The organotin compounds (OTC's) are considered a threat to marine life in areas with intense maritime activities and can act as endocrine disruptors in marine invertebrates, mainly gastropods (Axiak, Micallef, Muscat, Vella, & Mintoff, 2003; Fioroni, Oehlmann, & Stroben, 1991; Gagné, Blaise, Pellerin, Pelletier, Douville, M., Gauthier-Clerc, S. et al., 2003; Matthiessen, & Gibbs, 1998). One of the most studied toxic effects of OTC's on endocrine disruption in gastropods is imposex, which consists in the imposition of male sexual characters, like penis and vas deferens in females (Gibbs, & Bryan, 1987; Horiguchi, Shiraishi-Shimizu, & Morita, 1994; Mensink et al., 2002; Smith, 1981). Imposex is a widespread phenomenon known to occur in more than 190 species of marine gastropods throughout the world (Pessoa, Fernandez, Toste, Dores, & Parahyba, 2009). Due to its high sensitivity, imposex has been used as a biomarker of TBT contamination in several coastal countries such as the United Kingdom (Bryan, Burt, Gibbs, & Pascoe, 1993; Gibbs, Pascoe, & Burt, 1988), Japan (Horiguchi et al., 1994), Canada (Tester, & Ellis, 1995), Australia (Gibson, & Wilson, 2003), Patagonia (Bigatti & Penchaszadeh, 2005), Brazil (Castro, Alves De Lima, Braga, & Rocha-Barreira, 2007), Malaysia (Mohamat et al., 2010) and Mexico (Rodríguez-Romero, 2010). This study represents the first published report on Plicopurpura pansa ; this species is an intertidal carnivorous gastropod, which inhabits rocky intertidal beaches exposed to strong wave action. It is distributed in the Pacific Ocean from the northwestern Mexican coast (Keen, 1971) to northern Peru (Paredes, Huamán, Cardoso, Vivar, & Vera, 1999; Peña, 1970) and has been used by indigenous people to dye cotton and traditional fabric clothing (Turok et al., 1988). In this study, a higher incidence of imposex in areas close to human settlements was observed.
Regions with high human activity are expected to render zones with higher pollution levels than regions farther away from human settlements. Keeping this in mind, 5 habitats of P. pansa along the Pacific coastline of Mexico (Nayarit and Sinaloa states) with different proximity to human settlements were chosen (Fig. 1). In order to define the sampling area, a line of 50 m long parallel to the coast with 2 m width was studied.

Figure 1 Sampling sites in Nayarit and Sinaloa in the Pacific coast. Ocaso, Monas, Santa Cruz and Chacala in Nayarit; Olas Altas in Sinaloa.
According to the criterion of Stephenson and Stephenson (1949), the sampling was carried out in the mid-littoral and infra-littoral fringe, collecting all the specimens in the area during 2010 and 2011. The shell length, sex ratio (male:female) and imposex incidence (I%) within the population were obtained; then afterward, individuals were returned to the habitats. Imposex incidence was estimated through the percentage of imposex females in each site, using the following equation:
Data were analyzed using software Sigmastat 3.5 ®. Chi-square (p = 0.05) was used to determine significant statistical differences between sampling stations and imposex levels.
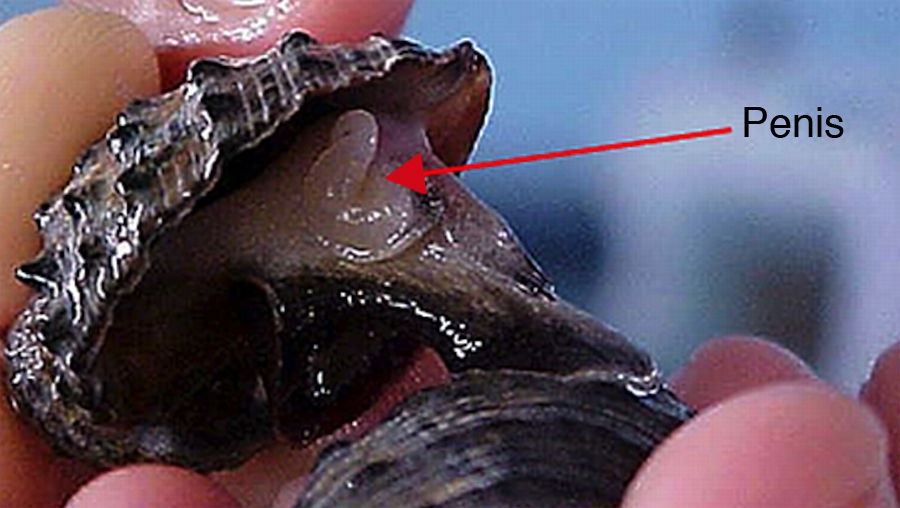
P. pansa males were identified by the penis, which is located behind the right cephalic tentacle, a common feature among neogastropods. This organ has a characteristic inverted-cedille form with 2-mm width becoming thicker at the base (Fig. 2). Females were identified due to (1) the absence of a penis, and (2) the presence of organs such as the albumen gland and capsule gland (Fig. 3).

Figure 3 Female anatomy of P. pansa in ventral view. Dg: digestive gland, Ag: albumen gland, Cg: capsule gland, Gon: gonad.
The female penis appears as a nub structure, no longer than 5 mm and no wider than 2 mm (Fig. 4). Thus, snails with a nub structure were considered as imposexed females. Ten females with this characteristic were examined under a stereomicroscope and all showed albumen and capsule glands, characteristic of females (Gibbs, & Bryan, 1994). Imposexed females found at the 4 sites showed no vas deferens and their penises were always smaller than those of the males.

Figure 4 (A) Imposexed female of P. pansa from Ocaso; (B) imposexed female of P. pansa from Olas Altas. Pseudopenis or imposex are marked with an arrow.
Imposex incidences were higher in the samples collected near harbor areas (Table 1). The highest percentage occurred in Olas Altas, the nearest site to the harbor in Sinaloa, with high anthropogenic activities with 28.1% of affected females, followed by Santa Cruz, the nearest site to the harbor in Nayarit with 21.3%. According to the chi-square test, significant differences were observed in the percentages of imposex between locations (p < 0.05) (Fig. 5).
Table 1 Imposex quantification in Plicopurpura pansa at the sampling sites. Data presented as mean±SD. SL: shell length; T: total snails collected; M: male; NF: normal females; IF: imposexed females; TF: total females; SR: sex ratio; I%: imposex percentage.
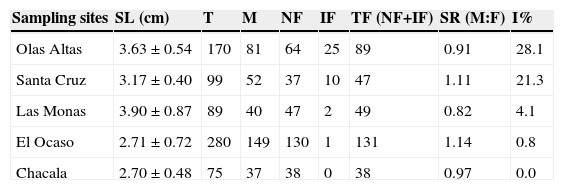

Figure 5 Percentage of imposex in Plicopurpura pansa of 5 study sites. *Statistically different with respect to Olas Altas and Santa Cruz. p<0.05 Chi square test.
In the study area, the size range of imposex affected females oscillated between 2.8 and 5.8 cm length. Evidence to date suggests that imposex is generally caused by tributyltin (TBT) (Davies, Harding, Bailey, Shanks, & Lange, 1997; Garaventa et al., 2008; Minchin, 2003; Smith, 1981), a chemical massively used in antifouling paints, which until recently was the most effective solution to prevent fouling on boat hulls (Terlizzi, Delos, Garaventa, Faimali, & Geraci, 2004). Several studies have linked imposex in neogastropods with TBT contamination from antifouling paint in the marine environment (DeFur, Crane, Ingersoll, & Rattersfield, 1999; Jenner, 1979; Li, & Collin, 2009; Ramón, & Amor, 2001; Short, Rice, Brodersen, & Stickle, 1989).
Despite the reduced percentage of imposex in the study area compared with other regions in North, Central and South America (Miloslavich, Penchaszadeh, & Bigatti, 2007; Pessoa et al., 2009; Tester, & Ellis, 1995), in the present study, imposex in P. pansa could be due to the presence of OTC's in the environment. Monitoring results suggest that harbors in the sampling area are the probable source of OTC pollution, demonstrated by a tendency of increased imposex levels in sampling points near the harbors.
OTC pollution is a major concern to the health of aquatic environments by the International Maritime Organization (IMO) which proposed a worldwide OTC ban, but the prohibition has not yet come into effect in many developing countries (Lousada, dos Santos, Castro, & Fillmann, 2013; Pessoa et al., 2009). Therefore, it is extremely important to control organotin contamination in order to review the enforcement of IMO rulings in Mexico. Although the sites chosen for this study showed low levels of imposex, Mexico needs to develop a monitoring policy to protect its coasts.
This is the first report of imposex in P. pansa in Mexico, but further research determining OTC dispersal would be valuable. Monitoring of the OTC's and the use of gastropods as sentinel organisms (Oehlmann et al., 1996) could be a useful tool for establishing the extent of OTC pollution on Mexican coasts, along with an analysis of the chemical composition of both water and sediments.
We thank Posgrado Ciencias Biológico Agropecuarias, Universidad Autónoma de Nayarit. This research was made possible through grants NAYARIT 2008-C01-93389, supported by Fomix-Nayarit.











 text new page (beta)
text new page (beta)