Introduction
Members of the copepod order Monstrilloida Sars, 1901, are rarely observed in regular plankton surveys as only 2 stages of their semi-parasitic life cycle are freeliving forms: the inconspicuous early nauplius and the adult (Suárez-Morales, 2011, 2017). Their intermediate postnaupliar and juvenile stages are endoparasites of benthic invertebrates like polychaetes (including members of the families Syllidae, Capitellidae, Serpulidae, and Spionidae), gastropod and bivalve mollusks, and sponges (Huys et al., 2007; Jeon et al., 2018; Suárez-Morales, 2017; Suárez-Morales et al., 2010, 2014). Typically, adult monstrilloids lack appendages between the antennules and swimming legs, they are non-feeding reproductive stages (Grygier & Ohtsuka, 2008; Suárez-Morales, 2011). They can be caught occasionally during plankton samplings in coastal lagoons and embayments (Suárez-Morales, 2011; Suárez-Morales & Dias, 2001), particularly in reef habitats where they can be abundant and diverse (Suárez-Morales, 2001). The order is currently represented by a single family (Monstrillidae) that contains 7 valid genera: Monstrilla Dana, 1849; Cymbasoma Thompson, 1888; Monstrillopsis Sars, 1921; Maemonstrilla Grygier & Ohtsuka, 2008; Australomonstrillopsis Suárez-Morales & McKinnon, 2014; Caromiobenella Jeon, Lee & Soh, 2018; and Spinomonstrilla Suárez-Morales, 2019 (Suárez-Morales, 2011, 2019; Suárez-Morales & McKinnon, 2014;). Cymbasoma and Monstrilla are currently deemed as the most speciose genera (Suárez-Morales, 2011). There is only one previous record of a monstrilloid copepod from Chetumal Bay, a species of the genus Monstrilla (Suárez-Morales & Castellanos, 2019)
There are several nominal species whose taxonomic status is doubtful; some of these have been recorded from different geographic regions but were recognized as species groups containing distinct species (Suárez-Morales, 2011; Üstün et al., 2014). One of these groups is the Cymbasoma longispinosum species-group, which is currently known to contain at least 7 species worldwide (Üstün et al., 2014). The purpose of this study is to describe a new species of this group from the Caribbean Sea Basin and compare it with the other members of the species-group. A general overview of the distribution of the species assignable to the Cymbasoma longispinosum species-group and a key to these species are provided.
Material and methods
Zooplankton samples were obtained on May 9, 2019 by performing nighttime surface hauls off Chetumal Bay, a binational embayment shared by Mexico and Belize, on the southernmost part of the Mexican Caribbean coast (Fig. 1). A standard plankton net of 0.5 m mouth diameter, and 0.333 mm filtering mesh was used. During a zooplankton trawl performed at station 10 (arrowed in Fig. 1), located off the District of Corozal, Belize, Central America an adult female specimen of a monstrilloid copepod was observed and sorted for further taxonomic examination. The specimen is assignable to the genus Cymbasoma. It was found to represent a new species which is herein described in full following the current upgraded descriptive standards in monstrilloid taxonomy (Grygier & Ohtsuka, 1995, 2008) and compared with its closest congeners. The morphologic terminology follows Huys and Boxshall (1991). The holotype was deposited in the collection of zooplankton held at El Colegio de la Frontera Sur (ECOSUR) at Chetumal, Mexico (ECOCHZ), where it is available for consultation.
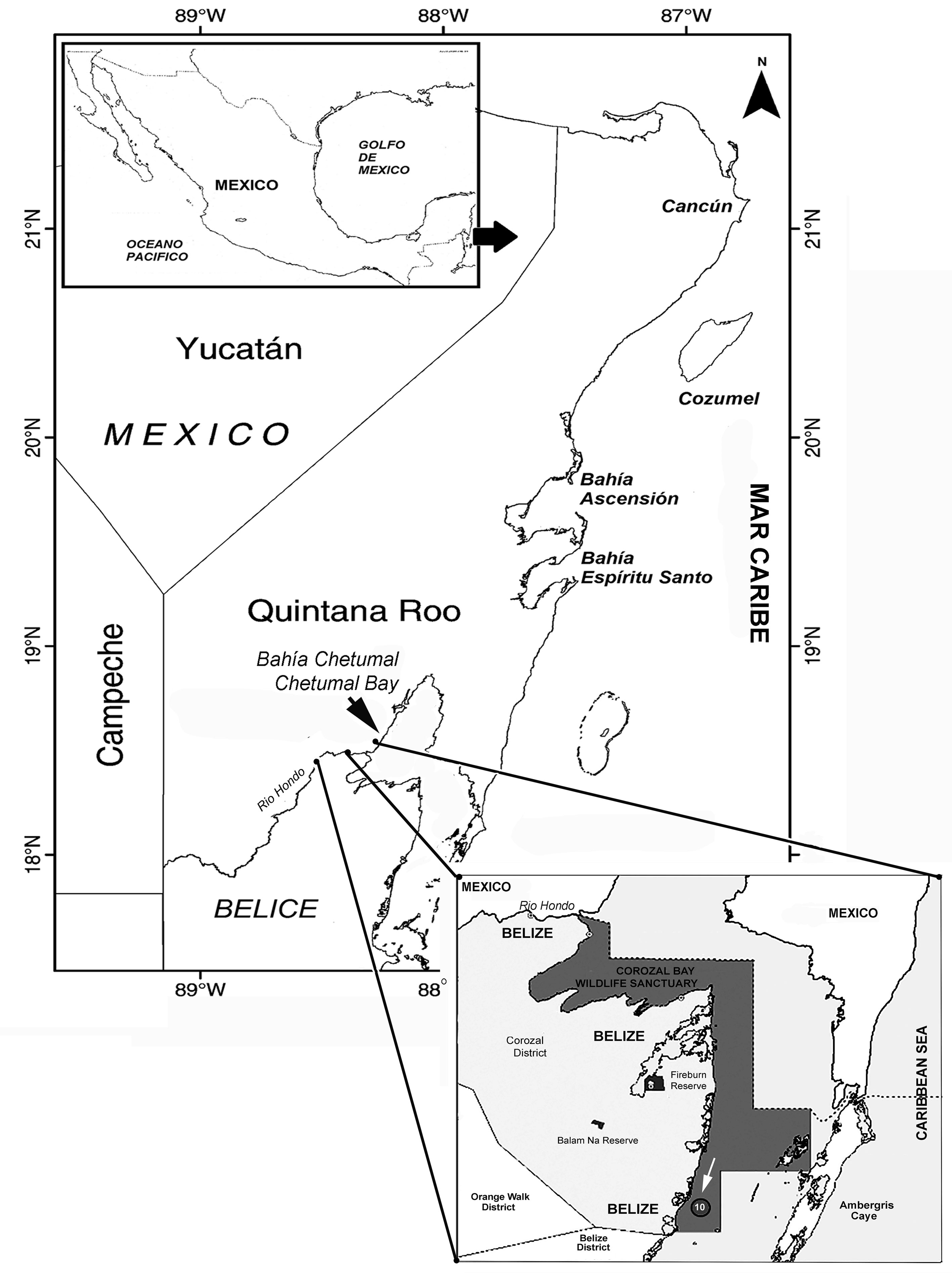
Figure 1 Study area and location of sampling station (10) in Corozal Bay, Belize, Central America, western Caribbean Sea.
Description
Order Monstrilloida Sars, 1903
Family Monstrillidae Dana, 1849
Genus Cymbasoma Thompson, 1888 Cymbasoma belizense sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:pub:E432BEE7-EA79-46AE-A96D-9A62978FBF65
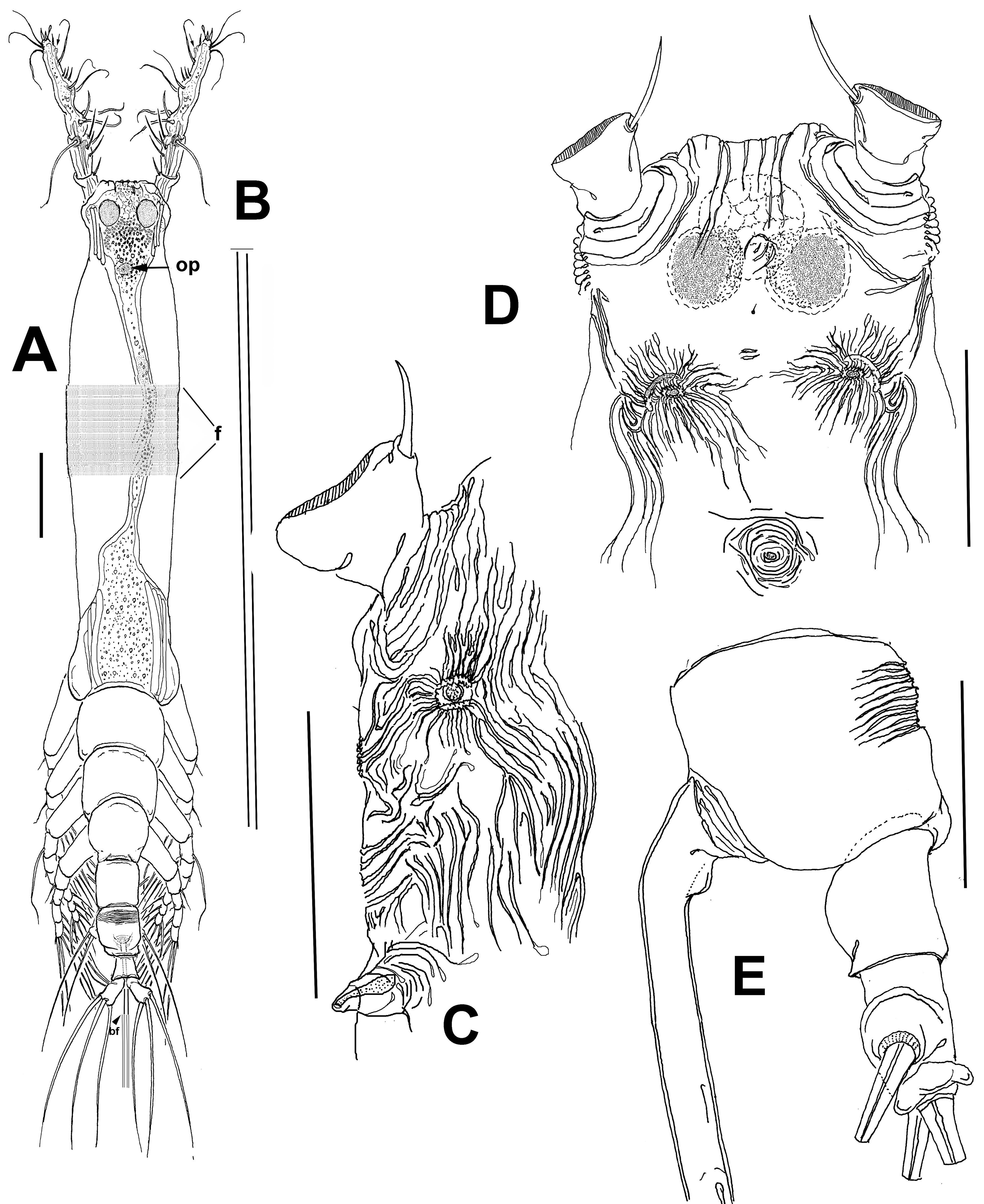
Figure 2 A-E. Cymbasoma belizense n. sp., adult female holotype from Belize. A, Habitus, dorsal view; B, ovigerous spine showing length with respect to body; C, ventral surface of cephalic region showing cuticular ornamentation, lateral view; D, same, ventral view; E, urosome showing ornamentation of genital somite, lateral view. op = Oral papilla, bf = bifurcation of ovigerous spines. Scale bars: A, B = 500 µm, C-E = 100 µm.

Figure 3 A-D. Cymbasoma belizense n. sp., adult female holotype from Belize. A, Urosome showing fifth legs and genital somite, ventral view; B, right antennule, dorsal view showing armature; nomenclature of setal elements follows Grygier and Ohtsuka (1995), ip = inner processes; C, detail of inner processes on left antennule; D, urosome and fifth legs, ventral view showing insertion of ovigerous spines and point of bifurcation of spines (arrowed); D, fifth leg, ventral view showing insertion of innermost setae (is) (arrowed); E, leg 4 exopodal ramus showing digitiform outer spine on first segment (arrowed). Scale bars: A, B, E = 100 µm; C, D = 50 µm.
Female. Body cylindrical, cephalothorax typically elongate, slender; body length of holotype female measured from anterior end of cephalosome to posterior margin of anal somite = 2.33 mm. Cephalothorax (incorporating first pedigerous somite) approximately 1.72 mm long, representing 64% of total body length. Oral papilla conical, located ventrally at anterior 1/5 of cephalothorax. Pair of relatively large ocelli present, pigment cups moderately developed, medially conjoined, weakly pigmented; ventral cup slightly larger than lateral cups (Fig. 2A). Frontal cephalic margin with conspicuous, longitudinally arranged cuticular ridges extending onto anterior ventral surface (Fig. 2D) between the antennule bases; sensilla not observed on “forehead” area. Additional cephalic cuticular ornamentation including small, semicircular group of medial cuticular scars overlying area between ocelli on ventral surface. Ventral surface with: 1) 2 large nipplelike processes surrounded by field of wrinkles on preoral area, 2) a small medial sensillum, and 3) perioral field of wrinkles (Fig. 2C, D). Other cuticular ornamentation represented by wide transverse fringe of faint, shallow striation on cephalothorax (“f” in Fig. 2A).
Urosome consisting of fifth pedigerous somite, genital double somite, anal somite, and caudal ramus, together representing 16% of total body length (Fig. 2A, E). Genital double- somite subquadrate, dorsal surface ornamented with field of deep transverse cuticular wrinkles on proximal half and field of wrinkles on posterior corners of somite (Fig. 2E). Anal somite smooth, with ventral surface protuberant in lateral view (Fig. 2E). Caudal ramus subrectangular, 1.3 times longer than wide, armed with 3 subequally long lightly setulated caudal setae (Figs. 2D, 3C). Ovigerous spines paired, long, about 1.2 times as long as body (Fig. 2A). Spines basally conjoined, individual spines bifurcate and arise slightly beyond posterior margin of caudal ramus (arrowed in Fig. 3A). Spines slender, straight at their base and along shaft, distal part of spines broken off, thus preventing us to determine its length (Fig. 3E).
Antennule 4-segmented (Fig. 3B); antennule representing about 22% of total body length and 32% of cephalothorax length. Relative length of segments, from base to top as: 13.7; 19.7; 15.3; 51.5 = 100. Armature formula (Arabic numerals = setae, Roman numerals = spines) as: 0-I; 1-V; 2-I; 8-VIII (Fig. 2C). In terms of pattern described by Grygier and Ohtsuka (1995) for female monstrilloid antennular armature, setae (Roman numerals) and spines (Arabic numerals), element 1 present on first segment; elements on second segment: 2d1, 2d2, 2v1-3, and IId. Third segment with setal elements 3, IIId, and IIIv. Fourth segment long, representing 51.5% of antennule length; segment bearing elements 4d1-3; elements 4v1-3 well developed, thick, with remarkably long, spiniform element 4v1 reaching beyond halflength of segment; setae IVd, IVv, Vd, Vv, Vm, and aesthetasc 4aes also present on same segment; element 5 spiniform, not reaching distal end of segment. Subterminal element b3 unbranched, aesthetasc 6aes short, slender; element 61 present, element 62 absent, probably broken off during collection or handling (Fig. 2C). Fourth segment with pair of subdistal rounded processes on inner margin, adjacent to insertion of elements Vv and 5 (arrowed, “ip” in Fig. 3B); processes observed in same position on left antennule as well (Fig. 3C).
Incorporated first pedigerous somite and succeeding 3 free pedigerous somites each bearing a pair of biramous swimming legs. Pedigerous somites 2-4 together accounting for 23% of total length in dorsal view. Structure, segmentation and armature of swimming legs 1-4 as in C. chelemense (Suárez-Morales & Escamilla-Sánchez, 1997).
Legs slightly increasing in size posteriorly. Endopodites and exopodites triarticulated. Intercoxal sclerites subrectangular, widest at base, tapering distally, surface and posterior margin smooth. Basis of legs articulating with large, rectangular coxa along diagonal line. Basis with hair-like lateral seta (Fig. 4A-D); on leg 3, this seta about 2.5 times longer, lightly setulated from proximal half, slightly thicker than those on other legs (arrowed in Fig. 4C). Ramus setae all biserially plumose except short spiniform outer seta on exopod segments 1 and 3 of legs 1-4 (Fig. 4A-D). Spine on first exopodal segment of leg 4 digitiform (arrowed in Fig. 3E). Outermost apical exopodal setae of swimming legs 1-4 with inner margin naked, lightly spinulose, outer margin spinulose. Leg 4 exopod with distinctive digitiform outer spine on first segment (arrowed in Fig. 3E). Overall, the armature and segmentation of legs 1-4 is conservative in species of the longispinosum group (Mageed, 2010; Üstün et al., 2014; present data; Table 1).
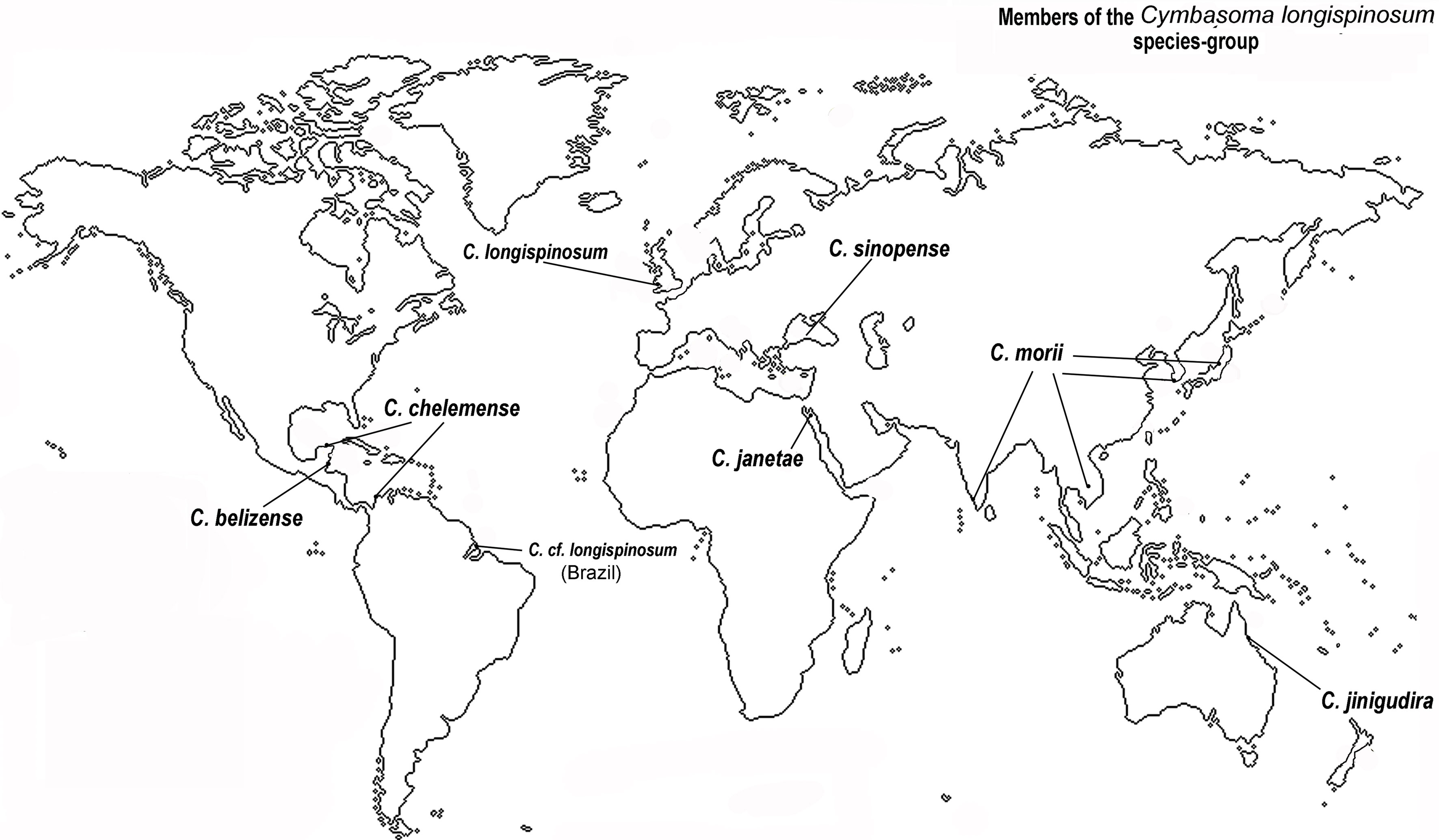
Figure 4 Worldwide distribution of known species of the Cymbasoma longispinosum species-group (Grygier, 1994; Suárez-Morales & Escamilla-Sánchez, 1997; Suárez-Morales & McKinnon, 2014; Suárez-Morales & Palomares-García, 1999; Üstün et al., 2014).
Table 1 Armature formula of swimming legs.
| Basis | Endopodite | Exopodite | |
| leg 1 | 1-0 | 0-1;0-1;1,2,2 | I-1;0-1;I,2,2 |
| legs 2-4 | 1-0 | 0-1;0-1;1,2,2 | I-1;0-1;I,1,2,2 |
Fifth legs medially conjoined, bilobed, inner (endopodal) lobe thumb-like, rounded distally. Outer (exopodal) lobe with rounded protuberance on distal inner margin, at insertion of innermost seta (“is”; Fig. 3D); exopodal lobe armed with 3 setae, 1 distal, 2 inserted subdistally. Outermost and medial setae long, biserially and lightly setulated; innermost seta shortest, slender, not reaching posterior margin of genital double-somite (Fig. 3A). Male. Unknown.
Taxonomic summary
Type material. Adult female holotype, dissected, mounted in 2 slides (ECO-CHZ-10341). Specimen collected from coastal waters off the Corozal Bay Wildlife Sanctuary, Corozal District, Belize (18°22’35.25” N, 88°21’03.62” W), NW Caribbean Sea; specimen partially dissected. Selected appendages, body and antennules on slides mounted on glycerine, sealed with Entellan®. Date of collection: 9 May, 2019 by L. Santoya (SACD- Belize) and Lourdes Vásquez-Yeomans (ECOSUR-Chetumal). Plankton sample. Slides deposited in the collection of Zooplankton at El Colegio de la Frontera Sur (ECOSUR), in Chetumal, Mexico.
Type locality. Corozal Bay (18°22’35.25” N, 88°21’03.62” W), off the Corozal District, Belize, Central America, on the western Caribbean Sea.
Etymology. The specific epiteth makes reference to the country in which the type locality is located, Belize, Central America. The name is proposed in genitive form with a neuter ending suffix to match the genus gender.
Remarks
This copepod from Belizean waters was assigned to the monstrilloid genus Cymbasoma Thompson, 1888 by virtue of the presence of a single free somite between the caudal rami and the genital double-somite, and its caudal rami being armed with only 3 caudal setae, both distinctive characters of Cymbasoma (Boxshall & Halsey, 2004; Isaac, 1975; Suárez-Morales, 2011; Suárez-Morales & McKinnon, 2014). It is assignable to the Cymbasoma longispinosum species-group by its size, body proportions with an elongate cephalothorax (+ 63% of total body length), relatively short urosome, proximally fused ovigerous spines, bilobed fifth legs with unarmed inner lobe and outer lobe bearing 3 setal elements, and the presence of conspicuous cuticular ornamentation on the genital double-somite (Grygier, 1994; Suárez-Morales, 2011; Üstün et al., 2014).
After the publication of Bourne’s (1890) original description of this species from the English Channel, the nominal C. longispinosum has been recorded from many different geographic areas and latitudes including the Mediterranean (Giesbrecht, 1893; Rose, 1933), Norway (Sars, 1921), the Australian coast and Philippines in the southern Pacific (Dakin & Colefax, 1940; Wilson, 1950), the eastern tropical Atlantic, the Red Sea (Gurney, 1927), India (Martin-Thompson, 1973), and Brazil (Dias, 1996; Dias & Bonecker, 2007; Duarte, 1999; Leite et al., 2010; Suárez-Morales et al., 2020, in press). Aside the improbability of a single monstrilloid species being so widely distributed some of these authors noted differences between their specimens and the original description of C. longispinosum. It was later realized that this nominal species contains a group of morphologically close cryptic species, each with a limited geographic distribution (Fig. 4). This notion supported the proposal of the longispinosum species-group and the subsequent description of new species linked to it (Grygier, 1994; Mageed, 2010; Suárez-Morales & Escamilla-Sánchez, 1997; Suárez-Morales & McKinnon, 2014; Suárez-Morales & Palomares-García, 1999). Only a few of the numerous records of C. longispinosum worldwide have been advanced as independent species and members of this group (Grygier, 1995). It is currently known to contain at least 7 nominal species showing subtle but consistent differences (Üstün et al., 2014) and are distributed in distinct geographical areas, including Europe (C. longispinosum s.str.), the Gulf of Mexico (C. chelemense Suárez-Morales & Escamilla-Sánchez, 1997), the Gulf of California (C. californiense Suárez-Morales & Palomares-García, 1999), Japan, Korea, Vietnam, India (C. morii Sekiguchi, 1982) (Chang, 2014; Grygier, 1994;), the Red Sea, Egypt (C. janetae Mageed, 2010), Turkey (C. sinopense Üstün, Suárez-Morales & Terbiyik, 2014), and western Australia (C. jinigudira Suárez-Morales & McKinnon, 2014) (Fig. 4). A Brazilian species of the group is currently being described (Suárez-Morales et al., in press).
Records of C. longispinosum from India (MartinThompson, 1973) were tentatively assigned to C. morii (Grygier, 1994), and the Brazilian specimens reported as C. longispinosum by Leite et al. (2010) and by Dias and Bonecker (2007) probably represent an undescribed species (Suárez-Morales, 2011; Suárez-Morales et al., in press; Üstün et al., 2014).
The new species from Belize most closely resembles C. morii, C. jinigudira, and C. californiense, mainly by its body size (female longer than 2 mm), relatively long cephalothorax (more than 65% of body length), the relatively long ovigerous spines bifurcating at distal end of caudal rami, and the position of oral papilla, among other characters (Suárez-Morales & McKinnon, 2014; Üstün et al., 2014). Distinctive characters of 6 known species of the C. longispinosum species-group are provided by Üstün et al. (2014).
Particularly, the new species C. belizense differs from its closest congeners, C. chelemense, C. morii, C. jinigudira, and C. californiense in several characters: 1) the peculiar arrangement of the forehead ridges, which are absent in C. morii, swirl-like in C. chelemense (Suárez-Morales & Escamilla-Sánchez, 1997), and transverse in C. californiense (Suárez-Morales & Palomares-García, 1999), thus diverging from the longitudinal ridges observed in the new species. This is a character that is probably more informative than previously thought when comparing species in this group (Suárez-Morales & Palomares-García, 1999); 2) the presence of a shelllike ventral cuticular process in the preoral surface is a relevant, unique character of C. sinopense from Turkey (Üstün et al., 2014), but it shares with most other species of the group including C. belizense, the pair of large ventral nipple-like processes (Fig. 2D); details of the cephalic ornamentation were not provided for C. janetae (Mageed, 2010) or C. longispinosum from Brazil (Leite et al., 2010); 3) in C. belizense, the last antennulary segment (51.2% of antennulary length) is among the longest of the longispinosum group (ranging between 46 and 51%), thus resembling both C. chelemense (51%) and C. californiense (51%) in this respect (Table I in Üstün et al., 2014); 4) a relatively short fifth leg innermost seta, which is longer than the fifth leg segment but not reaching the posterior margin of the genital double-somite (Fig. 3A). In other species of Cymbasoma the fifth leg innermost seta is absent or is smaller than the bearing segment; the longest fifth leg innermost seta among species of the longispinosum group was observed in C. sinopense, it is almost as long as the other 2 fifth leg setae (Üstün et al., 2014; Fig. 3D); 5) in the new species the point of bifurcation of the ovigerous spines is located slightly beyond or at the distal end of the caudal ramus. This is a character shared only with the Australian C. jinigudira (Suárez-Morales & McKinnon, 2014; Fig. 4B) and C. morii from Japan (Grygier, 1994; Fig 2b, c); 6) the inner distal rounded processes of the fourth antennulary segment observed in the new species is a character that has not been reported in other species of the group (Fig. 3B); 7) the produced, wrinkled corners of the genital somite is also advanced as a character unique to C. belizense (Fig. 3A). In addition, with a total length of 2.62 mm, the female of Cymbasoma belizense n. sp. is arguably the longest species known in the C. longispinosum species group. Its body length is comparable with that of C. sinopense from Turkey (2.5 mm) and C. morii from Japan (1.9-3.2 mm) (Grygier, 1994; Martin-Thompson, 1973; Üstün et al., 2014). It is smaller than specimens assigned to C. longispinosum by Giesbrecht (1893) and Rose (1933) from the Mediterranean and by Sars (1921) from Norway (2.3-3.16 mm). Leite et al. (2010) reported specimens from northern Brazil with a size range between 1.6 and 2.8 mm.
The evidence presented in this paper seems to be enough to recognize a new species, C. belizense, which represents the eighth nominal species assigned to the C. longispinosum species-group. It is also the second confirmed species record of the group in waters of the Caribbean Sea (Üstün et al., 2014). Overall, it is confirmed that the diversity of monstrilloid copepods is still underestimated in this region (Suárez-Morales, 2011).
The original description and most of the subsequently published records of this species lack the details that have been used recently in distinguishing the species assigned to the longispinosum group (Üstün et al., 2014). This is the second record of a species of the C. longispinosum speciesgroup in the Caribbean Sea after the finding of C. chelemense by Dorado-Roncancio and Dorado-Roncancio (2018) off the Caribbean coast of Colombia. Monstrilloids have been reported as parasites of different polychaete families, i.e.: Spionidae, Syllidae, Serpulidae, Capitellidae. Members of the Capitellidae and Spionidae are abundantly distributed along the coastal area of Chetumal Bay (Kuk-Dzul, 2007); these polychaetes are potential hosts for local monstrilloid copepods and their populations should be examined more closely to determine their host-parasite associations.
Key to the species currently assignable to the Cymbasoma longispinosum species-group (modified from Üstün et al., 2014).
Strong cuticular ridges present on forehead between antennule bases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
-- Cuticular ridges absent, forehead surface between antennule bases smooth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2. Urosome cuticular striae on genital double somite only. Relative length of cephalothorax with respect to total body length more than 68% . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
-- Urosome cuticular striae on genital double, anal, and fifth pedigerous somites. Relative length of cephalothorax with respect to total body length about 65%; fifth leg innermost seta as long as segment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C. californiense Suárez-Morales & Palomares-García, 1999 (Gulf of California, Mexico)
3. Forehead ridges arranged in longitudinal parallel pattern, medial ventral process between antennule bases absent; if present, shell-like. Ovigerous spines 1.2-2.0 times the length of body . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
-- Forehead ridges arranged in complex, swirl-like pattern, medial ventral process between antennule bases absent, ovigerous spines relatively short, about as long as body . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. chelemense Suárez-Morales & Escamilla-Sánchez, 1997 (Gulf of Mexico, Colombia)
4. Posterior margin of genital double-somite straight in dorsal view . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
-- Posterior margin of genital double-somite clearly curved, convex in dorsal view . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C. morii Sekiguchi, 1982 (Japan, India)
5. Cephalothorax representing less than 66% of total body length; point of bifurcation of ovigerous spines not reaching distal end of caudal rami. . . . . . . . . . . .C. cf. longispinosum from Brazil (Leite et al., 2010) (off Curuçá River, Brazil)
-- Cephalothorax representing more than 66% (usually 68-72%) of total body length; point of bifurcation of ovigerous spines beyond distal end of caudal rami . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
6. Point of bifurcation of ovigerous spines slightly beyond distal end of caudal rami; medial ventral process between antennule bases absent; fifth leg outer/inner lobes length ratio=1.9 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. longispinosum s.str. (Bourne, 1890) (Plymouth, United Kingdom)
-- Point of bifurcation of ovigerous spines well beyond distal end of caudal rami; medial ventral process between antennule bases present, rounded; fifth leg outer/inner lobes length ratio = 3.3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. janetae Mageed, 2010 (Red Sea, Egypt)
7. Point of bifurcation of ovigerous spines slightly beyond posterior margin of caudal rami; medial ventral cuticular process between antennule bases distinctively shell-like; fifth legs innermost seta long, about 0.9 times as long as other setae ... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. sinopense Üstün, Kurt & Suárez-Morales, 2014 (Turkey, Black Sea)
-- Large shell-like medial ventral cuticular process between antennule bases absent; fifth legs with thumb-like or globose inner lobe; cephalothorax smooth; fifth leg innermost seta 0.4-0.7 times as long as other fifth leg setae; lateral ocelli pigmentation weak or strong. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8. Fifth legs with innermost seta 0.4-0.7 times as long as other fifth leg setae, not reaching posterior margin of genital double- somite; cephalothorax with fringe of faint cuticular striae; fifth leg inner lobe of moderate size, thumb-like; lateral ocelli weakly pigmented; posterolateral corners of genital double-somite moderately produced, with field of deep wrinkles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C. belizense sp. n. (Belize, Central America)
-- Fifth legs with innermost seta 0.5-0.7 times as long as other fifth leg setae, reaching beyond posterior margin of genital double-somite; fifth leg inner lobe relatively small, globular; lateral ocelli strongly pigmented on inner margin; posterolateral corners of genital double-somite not produced, smooth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. jinigudira Suárez-Morales & McKinnon, 2014 (Western Australia)











 text new page (beta)
text new page (beta)


