Introduction
The environmental thermal quality and the organism’s ability to regulate heat exchange with the microclimate directly affect the body temperature (Tb) in reptiles. Most lizards control Tb within a relatively narrow range, mainly through basking behavior; however, in habitats with high environmental temperature, there is a risk of overheating (Avery, 1982; Bartholomew, 2005; Cowles & Bogert, 1944; Huey, 1982). Individuals may hide under shelters (e.g., rocks, fallen trunks) during some of the daylight hours in potential overheating situations, experiencing different temperatures from soil surface temperatures. Protecting under shelters is an obligated behavior of nocturnal species, which hide underneath retreats sites during the hours of the strongest solar radiation. This so-called diurnal “inactivity” lapse is not strictly inactive in reptiles, because during this period individuals may have postural changes and changes in location (like going deeper into a crevice) (Huey, 1982; Kearney & Predavec, 2000). Additionally, during the day, some reptiles like night geckos select retreat sites with adequate temperatures to improve locomotion performance or other parameters at night (Autumn et al., 1994; Huey et al., 1990; Ibarguengoytia et al., 2007).
Shift in the cost-benefit balance of thermoregulation occurs with thermal environmental variation, like seasonal and daily fluctuations (i.e., night vs. day thermal landscape); so, we can expect that ectothermic individuals exhibit different thermoregulatory strategies in different seasons and during day and night, based on the thermal offer of the environment, as has been broadly stated for reptiles (Blouin-Demers & Nadeau, 2005; Herczeg et al., 2008; Huey & Slatkin, 1976). The tropical northern Andes display strong changes in precipitation level throughout the year (Hurtado, 2012; Pabón-Caicedo et al., 2001); additionally, high-mountain environments in the northern Andes exhibit strong daily temperature fluctuation (Benavidez & Rocha, 2012; Hurtado, 2012). A bimodal pattern of precipitation that may affect the thermal quality of the micro-habitats, characterizes the geographic distributional range of the thickhead ground snake Atractus crassicaudatus (Duméril, Bibron and Duméril, 1854) (Fukuoka, 1971; Nobre et al., 1991). Therefore, when a high mountain nocturnal snake like A. crassicaudatus is sheltering during the day, it might experience higher environmental operative temperatures in comparison with the Te’s available at night. Conversely, during the night individuals of this species might experience “sub-optimal temperatures” or temperatures close to their minimum voluntary temperatures, whether under the shelters or foraging over the soil surface, as environmental night temperature at the altiplano drops dramatically up to 3-4 °C (see Te at Table 1, and Hurtado, 2012).
Table 1 Summary of thermoregulatory indices for A. crassicaudatus (2014, 2015-2019).
| Season | Tb Mean (SD) | Te Mean (range) | T sel Mean (SD) | Tsel range | db Median (range) | de Median (range) | E | Ex | |
|---|---|---|---|---|---|---|---|---|---|
| 25-75% | 10-90% | ||||||||
| Dry | 19.58 ± 2.00 (15.9-23.9 n = 70) | 15.88 (10.7-41.4 n = 3, 305) | 20.36 ± 2.15 (7.02-26.6 n = 21) | 19.5-21.3 | 18.7-24.8 | 1.57 (0.0-8.0 n = 242) | 4.22 (0-20.0 n = 2,398) | 0.74 | 12.7% (6.06% photophase - 60.8% scotophase) |
| Wet | 20.72 ± 2.19 (16.6-24.9 n = 38) | 15.67 (3.14-38.4 n = 1,865) | 18.5 ± 3.22 (8.4-27.69 n = 22) | 17.2-19.2 | 16.0-21.6 | 2.17 (0.0-6.2 n = 38) | 2.19 (0-19.2 n = 2,484) | 0.25 | 8.97% |
| Total | 19.98 ± 2.13 (15.9-24.9 n = 108) | 15.80 (3.1-41.4 n = 5,170) | - | 18.3-20.2 | 17.3-23.2 | 1.65 (0.0-8.0 n = 280) | 3.08 (0-20.0 n = 4,882) | 0.68 | 0.82% |
The particular and fluctuating habitat characteristics, body size, docile behavior, non-toxicity and easy access of individuals, make us consider this snake as a model to study the thermoregulatory strategy of a tropical highland nocturnal reptile. In this study, we characterized the thermal biology of the nocturnal snake A. crassicaudatus inhabiting a region of high elevation located in the eastern Cordillera of the Colombian Andes. We analyzed the relationship among field body temperatures (Tb’s), laboratory selected temperatures (Tsel), and environmental operative temperatures (Te’s). We also contrasted the accuracy and effectiveness of snake thermoregulation and habitat thermal quality during wet and dry seasons.
Materials and methods
Atractus crassicaudatus is an endemic snake to the Cundinamarca and Boyacá departments plateau, in the eastern Cordillera of Colombia, with an altitudinal distribution of 2,000 to 3,200 m asl. Snake body size is small with a total length of 400-440 mm (Paternina & Capera, 2017), and the snakes we collected had body weigh between 1.5 and 29 g (n = 32). Individuals are usually found near human settlements and under limestone rocks over a peaky soil (i.e., rich in organic matter) substrate with a net of grass and/or kikuyu roots, then we usually had to remove the rocks to capture snakes. All the study locations are characterized by a homogeneous vegetation composed of introduced grasses (e.g., Pennisetum clandestinum (Hochst. ex Chiov., 1903)), and agroecosystems with some species of native and exotic trees (Fraxinus chinensis (Roxb., 1820), Ficus andicola (Standl., 1937), Tecoma stans ((L.) Juss. ex Kunth, 1818), Sophora japonica (L., 1767), Sambucus nigra (L., 1753), Pinus patula (Schltdl. and Cham., 1831), etc.).
We recorded field body temperature (Tb) of snakes from a population at the campus of the Universidad Nacional de Colombia located in Bogotá D.C., Cundinamarca at 2,630 m asl. We recorded additional Tb’s in Sibaté, Cundinamarca at 2,700 m asl., and 27 km southeast of the capital city (Bogotá). The weather in the Cundinamarca/ Boyacá plateau is moderately wet with 2 rainy seasons -April to May, and October to November- with mean annual precipitation of 1,000-1,500 mm (Pabón-Caicedo et al., 2001). Likewise, the multi-annual mean temperature is 14-14.5 °C, and the lower mean temperatures are observed in the December-February trimester; conversely, the highest temperatures occur in April-May, and October-November rainy months (Benavidez & Rocha, 2012; Hurtado, 2012).
We obtained data between August 2014 and May 2015 (34 additional snakes were sampled during January 2019 to validate Tsel data from dry season 2015). We collected 108 individual adult snakes from Universidad Nacional campus from 00:00 to 23:00 h. Additionally, between August and September 2014, 12 individuals were collected in Sibaté (from 09:30 to 16:30 h). We recorded body temperature (Tb) for each captured snake using a digital thermometer (Supco® EM-90) and an ultrathin thermocouple type K (± 0.1 °C) (ICONTEC known-standard Calibration), inserted approximately 5 mm inside the cloaca and recorded within the first 60 seconds after the capture. We used nitrile gloves to prevent the heat exchange from the collector to the snake.
During August-November 2014, and January-May 2015, we recorded field Te at 9 localities (Appendix 1). The operative temperatures are the predicted equilibrium temperatures of non-regulating ectotherms (i.e., control organisms), characterization of these temperatures is an approach to the thermal environment experienced by the organism (Bakken 1992; Bakken et al., 1985). Previous studies have shown that copper pipe models closely resemble the body temperatures for small ectotherms (Dzialowski, 2005; Isaac & Gregory, 2004; Shine & Kearney, 2001). We used 6 unpainted hollow copper pipe models (160 mm length, 12.7 mm diameter) connected to external temperature data-logger sensors (HOBOTEMP®) and used cork to lock down the ends of the tubes. Calibration of the biophysical model of Te (see Dzialowski, 2005) was done on the field by recording Tb’s of an adult A. crassicaudatus and the temperature of a hollow copper pipe model connected to a digital thermometer. The temperature of the model and the snake was recorded every 5 minutes for 3 hours; the snake and the model were placed inside an artificial shelter completely under the shadow. Tb and model Te were significant and positively correlated (r = 0.84; n = 36; p <0.0001). The models were located under and next to rocks (sunny; semi-shady and shady conditions) (Sinervo et al., 2011); temperature of the models was recorded every 30 minutes during the logging ranges (Appendix 1), to estimate the distribution of Te throughout the potential activity and inactivity period of the species (Paternina, 2016).
We used a subsample of the individuals captured from the field, n = 43 (22 snakes for wet seasons 2014/2015, and 21 snakes for dry seasons 2015/2019). Groups of 5-6 snakes were housed in independent terraria (1,000 mm × 450 mm × 500 mm) under natural light conditions. The selected body temperatures (Tsel) were recorded in a thermal gradient constructed from wood walls and metal ground (100 cm × 60 cm × 15 cm). The gradient structure had 2 tracks (30 cm wide each) separated by an opaque craft barrier to allow multiple simultaneous runs while preventing the interaction of adjacent individuals. The gradient was housed in a room at an approximately room temperature of 21°C. We placed a non-luminous heat source (Ceramic Heat Emitters, Percell Pet System Co., Ltd.) at one extreme, and 3 metallic flasks with ice cubes (replaced every 30 minutes) in contact with the metal ground, at the opposite end, to generate a linear thermal gradient from 0 to 40 °C. We recorded the temperature of the metal ground every 20 cm (20 cm = 10 °C, 40 cm = 20 °C, 60 cm = 30 °C, 80 cm = 40 °C) during the experiments to control possible gradient fluctuations of temperature. Each point of the track had similar temperature in all the experiments (Dunn’s multiple comparisons, Appendix 2). We put 10 mm of natural organic soil and some rocks (uniformly distributed) along the tracks. We assume individuals feed infrequently in the wild, since there is no factual evidence of its foraging behavior, therefore the snakes were deprived of food 2 days before the experiment, and water was provided ad libitum (Webb & Shine, 1998). Before recording Tsel, each individual was allowed to acclimatize to the thermal gradient for 40 minutes. The Tsel was registered with an Omega® USB TC08 data-logger, which has 8 channels connected to ultrathin thermocouples (Omega 5SC-TT-T-40-72) that were inserted 5 mm inside the cloaca and secured with surgical tape (micropore®). The body temperatures of the individuals were recorded every 15 minutes for 3 hours (17:30 to 20:30 h). Additionally, during the dry season of 2019, we run gradient experiments for 24 hours. At 18:00 h the track was covered with cardboard to simulate the natural photoperiod and thus obtain the Tsel’s in the phases of light and dark but maintaining the gradient in both phases. Finally, following the scheme developed by Hertz et al. (1993), the median interquartile range (i.e., the central 50% of the measurements) of selected temperature was used as the set-point range to facilitate the comparison with related studies (Blouin-Demers & Weatherhead, 2001; Fitzgerald et al., 2003; Webb & Shine, 1998). All the experiments adhered to the international specifications (Regulation 86/609/EEC of the European Economic Community, 1986; Guidelines for use of live Amphibians and Reptiles in fields and Laboratory Research, HACC - American Society of Ichthyologists and Herpetologists, Beaupre et al., 2004), and were approved by the Ethics Committee of the Science Faculty, Universidad Nacional de Colombia.
We used data of field Tb, laboratory Tsel, and Te to compute the indices of thermoregulation (db), and habitat thermal quality (de) according to Hertz et al. (1993). High values of db and de indicate a low accuracy of Tb regulation, and a low habitat thermal quality from the organism’s perspective, while values equal or close to zero indicate a high accuracy of Tb and thermally ideal environments. Subsequently, thermoregulation effectiveness was calculated using the following equation: E = 1 - (average db/average de). An E index value close to 1 indicates that individuals exhibit active thermoregulation, with environmental temperature availability far from its physiological requirements; that is, an organism is under thermal stress and must react to increase or decrease Tb relative to Te. In contrast, an E value equal or near 0 identifies a thermoconformer strategy, it is, individuals do not use behavior to actively regulate body temperature, see details in Hertz et al. (1993). Following Blouin-Demers and Weatherhead (2001) and Blouin-Demers and Nadeau (2005), we measured the index of effectiveness of thermoregulation (de - db) to consider the magnitude of db and de for-interpreting E, and to quantify the deviation from perfect thermoconformity. We defined the direction of the Tb deviation from Tsel interquartile range, as well as Tb values percentage kept within the Tsel interquartile range, based on the proportion of field Tb data that fell above or below the Tsel interquartile range (Hertz et al., 1993). Thermal gradient for individuals captured on 2014-2015 was implemented at crepuscular time, and then, thermoregulation indices estimated from these data correspond to this phase of the day. However, in 2019 we obtained Tsel data for a 24 h period; therefore, we calculated and discussed thermoregulatory indices for day and night, separately, at that year.
Finally, the exploitation index (Ex), which determines the extent to which organisms take advantage of the thermal environment, was developed by Christian and Weavers (1996). The Ex-index is defined as the amount of time that an individual spends within its Tsel range, divided by the time available for the animal to have its Tb’s within the Tsel range; it is expressed as the percentage of time it is possible for the animal to perform this “match” (from the Te data). Therefore, Ex was calculated as the proportion of Tb measurements included within Tsel range, it is when de = 0, in at least one of the habitats. Following Brown and Weatherhead (2000), this index was also slightly modified to calculate the proportion of Tb data falling above or below Tsel when de = 0 in at least one of the habitats.
The data of Tb, Te, and db and de indices, that met the requirements for the use of parametric tests (normality and homogeneity; Shapiro-Wilk and Levene’s Tests, respectively), were compared using t-tests of Student; otherwise, the corresponding non-parametric test was used (Mann-Whitney U test). We used a Student t-test with Welch correction, given their dissimilar standard deviation, to compare Te between 2 seasons. This test was also used to contrast Tsel between seasons. PAST3 and Graphpad Prism 6 software were used for statistical analysis and graphing. The significance value for all statistical tests was p < 0.05 (Sokal & Rholf, 2000). Average ± standard deviation (SD), sample size (n), and the range between the minimum and maximum values are shown within text and figures.
Results
We captured 108 individuals of A. crassicaudatus (Appendix 3). Fourteen snakes were collected in the wet season of 2014, 34 in the wet season of 2015, 36 in the dry season of 2015, and 34 in the dry season of 2019. We also recorded Tb for 12 specimens in Sibaté locality during the daylight hours.
Body temperature from individuals captured at the university campus differed among seasons (t = 2.666; p = 0.0095). The body temperature of these individuals was not related to body size (r2 = 0.0076). Additionally, when we compared separately wet and dry seasons among different years (wet season 2014 (n = 14) vs. wet season 2015 (n = 24); dry season 2015 (n = 36) vs. dry season 2019 (n = 34), and wet season 2014 and dry season 2015, we found Tb was different (Mann Whitney U = 76.50, p = 0.0392; U = 90.50, p = 0.0389, and U = 492, p < 0.0001, respectively). In contrast, no significant differences were found between wet and dry season of the same year (i.e., El Niño year 2015) (Mann Whitney U = 196.5, p = 0.7512). Additionally, during twilight, between 17:30 and 19:00 h, Tb was within or close to Tsel interval (Fig. 1).
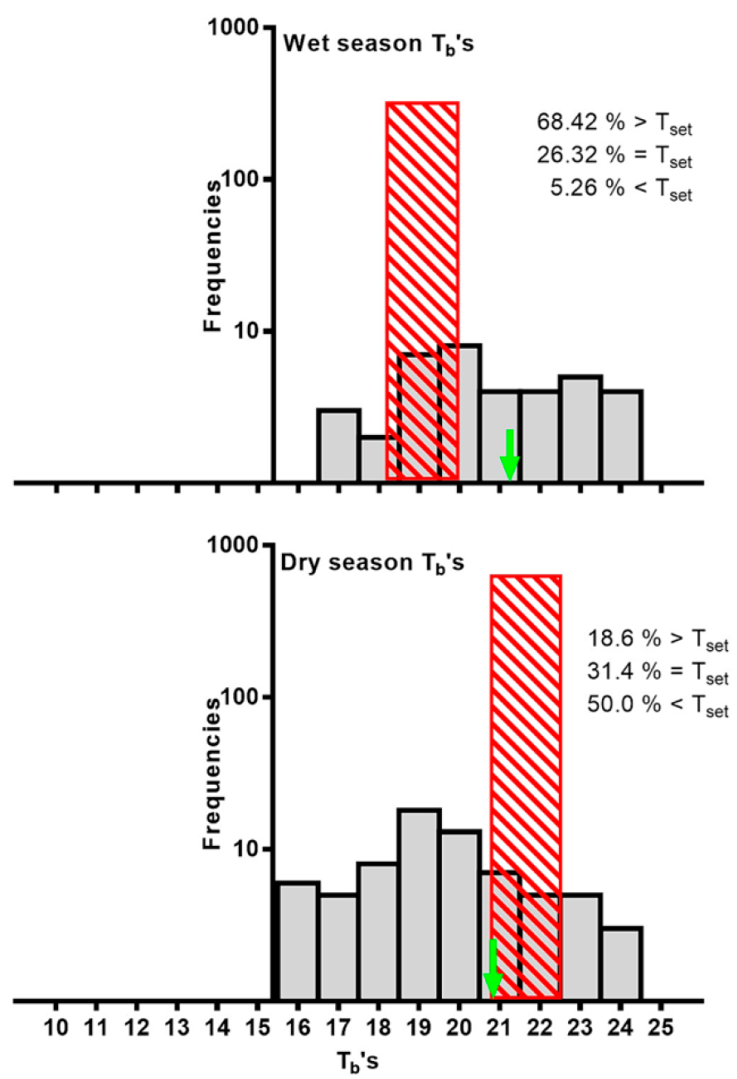
Figure 1 Atractus crassicaudatus body temperatures (Tb) in dry and wet season. The striped area indicates the selected temperature range (Tsel) in each season. The arrow indicates the average temperature (wet season data corresponds to twilight).
Individuals from Sibaté had diurnal Tb data closer to the diurnal Tb data of university campus from dry season (Mean rank difference = 8.841; alpha = 0.05, Dunn’s multiple comparison). The average of diurnal Tb’s from Sibaté was 23.5 °C, with a minimum of 19.9 °C, and a maximum of 32.4 °C, while the average of diurnal Tb’s from campus in the dry season was 21.48 °C, with a minimum of 19.1 °C and a maximum of 24.8 °C.
The data for selected body temperature recorded in the thermal gradient is presented in Table 1. We had data from crepuscular Tsel for the wet season of 2014 (n = 11)/2015 (n = 11) and the dry season of 2015 (n = 14)/2019 (n = 7). We found a significant effect of the climatic season over Tsel’s (λ de Wilk = 29.80; F1,12 = 10.72; p = 0.0066). Then, the difference in average Tsel among seasons is 1.9 °C. We found no differences between the crepuscular phase of the Tsel of dry season of 2019 and the dry season of 2015 (λ de Wilk = 10.22; F1,12 = 2.09; p = 0.1733). When we analyze the whole-day Tsel data from 2019, we found that Tsel central interquartile range was 18.7 - 20.2 °C during daylight hours (photophase) and 19.5 - 20.4 °C at night.
Operative temperatures differ among seasons (Mann Whitney U = 2.76 × 106; p < 0.0001, n = 5170). However, differences are minor if we examine the median and range values of Te per season (Table 1), with difference below 0.5 ºC. Interestingly, when we compared separately day (photophase) and night (scotophase), we found that dry season days were significantly hotter than wet season days (Mann Whitney’s U = 7.77x106, p = 0.0001, n = 9600). During the nights of the dry season there were not significant differences from the nights of the wet season (Mann Whitney’s U = 3.93x107, p = 0.1197, n = 17861). Also, there is a difference between day and night in the dry season (Mann Whitney U = 2.17 × 106; p < 0.0001, n = 12120), and during the wet season (U = 1.54 x107; p < 0.0001, n = 15341) (Appendix 4).
Comparing models exposed to direct sunlight versus models under shade, we found seasonal differences in both, rocky refuges (t = 5.5978; p < 0.001, n = 11652) and in grass near rocks (t = -2.5854; p = 0.00973, n = 25346). Higher Te’s are usually observed in sunny microhabitats in both seasons (Table 1, Fig. 1). During the dry season, higher values of Te’s are observed from 12:00 to 18:00h. On the other hand, during the wet season higher Te’s are between 11:00 to 12:00h and 14:00 to 17:00h.
Therefore, the thermal quality -de- (including all models, under shady and sunny conditions) varied seasonally (Mann Whitney U = 1.622 × 106; p < 0.0001; n = 4882). Besides, days have better thermal quality (dry day de = 2.96 and humid day de = 1.78) than nights (dry night de = 4.8 and humid night de = 3.0), and difference is significant both during dry season (dry day vs. humid day U = 488129, p < 0.0001, n = 2457) and wet season (dry night vs. humid night U = 280459, p < 0.0001, n = 2425). We compared Te among full sun, partial sun and shade microenvironments and found significant differences in both seasons (Dunn’s multiple comparison’s, Appendix 5), with partial sun microenvironments having highest thermal quality, particularly during daylight hours (photophase) in wet season (Fig. 2).

Figure 2 Circadian variation of operative temperatures (Te) of light exposed, semi-shady and shady models, throughout 24 h lapse. The colorful rectangles represent the Tsel interquartile range for each season (during the dry season there is a diurnal and a nocturnal range).
The relation between field body and crepuscular selected temperatures was evaluated by means of the thermal accuracy index db. In the dry season, this snake species had an average db index of 1.04 ºC, which is congruent with the thermal effectiveness index (E = 0.72) that identifies A. crassicaudatus as a slightly active thermoregulator during this period. In the dry season, the thermal behavior of snakes varies throughout the day, with the highest accuracy overnight (db day = 1.40 vs. db night = 1.25), despite the variation observed in thermal quality between day and night (de day = 2.96 vs. de night = 4.87). Thus, A. crassicaudatus behaves as a mild active thermoregulator during the photophase (E = 0.54), and a high active thermoregulator during the scotophase (E = 0.71).
Conversely, the snake behaves as a thermoconformer during the twilight in the wet season (E = 0.25), with accuracy decreasing during this season (db= 1.77 °C) and significant different with accuracy of the dry season db (U = 520.5; p = 0.0002, n = 108). Finally, in the dry season, the exploitation index during the day was 6.06%, and 60.8% during the night, while in the wet season for the daylong (24 h) was 8.97%.
Discussion
Variation in selected temperatures range (Tsel). The thickhead ground snake exhibits thermal plasticity, with different thermoregulatory patterns between climatic seasons. Average body temperature of A. crassicaudatus (20 °C) was slightly lower than data reported for species of tropical or subtropical distribution like Bothrops insularis (Amaral, 1922) (22 °C, small viper snake, Bovo et al., 2012), Crotalus triseriatus Wagler, 1830 (24.1 °C, Jaramillo-Alba et al., 2020) and Boiga irregularis (Bechstein, 1802) (25 °C a nocturnal-arboreal snake, Anderson et al., 2005). Atractus crassicaudatus shows average Tb much lower than average Tb of some temperate species (Blouin-Demers & Weatherhead, 2001; Brown & Weatherhead, 2000; Fitzgerald et al., 2003; Peterson, 1987; Weatherhead et al., 2012; Webb & Shine, 1998).
On the other hand, A. crassicaudatus average Tsel is lower than the Tsel of the temperate snake species of the studies mentioned above (Lutterschmidt et al., 2002; Row & Blouin-Demers, 2006), and its Tsel interquartile range is narrower than those of similar species, like B. irregularis with Tsel range of 28.2-31.4 °C. However, B. insularis with a Tsel range was 20.4 - 21.7 °C in winter and 24.9-26.3 °C in summer is the only species with a narrower range than A. crassicaudatus . Additionally, Tsel range of A. crassicaudatus is like Tsel of other ectotherms with similar geographical distribution to the thickhead ground snake; for example, Anolis heterodermus Duméril, 1851 (interquartile range 23.5-25.5 °C, Méndez-Galeano & Calderón-Espinosa, 2017) and Dendropsophus molitor (Schmidt, 1857) (selected temperatures in a gradient from 24 to 35 °C, Lüddecke, 1995). Likewise, during the dry season, A. crassicaudatus follows the general pattern of reptiles (i.e., the selected temperatures are over the field body temperatures), however, in the wet season, we observed an opposite pattern. Atractus crassicaudatus exhibits greater similarities with some temperate reptiles, like Crotalus cerastes Hallowell, 1854 (although comparisons with this species is limited due to habitat differences between both species), Charina bottae (Blainville, 1835), and Hoplocephalus bungaroides Schlegel, 1837, which spend daylight hours in refuges, and have a Tb higher than air temperature (Dorcas et al., 1997; Secor & Nagy, 1994; Webb & Shine, 1998). Like these species, the thickhead ground snake can also forage overnight outside its shelters (personal observation), being exposed to similar thermal constraints. This finding contradicts the pattern proposed by Anderson et al. (2005), and Luiselli and Akani (2002), in which the tropical species experiment fewer thermal constraints given that energy budget in tropical environments is greater than that of temperate habitats. The environmental conditions of the thickhead ground snake habitat (i.e., high altitudinal distribution, circadian thermal variations, and seasonal variation in precipitation) may be related to its low values of Tb and Tsel, and its relatively wide range or eurythermal condition (Tb’s general variation of almost 9 °C).
Generally, the body temperatures of some reptiles vary significantly between seasons. In contrast, the selected body temperature range (Tsel) has been considered as highly stable within some lizard populations despite thermal environmental variation (e.g., altitudinal gradient) (Gvoždík, 2002). There is just one report on geographical variation of Tsel, in the lizard Takydromus septentrionalis Gunther, 1864 (Du, 2006), but several cases of seasonal variation have been reported in lizards and the snake B. insularis (Bovo et al., 2012; Firth & Belan, 1998; Patterson & Davies, 1978; Van Damme et al., 1986). The Tsel range observed in A. crassicaudatus fluctuates between seasons, getting wider and with both extremes of the Tsel range decreasing during wet season. Previous studies about seasonal variation on Tsel suggest that such variation is an expression of reproductive phenology and seasonal differences of the physiological state of the organisms (Firth & Belan, 1988; Patterson & Davies, 1978; Rock et al., 2000; Van Damme et al., 1986); therefore, the potential influence of reproductive condition on Tsel of A. crassicaudatus should be studied. Interestingly, Bovo et al. (2012) observed a higher Tsel range of B. insularis during the summer in a southern Brazil island and suggested that it may be related to prey capture or foraging activities. However, the foraging strategy of 2 nocturnal snakes (H. bungaroides and Cryptophis nigrescens (Gunther, 1862)) does not seem to influence the selection of the retreat sites or the thermoregulation strategy (Webb et al., 2004), but it is necessary to evaluate the relationship between food availability (affected perhaps by precipitation levels) and thermoregulatory behavior in the thickhead ground snake.
On the other hand, it is possible that flooding along the geographic distribution of A. crassicaudatus affects refuge availability, mainly during the rainy season since this species inhabits flood-prone areas. Fluctuations of the number of shelters available for these snakes may influence its thermoregulation strategy. This hypothesis (seasonal variation of shelter availability influencing Tsel in this species) needs further evaluation.
The thickhead ground snake is highly sensitive to the thermal conditions of its retreat sites; during the dry season the Te is more constant (Te’s standard deviation 2.19 and maximum variation coefficient 17.4%), maybe due to the low cloud coverage. Unlike the dry season, during the wet season cloud coverage is more variable, and likewise the temperatures under the rocks (Fig. 2, Te sunny vs. Te shade), Te’s standard deviation 2.84 and maximum variation coefficient 29.11%. Likewise, we found that shelters under sunny and semi-shaded conditions offer better thermal quality during the dry season and preserve this thermal offer during the first hours of darkness (Fig. 2).
Nocturnal reptiles tend to spend the day in their shelters -especially in habitats with oscillating thermal conditions- and their Tb values are higher than those of air temperature at night (Autumn & De Nardo, 1995; Avery, 1982; Bustard, 1967; Huey et al., 1989; Rock et al., 2002). Therefore, it is possible that the lower Tsel observed during the wet season is explained by the Te variation and the thermal quality of shelters at night that increases uncertainty in the process of selection of near-optimum or body temperature range (Tsel), as suggested by Patterson and Davies (1978). It is remarkable that A. crassicaudatus does not exhibit differences in Tb between night and day, maintaining Tb near an optimum range during the scotophase.
The thermoregulation strategy of A. crassicaudatus during dry season showed indices values like other tropical species such as the lizards Anolis cristatellus Duméril and Bibron, 1837, Anolis cooki Grant, 1931, Anolis gundlachi Peters, 1877, and Christinus marmoratus (Gray, 1845), which also show seasonal variation in thermoregulation effectiveness (Hertz, 1992; Hertz et al., 1993; Huey & Webster, 1976; Kearney & Predavec, 2000). However, unlike these species, in the thickhead ground snake, the seasonal variation of the values of index E (thermoregulation effectiveness) is similarly explained by seasonal variation in the thermal quality of the environment as well as seasonal fluctuation in Tsel. This pattern suggests that thermoregulatory strategies vary in each season, probably because of the variation of direct solar radiation over the retreat sites, as indicated by the seasonal thermal quality variability observed. A similar pattern has been reported for B. insularis, which thermoregulates more effectively when the thermal quality of the habitat decreases during cold seasons (Bovo et al., 2012). Then, in A. crassicaudatus there are both, seasonal variations in thermoregulatory strategy through behavioral adjustment, and by means of physiological response to environmental climatic fluctuation. This double source of variation in thermoregulatory strategy remains poorly explored and suggests that evolution of plasticity in the thermoregulatory strategy may involve other mechanisms than behavior, in response to factors that affect the thermal quality of the habitat (Huey et al., 2012; Tejedo et al., 2012).
Otherwise, exploitation index may indicate the ability to exploit high optimum environmental temperatures, and it has been suggested that the low thermal resource exploitation or limited thermoregulatory behavior is an adaptation to cold climates (Saint Girons, 1980). Lower Ex indices may suggest that A. crassicaudatus does not actively exploit its thermal environment (when Te close to Tsel are available for a long time, i.e., when the environment shows a higher thermal quality), but it seems to spend a considerable amount of time searching for thermal opportunities if habitat or time of the day has low thermal quality. During the dry season diurnal Ex values are higher than diurnal Ex in the wet season, revealing an indispensable need to exploit high temperatures in a season with extremely cold nights and dawns. This behavior suggests that there are physiological advantages from maintaining Tb values inside Tsel range for at least a few hours per day. This pattern is like the exploitation behavior of young tuataras (Besson & Cree, 2010). The tuatara, Sphenodon punctatus (Gray, 1842) is a cold-adapted reptile with low thermal requirements (Barwick, 1982; Hill, 1980; Walls, 1983). Therefore, it seems to be enough for A. crassicaudatus to have a few hours of Tb within Tsel range for a relatively adequate performance.
It has been proposed that tigmothermic nocturnal lizards use to show a eurithermal pattern, because during the day they keep high body temperatures while in the shelter to increase its metabolic efficiency, but at night they perform under suboptimal lower temperatures (Kearney& Predavec, 2000; Lara-Resendiz et al., 2013). For example, the nocturnal lizard, Phyllodactylus bordai Taylor, 1942, shows a pattern where Tsel range are higher and wider during day than night (Lara-Resendiz et al., 2013). Also, Kearney and Predavec (2000) reported that the marbled geckos, C. marmoratus, follow the pattern where optimum body temperatures, thermal preferences, and tolerances, are much higher during the day than during their nocturnal activity. Some Scincidae and Gekkonidae species are nocturnal and have optimum temperature (Topt) for locomotion performance similar or higher than those of diurnal lizards (Huey et al., 1989).
We observed that optimum temperature for respiratory performance of A. crassicaudatus (Paternina et al., in preparation) is lower than the average habitat air temperature (14 - 14.5 °C), for instance, optimum temperature for length of the ventilation cycles is lower than the average of diurnal Tair.; also, we observed that the Tsel range of A. crassicaudatus does not follow the pattern mentioned above for P. bordai, since snakes selected very similar and narrow interval of temperature during the day (photophase) and during the night (scotophase). Unlike these nocturnal lizard species, the thickhead ground snakes spend most of the day and night under shelters, and then, they presumable are capable of behaviorally access to Te suitable for reaching its thermal preference all day long; therefore, this ¨day-inactive/night-field active” paradigm is not applicable for this nocturnal snake.
This suggests that, unlike other nocturnal reptiles that inhabit open environments, A. crassicaudatus is prone to have relatively low optima. Atractus is a species rich genus, with 147 species (Uetz et al., 2021), and information about habits (nocturnal/diurnal) for many species is unknown; however, it seems that species have nocturnal as well as crepuscular habits, for instances, see Cisneros-Heredia and Romero (2015), Ferreira-Silva et al. (2019), Passos et al. (2010, 2013). Thus, the question about the evolutionary relationship of nocturnal habits and the low thermal optimal that occurs in this genus deserves to be studied.
On the other hand, some previous studies have shown that A. crassicaudatus could be under a moderate threat because of increasing temperatures and other variables related to climate change; for example, Paternina (2016) suggested that the extinction risk of natural populations would be between 11.9%-18.05%, according to different future climatic scenarios by 2070, and concluded that the lowest populations would exhibit the highest risk of extinction. In contrast, Huertas-Barrera and Rey-Pulido (2018) reported lower extinction risk of 1%-2%, under 2 different future climatic scenarios (rcp26 and 85) by 2070. However, these authors account for extinction risk of the species using correlative models, whereas Paternina (2016) used both, correlative and physiological models. Thus, accounting for the thermal ecology of A. crassicaudatus may reveal more precisely the effect of climatic change by global warming on its natural populations.
Accordingly, A. crassicaudatus is a cold-adapted and thermal plastic reptile, characteristics which might be beneficial to deal with climate change (Lara-Resendiz, 2020; Tewksbury et al., 2008). This snake is usually found under rocks from open to semi-open high mountain environments, exploiting the thermal heterogeneity of the habitat, as has been suggested for other ectotherm species (Huey et al., 2012; Stevenson, 1985); however, solar radiation will increase in these open habitats and by consequence the temperatures will be higher under rocks. This situation may force the snakes to move into the forest or nearby environments, potentially competing with related species from the forest. However, there is no ecologically similar species to A. crassicaudatus at the same high elevations in the Colombian Andes, but Atractus wernerii Peracca, 1914 occurs in sympatric with A. crassicaudatus at low altitudinal distribution. Thus, evaluating the potential response of A. wernerii to climate change would be necessary to validate the prediction of greater pressure at lower altitudinal range. Our study provides thermoregulatory strategy data of a tropical highland and nocturnal snake for the first time; thermal attributes described here for this species shed light over future studies on extinction risk for this mountain reptile that could face global warming.
In conclusion, the thermal biology of A. crassicaudatus suggests that it is a thigmothermic snake, inhabiting a tropical highland environment characterized for a daily and seasonal variability on thermal quality, showing a lower thermal quality during the nights and seasonally during the dry season. Therefore, this species behaves as an active thermoregulator, E = 0.74 (particularly at night, Eday = 0.54 versus Enight = 0.72) in the dry season. During the wet season the habitat offers a more homogeneous and high-quality thermal environment, hence A. crassicaudatus behaves as a thermoconformer, E = 0.25. Characterizing the thermal ecology of related species living at different altitudes and employing an eco-physiological approach to evaluate the thermal performance of these species, would provide key knowledge to estimate the impact of global climate change over A. crassicaudatus.











 text new page (beta)
text new page (beta)




