metcalf, 1961: 140; Clark et al, 1976: 21.
Aeneolamia contigua: Fennah, 1968: 178; Clark et al., 1976: 21; Carvalho & Webb, 2005: 50.
Diagnosis. Length 6.5-8.5 mm. Head black; postclypeus rounded. Pronotum and scutellum black. Tegmina background dark brown to black, ornamented with 1 or 2 orange transverse lines (Fig. 13a), sometimes with orange lines on the claval edges that form a “v” (Fig. 13a). Subgenital plates are oblique and truncated (Fig. 15a).
Taxonomic summary
Distribution. Nuevo León, Tamaulipas, San Luis Potosí, Veracruz, Tabasco, Campeche, Yucatán, Quintana Roo, Puebla, Hidalgo. Likewise, on the Pacific coasts in the states of Sinaloa, Sonora, Colima, Nayarit, Guerrero, Oaxaca and Chiapas (López-Collado & Pérez-Aguilar, 2012), Chihuahua, Jalisco, Michoacán, Morelos.
Material examined. Holotype, 1♂, Honduras (BMNH). Campeche. Candelaria, 15/V/2001, Mario A. Poot, pasto, 1♂ (CEAM); El Tormento, 13/VI/1985, V. Arias, H. Velasco y V. Vértiz, 1♀, 1♂ (CNIN). Quintana Roo. Km 146 carr Chetumal-Cancun, 16/VIII/1982, V. Melendez, 1♀ (CNIN). Chiapas. Rancho San Ramón, Villaflores, 30/VIII/2008, Isaac Espinosa Velásquez, Phaseolus vulgaris L., 1♂ (CACH); Revolución Mexicana, 06/ XI/2008, Javier Escobar M., pasto, 1♂ (CACH); S. P. Buenavista, 01/XI/2008, B. D. Reyes R., Glycine max (L.) Merr., 1♂ (CACH); San Ramón, Villaflores, 5/X/1998, J. C. Abadia G., Zae mays L., 1♂ (CACH); San Sebastián Socoltena, 31/X/2009, Moreno M. B. Orbey, Zae mays L., 1♀ (CACH); UNACH, Villaflores, 12/XI/2005, Cynthia Paola Galdamez Figueroa, 1♂ (CACH); Villa Corzo, 15/IX/2005, Manuel A. Cristancho y León R. Lara, Zae mays L., 1♀ (CACH); Villacorzo, Santa Fe, 22/X/2008, Samuel Gómez, Cynodon nlemfuensis Vanderyst, 1♂ (CACH); Benito Juárez, El Plan, Cacahoatán, 3/VI/2013, O. Mikery, 1♂ (CCFT); Llano La Lima, Tapachula, 9/VI/2013, R. Muñoz, 1♀ (CCFT); Reserva El Triunfo Campamento, 14/V/2005, C. Pérez, 1♂ (CCFT); Tapachula, Col. Las Delicias, 09/VI/2012, E. Díaz, 1♂ (CCFT); km 35 carrtera Amacohite-Rómulo Calzada, 24/IX/2007, 230 m, J. Nápoles, 17°23’03.7’’ N, 93°32’48.4’’ W, 1♂ (CEAM); Reserva El Ocote, Las Palmas, 24337 ECO-SC-E, Ocozocoautla, 7/VII/1994, O. Gómez-N., 1♀ (CEFS); Reserva Montes Azules, Ocosingo Chajul, 5/V/1986, F. Arias, R. Barba y L. Cervantes, 1♀ (CNIN). Guerrero. Almolonga, 30/VII/1962, F. Pacheco M., 1♀, 3♂♂ (CEAM). Jalisco. Ameca, 23/VII/1999, A. Miranda A., 1♂ (CEAM). Michoacán. Morelia, 1/VI/1963, F. Pacheco M., 1♂ (CEAM). Morelos. Cuautla, 6/II/1996, S. López H., 1♀, 1♂ (CEAM); Reserva de la Biosfera Huahutla, 27/V/2000, 1,090 m, J. Romero Nápoles, 18°28’26’’ N, 99°02’27’’ W, 14♀♀, 2♂♂ (CEAM); San Isidro, Yautepec, 29/IX/1993, 1,100 m, Víctor R. Castrejón, Weed, 1♂ (CEAM). Nayarit. Guayabitos, 2/X/1980, Jorge Valdez, Weed, 1♀ (CEAM). Oaxaca. Tehuantepec, 10/VII/1966, Alberto Ortiz B., 1♀ (CEAM); Tuxtepec, VIII/1978, Brigada M. P. Tuxtepec, 1♂ (CEAM). Quintana Roo. 2 km S Rancho El 24, 20/XII/1984, O. Canul, 1♂ (CNIN); Cancún, 11/VIII/1982, V. Hernández, 1♀ (CNIN); Felipe Carrillo Puerto, Buenavista, 22/VI/1989, A. Cadena y L. Cervantes, 1♀, 2♂♂ (CNIN); Pino Suárez, 19/VI/1984, V. Hernández, 1♂ (CNIN); Pto. Morelos, 11/VIII/1982, V. Meléndez, 1♀ (CNIN); Rancho El 24, 21/XII/1983, O. Canul, 2♀♀, 1♂ (CNIN); Rancho San Isidro, Pto Morelos, 13/VIII/1982, V. Hernández, 1♀ (CNIN). Tabasco. Cárdenas, 14/VII/1994, S. Sánchez S., 1♂ (CEAM); 20 km W Cárdenas, 21/VII/1980, D. Letourneau, Zea mays L., Phaesolus vulgaris L., Cucurbita sp.1♂ (CECT); Bolanegra, 4/X/1976, A. López B., 1♀ (CECT); Campo Exp. CSAT, Cárdenas, 9/XII/1977, A. González Hdz, 1♀ (CECT); Cunduacan, 27/VII/1982, F. Rivera, 1♂ (CECT); Huimanguillo, 17/VII/1982, F. Rivera, 1♀ (CECT); Jardín Botánico, Cárdenas, 16/VII/1974, A. González Hdz, 1♀, 1♂ (CECT); Laguna del Rosario, Humanguillo, 18/II/1982, R. Lamas, 1♀, 1♂ (CECT); Mecatepc, 5/III/1982, R. Murillo, 1♀, 1♂ (CECT); Paraíso, 23/X/1976, R. Rómulo, 1♀ (CECT); Tapijulapa, 13/III/1982, F. Rivera, 1♀ (CECT). Veracruz. Cd. Mendoza, 15/IX/1994, Liévano y Calderón, Weed, 1♀, 1♂ (CEAM); Colegio de Postgraduados, Campus Córdoba, 15/IX/1994, G. Pérez S., Saccharum officinarum L., Zea mays L., 1♀, (CEAM); Cosamaloapan, 20/VII/1962, Hiram Bravo M., 3♀♀, 1♂ (CEAM); Córdoba, 24/VII/1998, R. Lucio, Weed, 1♂ (CEAM); El Encanto, 19/VI/1965, J. Kikushima, 1♂ (CEAM); Estación Biológica Los Tuxtlas, San Andrés Tuxtlas, 21/IX/2007, 152 m, J. Romero Nápoles, 18°35’06.3’’ N, 95°04’30.9’’ W, 1♂ (CEAM); Isla, 13/VII/2002, Magali Odi L., 2♀♀ (CEAM); km 14 Aut. Cárdenas-Minatitlán, Rancho La Majada, 4/X/2016, 9 m, U. Castro y J. Romero N., Brachiaria sp., 18°03’0.7’’ N, 94°05’0.27’’ W, 10♀♀, 5♂♂ (CEAM); km 18 Aut. Minatitlán-Acayucan, 4/X/2016, 19 m, U. Castro y J. Romero N., Dichanthium aristatum (Poir.) C.E.Hubb., 17°55’43.9’’ N, 94°46’17.3’’ W, 12♀♀, 2♂♂ (CEAM); La Antigua, 18/VIII/1978, Brigada M. P. Jalapa, Cynodon nlemfuensis Vanderyst, 1♀ (CEAM); Tinája, 18/IX/1994, F. Santos G., 1♂ (CEAM); Tlacotepec de Mejía, 15/VII/2016, 19♀♀, 37♂♂ (CEAM); Uxpanapa, 6/IX/1978, Cynodon nlemfuensis Vanderyst, 1♂ (CEAM); Villa Juárez, 20/VI/1965, J. Kikushima, 1♀ (CEAM); Estación Biológica Los Tuxtlas, San Andrés Tuxtlas, 18/VI/1985, P. Sinaca, 1♂ (CNIN). Yucatán. Chichen Itza, 20/VI/1985, F. Arias, R. Barba y L. Cervantes, 1♀, 1♂ (CNIN); Cuncunul, 13/IX/1994, 60 m, E. Barrera, 20°38’19.26’’ N, 88°19’8.08’’ W, 1♀ (CNIN).
Hosts. Poaceae: Brachiaria fasciculata (Sw.) Parodi, Rottboellia cochinchinensis (Lour.) Clayton, Pennisetum purpureum Schumach., Echinochloa colona (L.) Link, Panicum maximum Jacq., Eleusine indica (L.) Gaertn and Saccharum officinarum L. (De la Cruz et al., 2016). In addition, Cynodon nlemfuensis Vanderyst, Dichanthium aristatum (Poir.) C.E. Hubb., Zea mays L., and, at low frequency, adults on dicotyledons such as Fabaceae: Glycine max (L.) Merr., and Phaseolus vulgaris L.; Euphorbiaceae: Jatropa sp.; Asteraceae: Helianthus annuus L.
Remarks
This species differs from the similar A. albofasciata (Lallemand, 1939) in body color and deailed of ornamentation of the tegmina (Fig. 13a, c, d). In addition, the subgenital plates are oblique-truncated (Fig. 15a), while those of A. albofasciata are acute (Fig. 15b).
Aeneolamia contigua campecheana (Fennah, 1951: 138) Aeneolamia postica campecheana Fennah, 1951: 138, 1953: 350; Metcalf, 1961: 139.
Aeneolamia contigua campecheana Clark et al., 1976: 21; Carvalho & Webb, 2005: 50.
Diagnosis. Length 6.5-8.5 mm. Head black; postclypeus rounded. Pronotum and scutellum black. Tegmina with narrow oblique transverse basal line, interrupted at claval suture, distal line straight (Fig. 13b); lines dark orange, red, or dark red; sometimes so dark in the female as to be almost obscured (Fennah 1951), with red lines on the costal edges that form a “v” (Fig. 13b). In addition, the subgenital plates are oblique and truncated (Fig. 15a).
Taxonomic summary
Distribution. Campeche (Fennah, 1951), Quintana Roo and Yucatán.
Material examined. Quintana Roo. Tecnológico de Chetumal, 15/V/2001, R. Chame, Grass, 1♂ (CEAM).
Host. Poaceae: Saccharum officinarum L (Fennah, 1951).
Remarks
The red color of the tegminal markings (Fig. 13b) distinguish this form from the conspecific typical form (Fig. 13a). It appears to be a limited distribution polymorphism in Quintana Roo and Campeche.
Aeneolamia albofasciata (Lallemand, 1939: 59) Monecphora albofasciata Lallemand, 1939: 59; Metcalf, 1961: 224.
Aeneolamia albofasciata: Fennah, 1968: 179; Clark et al., 1976: 21; Carvalho & Webb, 2005: 49.
Diagnosis. Length 6.8-8.7 mm. Head dark brown to black; postclypeus rounded. Pronotum and scutellum black. Tegmina background dark brown to black, ornamented with 1 or 2 yellowish or white transverse lines (Fig. 13c, d), that are sometimes accompanied by lines on the claval edges of the tegmina that form a “v” (Fig. 13c), though the “v” is not as evident as in A. contigua (Walker, 1851) (Fig. 13a) (see above). When the tegmina are dark brown, the lines are usually yellowish (Fig. 13c), when black, the lines are white (Fig. 14d). The subgenital plates are acute (Fig. 15b).
Taxonomic summary
Distribution. Tamaulipas, Nuevo León, San Luis Potosí, Veracruz, Hidalgo Puebla, Tabasco, Campeche, Yucatán, Quintana Roo, Chiapas, Oaxaca, Jalisco, Guerrero, Colima, Nayarit, Sinaloa y Sonora (López Collado & Pérez-Aguilar, 2012). Guanajuato, Morelos.
Material examined. Sintype, 1♀, Guadalajara, Mexico (BMNH). Campeche. Bolonchén de Rejón, 15/VI/1985, F. Arias, 1♂ (CNIN). Haltunchen, km 159.5 Ciudad de Carmen-Champotón, 2/X/2016, 7 m, U. Castro y J. Romero N., Panicum maximun Jacq., 19°29’57.4’’ N, 90°42’02.8’’ W, 10♀♀, 100♂♂ (CEAM). Chiapas. Comunidad Providencia, km 7.9 Carr. fed. Ocozingo-Altamirano, 6/VI/2011, J. Romero Nápoles, 16°28’26’’ N, 92°02’27’’ W, 8♀♀, 3♂♂ (CEAM); Guanajuato. Irapuato, 6/V/2002, Talina Santiago, 1♂ (CEAM). Guerrero. Petaquillas 9 km W Chilpancingo, 6/VI/1963, S. López B., 1♂ (CEAM). Michiacán. Charapendo, Carr. libre Uruapan-Lombardía, 18/VIII/2015, 1,018 m, Paul Méndez, 19°26’34.54’’ N, 102°06’34.40’’ W, 1♀ (CEAM); Tangamandapio, Borde Grande, parcela José Luis González, 14/IX/2017, 1,739 m, U. Castro y A. Manzo, Cynodon dactylon (L.) Pers., 19°55’54.25’’ N, 102°23’27.54’’ W, 7♀♀, 13♂♂ (CEAM); Taretan, 13/IX/1963, A. Sáenz, 2♀♀ (CEAM); Uruapan, VII/1998, G. Ascencio B., Maleza, 1♂ (CEAM). Morelos. 1.5 km N Est. Microondas, Reserva Huautla, 3/VII/2000, 1,645 m, J. Romero Nápoles, 18°27’51’’ N, 99°16’30’’ W, 1♀ (CEAM); Cuautla, 14/IX/1980, 1,250 m, Felipe Peralta, 1♀, 2♂♂ (CEAM); Temixco, 2/VII/1961, F. Pacheco M., 6♀♀, 3♂♂ (CEAM); Tétela del Volcán, 12/X/1987, V. Domínguez, 1♀ (CEAM); Cuernavaca, 2/IX/1984, Oryza sativa L., 1♂ (CLPV); Emiliano Zapata, 17/IX/1988, García, Oryza sativa L., 1♀, 1♂ (CLPV); Puente de Ixtla, 2/X/1988, Campos, Medicago sativa L., 1♂ (CLPV); Zacatepec, 12/IX/1984, Medicago sativa L., 5♂♂ (CLPV). Nuevo León. Apodaca, 4/VIII/1979, Cenchrus ciliaris L., 1♂ (CECT); Oaxaca. I. Bastida, 12/IX/1981, R. Bújanos, 1♀ (CEAM). PUEBLA. Microondas, Xuchiapa, 11 km SE Izucar de Matamoros, 1♂ (CEAM). San Luis Potosí. RB El Abra, Sitio 1, 5/IX/2017, E. Chamé, 1♀, 1♂ (CCFT). Sonora. Km 32 HermosilloSta Ana, 2/X/2020, 445 m, U. Castro-Valderrama, Cenchrus ciliaris L., 29°22’12.30’’ N, 110°58’16.67’’ W, 2♀♀, 5♂♂, SON-1 (CEAM); km 153 HermosilloSta Ana, 2/X/2020, 733 m, U. Castro-Valderrama, Cenchrus ciliaris L., 30°25’43.41’’ N, 111°05’20.68’’ W, 10♀, 9♂♂, SON-2 (CEAM). Sinaloa. Culiacán, 22/IX/1999, Primitivo Pérez Pérez, 1♀ (CEAM). TABASCO. Frontera, 1,103, 10/VI/1983, R. Murillo, 1♀ (CECT). Tamaulipas. Cd. Mante, 7/X/1983, 90 m, R. Garza, 1♀, 3♂♂ (CEAM); Camino al ejido Azteca, Gómez Farías, 18/IX/2003, Q. Santiago y L. Cervantes, Colecta nocturna, 2♂♂ (IEXA). Veracruz. 20 km de Cd. Cardel, carr. a Nautla, Superv. Gral de Campo, Cynodon nlemfuensis Vanderyst, 1♂ (CEAM); Coazintla, 2/VIII/1978, Brigada Gutiérrez Zamora, Digitaria eriantha Steud., 2♀♀, 1♂ (CEAM); Colegio de Postgraduados, Campus Córdoba, 10/VI/2000, Celina Palacios M., Saccharum officinarum L., 1♂ (CEAM); Estación Biológica Los Tuxtlas, San Andrés Tuxtlas, 29/VIII/2017, 230 m, J. Romero Nápoles, 18°35’14.7’’ N, 95°04’37.2’’ W, 1♀ (CEAM); Poza Rica, 6/IX/1973, William Olarte E., 1♀ (CEAM); km 4.5 Carr. Cardel-Salmoral, 4/IX/2003, 20 m, Youssef Utrera Vélez, Saccharum officinarum L., 19°23’33.95’’ N, 96°21’43.67’’ W, 1♂ (CEAM); Palma Sola, 2/X/1973, J. Mateu, C. Huertas y S. Gómez, Vegetación pionera 2♂♂ (IEXA); San Jóse de Tapia, Córdoba, 15/VII/2013, J. Refugio Lomelí F., 1♂ (CEAM). YUCATÁN. Uxmal, 11/IX/1994, E. Barrera, 20°21’0.97’’ N, 89°47’5.74’’ W, 2♂♂ (CNIN).
Hosts. Poaceae: Saccharum officinarum L., Digitaria eriantha Steud., Panicum maximum Jacq., Cynodon dactylon (L.) Pers., C. nlemfuensis Vanderyst, Paspalum sp., Oryza sativa L., Cenchrus ciliaris L. The hosts are generally grasses, but occasionally adults are found on dicotyledonous plants such as Fabaceae: Medicago sativa L.
Remarks
See remarks of A. contigua.
Aeneolamia aff. albofasciata (Lallemand, 1939).
Material examined. Oaxaca. Sola de Vega, 28/IX/2003, 1,715 m, U. Castro-Valderrama and Youssef Utrera Vélez, Paspalum sp., 16°27’44.48’’ N, 97°1’25.73’’ W, 33♀♀, 15♂♂ (CEAM); 5 km W San Martín Lachila, Mpio Zimatlán, 12/VII/2004, L. Cervantes, A. Delgado, C. Mayorga, S. Gámez, 16°35’39.18’’ N, 96°52’14.16’’ W, 1♀ (CNIN).
Taxonomic summary
Distribution. This species is found in the highlands of the State of Oaxaca. Especially, the “Central Valley” and the mountains between Sola de Vega and the city of Oaxaca.
Host. Poaceae: Paspalum sp.
Remarks
This species is in the process of description by some of the authors; provisionally, it is called Aeneolamia aff. albofasciata (Lallemand 1939) (Fig. 14). The subgenital plates are acute, like those of A. albofasciata (Fig. 15b). The locality, San Martín Lachila, is a Municipality and is not part of the Zimatlán Municipality.
Finally, 2 additional subspecies are mentioned in passing: A. contigua postica (Walker 1858: 177) (see Carvalho & Webb (2005): figure 660), Veracruz, from around the Orizaba volcano, and A. contigua sanctaerosae Fennah, 1953: 351, from Yucatán, in grasses (Fennah, 1953) (see Carvalho & Webb (2005): figure 371). Sideby-side comparison of the BMNH A. postica santaerosae and A. contigua holotypes strongly suggested that A. p. sanctaerosae, described from specimens from sugar cane in Sta. Rosa, Yucatán, is a variant of A. contigua. The same was true for A. contigua postica. Aeneolamia contigua exhibits geographic color variation and occasional color polymorphism, with the tegminal markings ranging from light orange to deep red. Orange is the predominant and typical form in most of Mexico and is the only form present in Costa Rica (Thompson & León, 2005). Forms with redder markings are common in parts of Nicaragua, Honduras, Belize, and adjacent areas. Following Clark et al. (1976) and Carvalho & Webb (2005), all of these variant color forms, some of which have been given subspecific status, are subsumed under A. contigua, with the possibility that, with further study, one or more might eventually prove to represent distinct taxa.
Key to Mexican species and subspecies of Aeneolamia Fennah, 1949
1´. Apex of subgenital plates obliquely truncate (Fig. 15a)................................................................................................2
1. Apex of the subgenital plates acute (Fig. 15b)................................................................................................................3
2. Tegmina background dark brown to black, ornamented with 1 or 2 orange transverse lines (Fig. 13a), sometimes with orange lines on the claval edges that form a “v” (Fig. 13a)...................................................A. contigua (Walker, 1851)*
2´. Tegmina background black with narrow oblique transverse basal line, interrupted at claval suture, distal line straight (Fig. 13b); lines sometimes so dark red in females as to be almost obscured.................................................................................................................A. contigua campecheana Fennah, 1951
3. Tegmina background dark brown to black, ornamented with 1 or 2 yellowish or white transverse lines (Fig. 13c, d), sometimes accompanied by lines on the claval edges that form a “v” (Fig. 13c)..........................................................................................................................A. albofasciata (Lallemand, 1939)
3´. Tegmina background dark brown to black, ornamented with 1 or 2 transverse lines, barely visible or faint or incomplete (Fig. 13) ................................................................................................................Aeneolamia aff. albofasciata
Genus incertae sedis Hamilton, 2016: 248
Olcotomaspis Lallemand, 1949: 29 (s. str. Hamilton, 2016: 243)
Olcotomaspis (Olcotomaspis) s. str. Hamilton, 2016: 243.
Olcotomaspis (Hyalotomaspis) Hamilton, 2016: 243.
Diagnosis. Length 6.5-8.7 mm. Head reddish brown or orange the anterior area and posterior black; postclypeus reddish brown or orange and rounded. Pronotum orange, orange brown or reddish brown and lateral margin whitish or black. Scutellum orange or reddish brown. Tegmen background dark brown with a whitish spot in costa vein or venation prominent on hyaline tegmen. Hamilton (2016) reported that the mesosternum has transversely ridged prominences, but actually appears triangular (Fig. 16b, d).
Hosts: Unknown.
Taxonomic summary
Distribution. This genus has 3 species distributed from Guatemala and southeastern Mexico. The type species is Olcotomaspis versicolor (Lallemand, 1927) from Guatemala.
Olcotomaspis (Olcotomaspis) laterinotata (Fowler 1897: 184, plate XI, Fig. 22a.)
Tomaspis laterinotata Fowler, 1897: 184; Metcalf, 1961: 101.
Incertae sedis laterinotata: Carvalho & Webb, 2005: 107.
Olcotomaspis (=Tomaspis) laterinotata: Hamilton, 2016: 244.
Olcotomaspis (Olcotomaspis) laterinotata: Hamilton, 2016: 248.
Diagnosis. Length 9.5 mm. Head reddish brown; postclypeus rounded. Pronotum orange brown or with anterior area reddish brown and lateral margin whitish. Scutellum orange or reddish brown. Although Hamilton (2016) generically defines the anterior margin of the scutellum as “usually nearly transverse”, it is slightly convex in this specimen revised (Fig. 16a), in contrast to his illustration of the same species (see Hamilton (2016): fig 6B). Tegmen background light brown and a triangular white lateral spot above the first third of the costal vein (Fig. 16).
Taxonomic summary
Distribution. Oaxaca (Fig.17f).
Type material. Female holotype deposited at HEC (Hope Entomological Collection, Oxford University Museum, Oxford, UK), Not examined in this research.
Material examined. Oaxaca. La Esperanza, 10/II/1982, A. Ibarra, 1♀ (CNIN).
Host. Unknown.
Remarks
The white triangular lateral spot in the first third of the costal margin differentiates this species from the other species of Cercopidae.
Olcotomaspis (Hyalotomaspis) clarissa (Jacobi, 1921: 41)**
Tomaspis clarissa Jacobi, 1921: 41; Metcalf, 1961: 117. Hyalotomaspis clarissa: Lallemand 1949: 30; Carvalho & Webb, 2005: 62; Hamilton, 2016: 206.
O. (=Hyalotomaspis) clarissa: Hamilton, 2016: 235 Olcotomaspis (Hyalotomaspis) clarissa: Hamilton, 2016: 248.
Diagnosis. Length 10 mm. Head orange the anterior area and posterior black; postclypeus rounded. Pronotum orange, with a black median line joining the black posterior margin. Scutellum orange with black margins. Tegmina hyaline with conspicuous veins and cells, without spots or lines (see Carvalho & Webb (2005): figure 330; Hamilton (2016): figure 7A).
Taxonomic summary
Distribution. Jacobi (1921) reported the species at Xalapa, Veracruz.
Type material. 3♂♂, syntypes (MTD = Museum für Tierkunde, Dresden, Germany), 1♂ (BMNH) (Carvalho & Webb (2005)). Not examined in this research.
Host. Unknown.
Remarks
It is differentiated from other Cercopidae by the black central line attached to the posterior margin of the pronotum, and the hyaline but conspicupously veined tegmina.
Key to species Olcotomaspis Lallemand, 1949
1. Tegmina background light brown, each ornamented with triangular white lateral spot above first third of costal margin (Fig. 16)..........................................................................................................O. (Olcotomaspis) laterinotata Fowler, 1897
1´. Tegmina hyaline with conspicuous veins and cells, without spots or lines (see Hamilton (2016): fig. 7).................................................................................................................O. (Hyalotomaspis) clarissa (Jacobi, 1921)
Distribution of the genera of Cercopidae in Mexico
Members of Aeneolamia and Prosapia are distributed along the Mexican coasts from Belize to the United States on the Gulf coast and from Guatemala to the State of Sonora on the Pacific coast. Some species are sympatric and inhabit lowlands on sugar cane and forage grasses for cattle. According to SIAP (2020), the sugar cane producing states are Campeche, Chiapas, Colima, Jalisco, Michoacán, Morelos, Nayarit, Oaxaca, Puebla, Quintana Roo, San Luis Potosí, Sinaloa, Tabasco, Tamaulipas and Veracruz, states where the presence of Aeneolamia and Prosapia species is confirmed by records in this study. In addition, our results indicate that species of both genera also are found in the valleys, mountains and plains of Sonora, Chihuahua, Nuevo León, Durango, Guerrero, Estado de México, Queretaro, Guanajuato and Hidalgo states (Fig. 17a, b). These genera occur in the southernmost USA very near the border with Mexico (VT, observations) and are widely distributed south to Panama (Carvalho & Webb, 2005). Aeneolamia is also distributed in northern South America and Prosapia simulans has been reported from Colombia y Venezuela (Peck et al., 2001).
Huaina inca, the only species in the monotypic genus Huaina, is distributed as far north as Chihuahua (Chihuahua City) and as far south as Tapachula. It is found in the highlands of the Sierra Madre Oriental (near Saltillo), Sierra Madre Occidental (near Ameca, Jalisco) and converges in the Trans-Mexican Volcanic Belt, as well as in lowlands including the Pacific and Gulf Mexican plains (Guerrero, Oaxaca, Veracruz). It is also found in Chihuahua, Coahuila, Tamaulipas, San Luis Potosí, Hidalgo, Nayarit, Jalisco, Michoacán, Guerrero, Estado de México, Morelos, Puebla, Oaxaca, Veracruz and Chiapas (Fig. 17d) and probably in Sonora (see References: Naturalista, 2020). The species distribution ranges south to Costa Rica.
Ocoaxo is the most diverse genus of Cercopidae in Mexico. It ranges from the north in the Sierra Madre Occidental in Chihuahua (Areponapochi), the Sierra Madre Oriental in Nuevo León (Cerro Chipinque), the Trans-Mexican Volcanic Belt, the Sierra Madre del Sur and the Sierra Madre de Chiapas to the lowlands of the Yucatán plains. It is also found in Chihuahua, Nuevo León, Sinaloa, Tamaulipas, San Luis Potosí, Guanajuato, Queretaro, Michoacán, Estado de México, Puebla, Guerrero, Oaxaca, Tabasco, Chiapas, Campeche, Yucatán and Quintana Roo (Fig. 17c). This genus is widespread throughout Central America and many species occur in highland South America (Nast, 1950).
Iphirhina has been recorded from 4 states (Morelos, Puebla, Veracruz and Oaxaca; Fig. 17e). The northernmost site is found in the Sierra Madre Oriental, near the city of Chicontla, Puebla, the southernmost in the Sierra Madre del Sur in Candelaria Loxicha, Oaxaca. In addition, there are specimens from the Trans-Mexican Volcanic Belt in Morelos state, as well as isolated specimens from the Tuxtlas Biosphere Reserve (Veracruz). The genus occurs south to Panama and Colombia.
Neaenus is distributed only on the Pacific slope (Fig. 17e) and extends from the Sierra Madre Occidental (El Palmito, Sinaloa) to the Sierra Madre del Sur and the Sierra Madre de Chiapas (near San Cristóbal de la Casas), but some species are be also found in the Trans-Mexican Volcanic Belt from Michocán (near Aguililla) to Morelos (Tepoztlán). This genus is confined to Mexico and Central America (Carvalho & Webb, 2005).
Mahanarva jurael, the only member of the genus known from Mexico, is distributed in the southern lowlands and has been recorded from only 2 sites: the Los Tuxtlas Biosphere Reserve (Veracruz) and the Montes Azul Biosphere Reserve (Chiapas) near the border with Guatemala (Fig. 17f). It is not known outside Mexico but is clearly closely related to 2 Costa Rican species, M. costaricensis and M. insignita (see below).
The species of Microsargane are found on the Pacific slope of the Sierra Madre Occidental in Guerrero and Oaxaca and in the Sierra Madre de Chiapas (Tuxtla Gutiérrez and near Ocosingo) (Fig. 17f). Finally, Olcotomaspis (Olcotomaspis) laterinotata is only found in the Sierra Mixteca around the town of La Esperanza (Fig. 17f). The genus Zulia, represented in Mexico by a single species known only from a single museum specimen, was not collected or examined for this study.
Discussion
Mexico is among the 17 countries that together account for 65-70% of the world’s species richness (Mittermeier et al., 1997). Based on the inventory of numerous taxa, it is estimated that more than 94,000 species have been recorded within the national territory and it is estimated that this number may reach 109,000 which corresponds to 8.5% of the world’s diversity (Martínez-Meyer et al., 2014). According to these data, the 40 Cercopidae species in Mexico represents 0.04% of the country’s diversity (Martínez-Meyer et al., 2014). Tropical regions harbor the greatest diversity of Cercopidae in the world, with more than a thousand species (Carvalho & Webb, 2005; Fennah, 1953, 1968; Hamilton, 1977). The diversity of this taxon in the Americas reaches almost one third of the world total of described cercopids, with 460 species and 65 subspecies grouped in 60 genera (Carvalho & Webb, 2005; Carvalho et al., 2016; Carvalho & Paladini, 2017; Castro-Valderrama, Carvalho et al., 2018; Castro-Valderrama, Peck et al., 2018; Castro-Valderrama et al., 2020; Hamilton, 2016; Meneghetti et al., 2019; Castro-Valderrama, Carvalho et al., 2018; Paladini & Carvalho, 2007, 2008, 2013; Paladini & Cavichioli, 2015; Paladini & Cryan, 2012; Paladini et al., 2016). This work demonstrates that Mexico encompasses 2.6% of the cercopid species in the world and more than 8% of the species in the Americas. At the generic level, Cercopidae in Mexico include 10 of 60 recorded genera in New World, corresponding to 16% of the total.
The most diverse tribe is Tomaspidini with 29 species distributed in the genera Aeneolamia, Mahanarva, Ocoaxo, Prosapia and Zulia. Aeneolamia is represented by 3 species; 2 of them, A. contigua and A. albofasciata, are distributed through almost the entire country (López-Collado & Pérez-Aguilar, 2012) (Fig. 17a). Both species are polyphagous and have been reported as important damaging pests in sugar cane areas (García-González et al.; 2017; López-Collado & Pérez-Aguilar, 2012; Morales-Pérez et al., 2014) and in pasture grasses (De la Cruz-Zapata et al., 2016; Martin et al., 1995; Oomen, 1975). Within A. contigua, 3 subspecies have been recorded in Mexico: A. contigua postica; A. c. campecheana and A. c. sanctaerosae. As noted in the above; sorting these species out is complicated because A. contigua exhibits considerable dorsal color variation over its distribution range. Based on examination of the BMNH holotypes; A. c. postica and A. c. sanctaerosae appear to be color variants of A. contigua and are treated as such, following Clark et al. (1976) (the holotype of A. c. campecheana could not be located in BMNH). However, given the lack of field data and possible morphological inconsistencies among subspecies (Carvalho & Webb, 2005), definitive conclusions at the species and subspecies level will require further studies, including analysis of molecular markers of specimens from varied geographic locations. This report includes a new undescribed species of this genus, Aeneolamia aff. albofasciata, found in the mountains and Central Valleys of Oaxaca, but current information does not support its status as a pest.
Mahanarva jurael is the first record of the genus Mahanarva in Mexico (Castro-Valderrama, Peck et al., 2018) (Fig. 17f). Mahanarva has been reported in Costa Rica with 3 species: M. costaricensis (Distant, 1879), M. insignita (Fowler, 1897) and M. stygia (Fowler, 1897); all of them grouped within subgenus Mahanarva (Carvalho & Webb, 2005). Nymphs of M. costaricensis and M. insignita are semi-aquatic because their developmental stages can feed within water filled Heliconia bracts (Thompson, 1997). In addition; adults feed on stems of Heliconia tortuosa and other Heliconia species (Carvalho & Webb, 2005; Thompson, 1997). Plants of this genus are probably hosts for nymphs and adults of M. jurael, as, as noted above, some specimens were reported from “platanillo”, the common name of heliconias in some places in Mexico. M. jurael is not a pest in Mexico; but in South America some congeneric species are serious pests of sugar cane (Azzi & Dodson, 1971; García et al., 2006; Peck et al., 2004; Thompson, 2004).
The genus Ocoaxo displayed the highest species richness in Mexico, 16, constituting half of the total species diversity of the genus (Carvalho & Webb, 2005; Fennah, 1979; Nast, 1950, 1975). In the last 10 years, the study of this taxon has taken on relevance in the national territory because 3 species, O. cardonai, O. assimilis and O. varians, developed infestations on pines (Pinus spp.) in the states of Puebla, Oaxaca and Veracruz, leading to a phenomenon called pine decline. The adults suck fluid from needles, leading them to die and fall off the trees (Castro-Valderrama et al., 2017; Cid-Muñóz et al., 2020). Based on their shared ecological requirements, adult feeding habits and morphological similarities, these 3 taxa are proposed as a pest species complex (Castro-Valderrama et al., 2017; Castro-Valderrama, Carvalho et al., 2018). The rest of the Ocoaxo species probably have a wide feeding spectrum because they have not been recorded on Pinus ssp., however most of them, including O. fowleri, O. femoratus, O. confusus and O. lineolatus, were collected near pine forests. Some species have wide geographical distribution records, such as O. cardonai and O varians, while others appear to be restricted to a single zone, such as O. femoratus (Temascaltepec, Estado de Mexico) and O. confusus (Huixtán Municipality, Chiapas). Nast (1950) reported, many aspects of the biology of this genus are unknown. Recently, studies of the natural history of O. assimilis have shown that it has a univoltine life cycle, 5 nymphal stages, feeds on diverse host plants in the nymphal stage (herbs, shrubs and trees), and often changes hosts during development (Castro-Valderrama et al., 2017; Cid-Muñoz et al., 2020). The various Ocoaxo species occupy different habitats (Fig. 17c). In the highlands, Ocoaxo is associated in habitats with conifers, holm oaks, and coniferous forest (Castro-Valderrama et al., 2017; Cid-Muñoz et al., 2020). In the plains of Yucatán, Ocoaxo is probably associated in habitats with low and medium deciduous forest and sub-evergreen forest (Durán García & García Contreras, 2010). In Los Tuxtlas Biosphere Reserve (Veracruz), Ocoaxo could be associated in habitats with tall and medium evergreen forest (CONANP, 2006).
The genus Prosapia is the second most diverse genus of Cercopidae in Mexico, with 8 species. Most species, such as P. isobar, P. ignifera, P. teapana, P. simulans, and P. inferens, display a broad distribution, while others have been recorded in only one state (P. hemelytra) or 2 (P. chiapana and P. morelosi). Mexican Prosapia include a newly described species, P. morelosi (Castro-Valderrama et al., 2020), increasing Mexican generic diversity from the 7 species reported by Hamilton (1977) to 8 reported here. P. morelosi was found in an ornamental Paspalum sp. and in the forage grass Pe. clandestinum (Castro-Valderrama et al., 2020), but its pest status in these grasses is unknown. Identification of Prosapia species in Mexico is complicated because intraspecific variation in their coloration combined with convergence of color forms among species, most notably the all-black variants of P. ignifera, P. inferens and P. morelosi. Consequently, the key to Prosapia species should be used in combination with geographical information and the specimen photographs, with reference to male genitalia as a last resort (see “Remarks” for P. morelosi above).
Zulia obscura is known only from the female holotype collected in the lowlands of southeastern Mexico and deposited at VMW (see Carvalho & Webb (2005): fig. 801). This species, belonging to the subgenus Zulia (Fennah, 1953), represent the northern boundery of the generic distribution going to south to Northern Argentina. Despite many national sampling campaigns targeted at cercopids, such as the “Campaña nacional de la mosca pinta” during the 1960s, and periodic sampling campaigns by universities and official organizations since, this species has not been collected again, suggesting that it is very rare, or that it occupies habitats rarely sampled.
The tribe Ischnorhinini is represented in Mexico by 4 species from the genera Huiana and Iphirhina. Huaina is monotypic, with H. inca distributed from Mexico to southern Costa Rica (Carvalho & Webb, 2005). This species is one of the most common cercopids in Mexico and has been recorded from north to south with numerous records in the Trans-Mexican Volcanic Belt, the Sierra Madre del Sur and the Sierra Madre de Chiapas, from altitudes of 300 to 2,850 m. Field records support feeding habits on corn and sugar cane. It also feeds on shrubs, weeds, wild papaya, secondary vegetation and graminoids. Peck (1998) reported adult males of H. inca feeding on the main stem of plants of an indeterminate herbaceous species of Solanaceae, causing the leaves to turn brown color and the plants to wither in just 2 weeks. Maes and Robleto (1988) reported it feeding on commercial tobacco, Nicotiana tabacum L. (Solanaceae), but did not report which life stages. Other species of the Solanaceae family are probably associated hosts because there are 32 solanaceous genera and 318 species, cultivated and wild, present in the 32 states of the country and many habitats, including tropical deciduous forest (Martínez et al., 2017). In addition, Bates (1942), and Maes and Robleto (1988) reported that H. inca feeds on cotton, Gossypium hirsutum (Malvaceae). Gossypium species are distributed throughout Mexico, with a high concentration of diversity of wild species in the Sierra Madre Occidental, the Sierra Madre del Sur and the Trans-Mexican Volcanic Belt (Pérez-Mendoza et al., 2016), places that coincide with the distribution of H. inca (Fig. 17c), but it has not been found in the Yucatán Peninsula. Despite the damage to hosts, its presence in various habitats and its polyphagous nature, this species is not considered an economically important pest.
Three species of Iphirhina are recorded from Mexico: I. sepulchralis, I. discontinua and I. limbata. This genus occurs from Mexico to Colombia ranging in altitude from 160 to 1,860 m in Mexico (Carvalho & Webb, 2005). Iphirhina sepulchralis has a few records around Huatusco and it is associated in habitats with coniferousoak vegetation, coniferous forest, fir forest and mountain mesophilic forest (Ellis & Martínez-Bello, 2010), while I. discontinua and I. limbata have been recorded from the Los Tuxtlas Biosphere Reserve, where both species could be associated in habitats with tall or medium evergreen forest (CONANP, 2006). Iphirhina limbata also inhabits low deciduous forest in the Xochicalco, Morelos archaeological zone (Semarnat-Conafor, 2014a), mountain mesophilic and medium deciduous forest in Oaxaca (Semarnat-Conafor, 2014b), and medium and high evergreen forest in Puebla (Semarnat-Conafor, 2014c). Peck (1998) reported males of I. quota (Distant, 1900) feeding on the trunk barkof Bourreria costaricensis (Standl.) A. H. Gentry (Boraginaceae) in Costa Rica, apparently without damage, an observation that has recently been repeated (VT, pers. observ.). Because there are reports of Bourreria spp. in low deciduous forests in Mexico (Campos-Ríos, 2005), members of this genus are probably associated as hosts for Iphirhina in the same states where insects have been collected (Fig. 17e); however, Iphirhina is not economically important.
The tribe Neaenini Fennah is represented in Mexico by the genus Neaenus (Hamilton, 2016: 220), of which N. (Neaenus) varius is most widely distributed (Michoacán, Guerrero, Oaxaca and Morelos). In addition, 2 species reported here are new to Mexico: N. (N.) hystricosus in Sinaloa and Oaxaca and N. (N.) natrix in Chiapas (Hamilton, 2016). Neaenus species are distributed in the states of the Pacific Coast, probably including Nayarit and Colima. Around El Palmito and Santa Lucía (Sinaloa) in the Sierra Madre Occidental, they are probably associated in habitats with conifers and oaks mixed with conifers (Semarnat-Conafor, 2014d). Neaenus varius might be associated in habitats with low deciduous forest or sub-deciduous forest near the small town of Cayacal, in the coast of Guerrero, and to oak forest, low deciduous forest and sub-deciduous forest in the Sierra Madre del Sur, between Chilpancingo and Almoloya (Semarnat-Conafor, 2014e). In Oaxaca, Neaenus species are probably associated in habitats with coniferous forest, mountain mesophilic forest and subdeciduous forest (Semarnat-Conafor, 2014b). In Chiapas, N. natrix is probably associated with oak-coniferous forest habitat (Arriaga et al., 2000), the only type of forest around Cerro Tzontehuitz, where all the specimens of this species were collected. The genus has not economic importance.
The tribe Microsarganini is represented by 2 species in the genus Microsargane. This genus was previously included in the family Aphrophoridae, but phylogenetic analysis based on molecular markers support its placement in Cercopidae (Cryan & Svenson, 2010). Mexican Microsargane are classified in the subgenera Microrhaphe and Microtholia (Hamilton, 2016). M. (Microrhaphe) martialis is found in the south of the country in Guerrero, Oaxaca, and Chiapas, with an altitudinal range of 500 to 1300 m. It is probably associated in habitats with oak forest, low deciduous forest, high sub-deciduous forest, coniferous forest and sub-deciduous forest (Semarnat-Conafor, 2014b, e, f). Microsargane (Microtholia) sobria has been collected only near Ocosingo, Chiapas in coniferous and mesophilic forests (Semarnat-Conafor, 2014f).
The genus Olcotomaspis is incertae sedis. Recently, it was redefined by Hamilton (2016), who created 2 new subgenera, Hyalotomaspis and Olcotomaspis. The Mexican species, O. (Hyalotomaspis) clarissa and O. (Olcotomaspis) laterinotata, are difficult to find. The few available specimens of O. clarissa comprise 3 male syntypes in MTD and at least 1 male in BMNH (Carvalho & Webb, 2005). This species was reported by Carvalho and Webb (2005) in Xalapa, Veracruz. O. laterinotata is known from 2 specimens. The holotype (see Carvalho & Webb (2005): fig. 797; Fowler (1897): plate 11, fig. 22a; Hamilton (2016): fig. 6A), deposited at HEC (not examined in this research), is without locality data. The second is a teneral female deposited in CNIN collected in the small town of La Esperanza, Oaxaca. The locality is predominantly mesophilic forest (Semarnat-Conafor, 2014b). Compared to the holotype, the chitin of the body seems to exhibit additional white spots, perhaps because it is teneral. This specimen is the first reported since 1897 (Fowler, 1897).
This work demonstrates that Mexico encompasses 2.6% of the cercopid species in the world and more than 8% of the species in the Americas. At the generic level, Cercopidae in Mexico include 10 of 60 recorded genera in New World, corresponding to 16% of the total. The most diverse tribe is Tomaspidini with 29 species distributed in the genera Aeneolamia, Mahanarva, Ocoaxo, Prosapia and Zulia. Aeneolamia is represented by 3 species; 2 of them, A. contigua and A. albofasciata, inhabit almost the entire Mexican Republic. The tribe Ischnorhinini is represented in Mexico by 4 species from the genera Huiana and Iphirhina. Huaina is monotypic, with the species H. inca distributed from Mexico to the south of Costa Rica. The tribe Neaenini Fennah is represented in Mexico by the genus Neaenus. The species are distributed in the states of the Pacific Coast, probably including Nayarit and Colima. Neaenus (Neaenus) varius is most widely distributed (Michoacán, Guerrero, Oaxaca and Morelos). In addition, 2 species reported here are new to Mexico: N. (Neaenus) hystricosus (Sinaloa and Oaxaca) and N. (Neaenus) natrix (Chiapas) (Hamilton, 2016). The tribe Microsarganini is represented by 2 species in the genus Microsargane. Microsargane are classified in the subgenera Microrhaphe and Microtholia (Hamilton, 2016). M. (Microrhaphe) martialis is found in the south of the country in Guerrero, Oaxaca, and Chiapas, with an altitudinal range of 500 to 1,300 m. The 40 Cercopidae species in Mexico represents 0.04% of the country’s diversity and adds 9 described species to the national fauna, M. martialis, M. sobria, N. hystricosus, N. natrix (Hamilton, 2016), M. jurael (Castro-Valderrama, Peck et al., 2018), O. sinai, O. cardonai (Castro-Valderrama, Carvalho et al., 2018) and P. morelosi (Castro-Valderrama et al., 2020), as well as one new species, Aeneolamia aff. albofasciata, which is pending description. We make this contribution to the knowledge of Cercopidae to support field professionals and phytosanitary officials in the agricultural and livestock industry in Mexico, and Central America, who now have a tool, for identifing the 40 Cercopidae species. This work complements earlier, partial keys (Clark et al., 1976; Hamilton, 1977, 2016).











 text new page (beta)
text new page (beta)



