Introduction
The oxidative stress condition can be defined as unbalanced between the production and consumption of reactive oxygen species (ROS). [1] significant number of health conditions such as cancer, atherosclerosis, Alzheimer, Parkinson, and cardiovascular disorders have been related to the stress oxidative condition. [2] The ROS are molecules capable of causing cellular damage through oxidized reactions involving biological molecules like amino acids and proteins, [3] lipids, [4] and DNA; [5] the ROS are molecules that show different characteristics and reactivities; these can be radicals, anions, or neutral molecules, for example, hydroxyl radical □OH, superoxide O2 □□ and singlet oxygen 1O2. [1, 2]
The pro-oxidant molecules can generate oxidative stress in the organism by increasing the ROS concentration by one of the subsequent two mechanisms, the production of ROS or the inhibition of the biological mechanism to decrease ROS concentration. [6] The photosensitizer molecules are pro-oxidants capable of inducing oxidative stress through increase the ROS concentration by any of two types of reaction mechanisms: the type I mechanism involves the electron transfer from the photosensitizer to 3O2 molecule, and in this way, the O2 (- molecule is generated, this last molecule is a ROS and trough several reactions could generate ROS more reactive as □OH, □OR, □OOH and □OOR; while in the type II mechanism the photosensitizer can generate the 1O2 molecule, through the catalytic cycle where is involved the energy transfer between the photosensitizer and the 3O2 molecules, the 1O2 is a ROS that has higher reactivity to react with many biological molecules; in both ones, the oxidative stress is the result. [7, 8, 9]
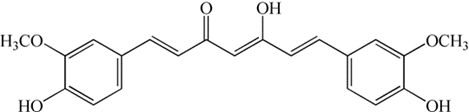
The curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] a yellow-orange dye isolated from Curcuma longa (Fig. 1). Curcumin is a molecule related to several biological activities: anti-inflammatory, [10] anti-fungal, [11] anti-cancer, [12] anti-Alzheimer, [13] antimicrobial, [14] and gastroprotection. [15] One of the most important activities of curcumin is the antioxidant activity, these have been reported in theoretical and experimental studies; [16-19] however, pro-oxidant activity has been reported from curcumin, and this activity has been related to some biological activities. It was observed that the curcumin act as a type II photosensitizer through the generation of 1O2 in ethanol media. [20] It was reported that the photosensitizer capability of curcumin could justify its antibacterial property against Streptococcus mutans in a study where it was considered in antibacterial photodynamic therapy- [21] In the same sense, curcumin accompanied with photodynamic therapy has been employed in osteosarcoma treatment. [22] In addition, studies related to antimicrobial photodynamic therapy have been reported that curcumin has an inhibitory effect on the Gram-positive and Gram-negative bacteria and Candida sp. [23]
According to the above, this work was carried out to study the photosensitizer capacity of curcumin in lipid media and employed quantum chemistry and computational kinetics methods. The single electron transfer (SET) mechanism was considered in all reactions proposed in this study. Furthermore, this study considered the presence of 3O2 and O2 □□ molecules due to the great concentration of the first one, and the second one is a metabolic product. This study presents kinetics data about the photosensitizer activity of curcumin to increase the knowledge about the pro-oxidant property of that molecule.
Experimental
Method and computational details
It was employed the conventional transition state theory (TST) in the calculations of rate constant, [24-26] according to the following equation:
where the k B, h, and R are the Boltzmann, Plank, and gas constant, respectively; T is the temperature, and ΔG ≠ is the Gibbs free energy of activation.
The reactions in this study considered the single electron transfer mechanism (SET); according to the Marcus theory, [27-29] the energy barrier was calculated from two thermodynamic parameters, the Gibbs free energy of reactions (ΔG 0) and the nuclear reorganization (λ), following the next equation:
The nuclear reorganization was calculated according to the following equation:
where E is the non-adiabatic energy difference between reactants and vertical products, this approximation is similar to that employed for many intramolecular electron exchange reactions. [30]
The apparent rate constant (kapp) cannot be obtained straight from the calculations obtained with the TST, especially when the rate constant calculated is close to or within the limit of the regime of the diffusion rate; hence, the colling-kimball theory was used, [31] following the next equation:
where the kD is the Smoluchowski rate constant of the stationary state, [32] for an irreversible bimolecular reaction controlled by diffusion and was calculated according to the following equation:
where r is the reaction distance, N A is the Avogadro number, and D AB is the coefficient of mutual diffusion of A and B reactants in a bimolecular reaction. D AB has been calculated from D A and D B, [33] and these last ones were calculated from the Stoke-Einstein approximation, following the next equation. [34,35]
where η is the solvent viscosity, in this case, pentyl ethanoate (η=8.62 x 10-4 Pa s) and
The geometry optimization and frequency calculation were carried out with the package of programs Gaussian 09, [36] employing the functional M06-2X and the basis-set 6-31G(d,p), employing the solvent model of continuum density (SDM)[37] with pentyl ethanoate as a solvent to mimic the lipid media. [38-40] The M06-2X is among the best performing functionals for calculating reaction energies involving free radicals and for kinetic calculations in solutions. [41,42] The geometry optimization and frequency calculations from the first singlet excited-state (1CUR*) employed TD-DFT calculations, including the solvent effects, in the TD-M06-2X/6-31G(d,p) level.
The methodology employed in this study is in line with the quantum mechanics-based test for overall free radical scavenging activity.[43] That is validated by experimental results comparison, and its uncertainty was probed that is not bigger than those arising of the experiments.
Results and Discussion
The study of the photosensitizer capability from curcumin was employed de enol form because this is the most important in keto-enol equilibrium in non-polar media. [43] The curcumin absorption was calculated in lipid media, and the maximum absorption was 373.41 nm; this is close to the experimental value observed. [44]
In the photosensitizer capacity of curcumin in lipid media were considered the electron transfer reactions between the curcumin in its basal (1CUR), excited (1CUR* and 3CUR), oxidized (1PN□+), and reduced (1PN□-) forms and the oxygen (3O2) and radical anion superoxide (O2 □-) molecules because they are abundant in the organism. The reaction considered in this study were the following:
| 1CUR + 3O2 → CUR•+ + O2 •‒ | (R1) |
| 1CUR* + 3O2 → CUR•+ + O2 •‒ | (R2) |
| 3CUR + 3O2 → CUR•+ + O2 •‒ | (R3) |
| 1CUR + O2 •‒ → CUR•‒ + 1O2 | (R4) |
| 1CUR + O2 •‒ → CUR•‒ + 3O2 | (R5) |
| 1CUR* + O2 •‒ → CUR•‒ + 1O2 | (R6) |
| 1CUR* + O2 •‒ → CUR•‒ + 3O2 | (R7) |
| 3CUR + O2 •‒ → CUR•‒ + 1O2 | (R8) |
| 3CUR + O2 •‒ → CUR•‒ + 3O2 | (R9) |
| 1CUR + 1CUR → CUR•+ + CUR•‒ | (R10) |
| 1CUR + 1CUR* → CUR•+ + CUR•‒ | (R11) |
| 1CUR + 3CUR → CUR•+ + CUR•‒ | (R12) |
| 1CUR* + 1CUR* → CUR•+ + CUR•‒ | (R13) |
| 1CUR* + 3CUR → CUR•+ + CUR•‒ | (R14) |
| 3CUR + 3CUR → CUR•+ + CUR•‒ | (R15) |
| CUR•+ + O2 •‒ → 1CUR + 1O2 | (R16) |
| CUR•+ + O2 •‒ → 1CUR + 3O2 | (R17) |
| CUR•+ + O2 •‒ → 3CUR + 1O2 | (R18) |
| CUR•+ + O2 •‒ → 3CUR + 3O2 | (R19) |
| CUR•‒ + 3O2 → 1CUR + O2 •‒ | (R20) |
| CUR•‒ + 3O2 → 3CUR + O2 •‒ | (R21) |
The study of the photosensitizer capacity of curcumin was begun with the thermodynamic study of the reactions proposed previously, and the results are shown in Table 1. The results showed that the R5, R6, R7, R8, R9, R13, R14, R15, R16, R17, R18, and R19 reactions were exergonic reactions, thus feasible reactions in lipid media and could contribute to the photosensitizer capacity of curcumin. While the reactions R1, R2, R3, R4, R10, R11, R12, R20, and R21 are endergonic reactions, they did not contribute to the photosensitizer capacity of curcumin in lipid media.
Table 1 Gibbs free energy of reaction (ΔG, kcal/mol) of the reactions considered in the photosensitizer capacity of curcumin in lipid media, at 298.15 K.
| Reaction | ΔG | |
| R1 | 1CUR + 3O2 → CUR•+ + O2 •‒ | 94.99 |
| R2 | 1CUR* + 3O2 → CUR•+ + O2 •‒ | 27.87 |
| R3 | 3CUR + 3O2 → CUR•+ + O2 •‒ | 46.61 |
| R4 | 1CUR + O2 •‒ → CUR•‒ + 1O2 | 24.73 |
| R5 | 1CUR + O2 •‒ → CUR•‒ + 3O2 | -13.37 |
| R6 | 1CUR* + O2 •‒ → CUR•‒ + 1O2 | -42.39 |
| R7 | 1CUR* + O2 •‒ → CUR•‒ + 3O2 | -80.49 |
| R8 | 3CUR + O2 •‒ → CUR•‒ + 1O2 | -23.65 |
| R9 | 3CUR + O2 •‒ → CUR•‒ + 3O2 | -61.75 |
| R10 | 1CUR + 1CUR → CUR•+ + CUR•‒ | 81.62 |
| R11 | 1CUR + 1CUR* → CUR•+ + CUR•‒ | 14.50 |
| R12 | 1CUR + 3CUR → CUR•+ + CUR•‒ | 33.24 |
| R13 | 1CUR* + 1CUR* → CUR•+ + CUR•‒ | -52.63 |
| R14 | 1CUR* + 3CUR → CUR•+ + CUR•‒ | -33.88 |
| R15 | 3CUR + 3CUR → CUR•+ + CUR•‒ | -15.14 |
| R16 | CUR•+ + O2 •‒ → 1CUR + 1O2 | -56.89 |
| R17 | CUR•+ + O2 •‒ → 1CUR + 3O2 | -94.99 |
| R18 | CUR•+ + O2 •‒ → 3CUR + 1O2 | -8.51 |
| R19 | CUR•+ + O2 •‒ → 3CUR + 3O2 | -46.61 |
| R20 | CUR•‒ + 3O2 → 1CUR + O2 •‒ | 13.37 |
| R21 | CUR•‒ + 3O2 → 3CUR + O2 •‒ | 61.75 |
These results showed the remarkable capacity of curcumin to be reduced and accept an electron; in this case, the reducing agent is the O2 (- molecule. On the other hand, the reactions involved in the photosensitizer capacity of curcumin consumed O2 •‒ and yielded a 1O2 molecule, mainly; hence, the photosensitizer capacity of curcumin in lipid media could be attributed to the type II mechanism.
According to the above results, the kinetic study was carried out in the exergonic reactions agree with the thermodynamic study; the reactions considered in the kinetic study are R5, R6, R7, R8, R9, R13, R14, R15, R16, R17, R18, and R19.
The results of the kinetic study on the photosensitizer ability of curcumin in lipid media are shown in Table 2. The barrier in the reactions R5, R8, R15, and R18 were 0.46, 0.39, 0.99, and 2.03 kcal/mol; due to the lower barriers shown by these reactions, the diffusion rate was limited to the reaction rate constants. On the other hand, the barrier of the reaction R19 was 6.40 kcal/mol, and the reaction rate constant calculated was 1.24 x 108 M-1 s-1. In addition, it was observed a barrier of 11.27 kcal/mol and a reaction rate constant of 3.39 x 104 M-1 s-1 by the R6 reaction. Finally, the highest barriers and slowest reaction rate constants calculated to be observed were R6, R7, R9, R13, R14, R16, and R17 reactions.
Table 2 Gibbs free energy of activation (ΔG ≠, kcal/mol), reorganization energy (λ, kcal/mol), the reaction rate constant of diffusion, and apparent (k D and k app, kcal/mol) from the photosensitizer capacity of curcumin in lipid media, at 298.15 K.
| Reaction | ΔG≠ | λ | kD | kapp | |
| R5 | 1CUR + O2 •‒ → CUR•‒ + 3O2 | 0.46 | 19.33 | 8.68 x 109 | 8.66 x 109 |
| R6 | 1CUR* + O2 •‒ → CUR•‒ + 1O2 | 11.27 | 15.75 | 8.83 x 109 | 3.39 x 104 |
| R7 | 1CUR* + O2 •‒ → CUR•‒ + 3O2 | 61.97 | 16.51 | 8.83 x 109 | 2.23 x 10-33 |
| R8 | 3CUR + O2 •‒ → CUR•‒ + 1O2 | 0.39 | 18.28 | 8.73 x 109 | 8.71 x 109 |
| R9 | 3CUR + O2 •‒ → CUR•‒ + 3O2 | 23.94 | 19.04 | 8.73 x 109 | 1.77 x 10-5 |
| R13 | 1CUR* + 1CUR* → CUR•+ + CUR•‒ | 119.77 | 4.78 | 7.66 x 109 | 1.00 x 10-75 |
| R14 | 1CUR* + 3CUR → CUR•+ + CUR•‒ | 24.14 | 7.31 | 7.66 x 109 | 1.25 x 10-5 |
| R15 | 3CUR + 3CUR → CUR•+ + CUR•‒ | 0.99 | 9.11 | 7.66 x 109 | 7.61 x 109 |
| R16 | CUR•+ + O2 •‒ → 1CUR + 1O2 | 23.89 | 16.81 | 8.58 x 109 | 1.93 x 10-5 |
| R17 | CUR•+ + O2 •‒ → 1CUR + 3O2 | 85.22 | 17.58 | 8.58 x 109 | 2.10 x 10-50 |
| R18 | CUR•+ + O2 •‒ → 3CUR + 1O2 | 2.03 | 21.80 | 8.58 x 109 | 8.23 x 109 |
| R19 | CUR•+ + O2 •‒ → 3CUR + 3O2 | 6.40 | 22.57 | 8.58 x 109 | 1.24 x 108 |
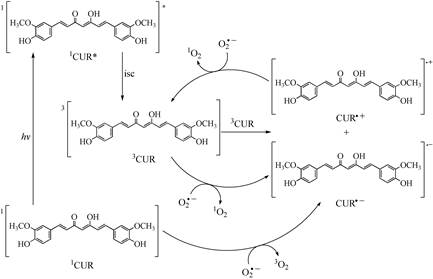
The most important reactions in the photosensitizer capacity of curcumin in lipid media were shown in Scheme 1. According to the kinetic results, the 1CUR* evolve to 3CUR through the intersystem crossing. The 3CUR can react through two routes of reaction: in the first one, the 3CUR was reduced by O2 □- to generate CUR□- and 1O2, this route of reaction supports the type II mechanism in the photosensitizer capacity of curcumin; in the second route of reaction, two molecules of 3CUR react to yield CUR(- and CUR(+ as products of the reaction. The CUR□+ can be reduced in the presence of O2 □- and this reaction yielded 3CUR and 1O2 as products; this reaction supported the type II mechanism of photosensitizer capacity of curcumin. In the R5, R8, R18, and R19 reactions, the property of curcumin was observed to accept an electron in the presence of O2 □- molecule.
The photosensitizer capacity of curcumin in lipid media showed that the main mechanism is type II and is favored by the R6, R8, and R18 reactions, while the reactions that support the type I mechanism were endergonic, according to the thermodynamic study.
According to the above, we can define a reaction rate constant to the type II mechanism, following the next equation:
The rate constant for the type II mechanism in photosensitizer capacity of curcumin in lipid media was 1.69 x 1010 M-1 s-1.
According to the above, curcumin is a pro-oxidant agent in lipid media because it is capable of generating oxidative stress through the type II mechanism, where the main product yielded was the 1O2 molecule; the total reaction rate constant by the type II mechanism was 1.69 x 1010 M-1 s-1. Also was observed that the curcumin was unable to generate oxidative stress through the type I mechanism.
Conclusion
It was carried out the study of the photosensitizer capacity of curcumin in lipid media employing the density functional theory. This study considered the single electron transfer reactions mechanism and the presence of 3O2 and O2 □□ were considered. The thermodynamic results observed the remarkable capacity of curcumin to reduce itself through the single electron transfer mechanisms. The kinetic results showed that the pathways are R6, R8, and R18 to support the mechanism type II; hence, the total reaction rate constant calculated for the photosensitizer capacity of curcumin in lipid media was 1.69 x 1010 M-1 s-1. The reaction related to the mechanism type I were not feasible reactions, and hence, these reactions were not contributed to the photosensitizer capacity of curcumin in lipid media. Finally, these results support the idea that the curcumin in lipid media is a pro-oxidant molecule capable of generating the 1O2 molecule and could generate oxidative damage through the photooxidative process.











 text new page (beta)
text new page (beta)