Introduction
Plants have developed the foundation of traditional systems of medicine that have been in existence for thousands of years due to the presence of vital bioactive constitutes [1]. According to World Health Organization (WHO), about 80 % population is dependent on herbal medicines as their prime health care resource. Greater use and recognition of traditional medicines demonstrate that in the worldwide market, traditional medications continue to be strong [2].
Therapeutic plants are the harbinger of numerous beneficial secondary metabolites such as alkaloids, flavonoids, saponins, anthraquinones, vitamins, terpenoids, minerals, coumarins, tannins, glycosides, inorganic, and phenolic compounds. Consumption of medicinal plants as food items is recommended for the management of diabetes mellitus. These herbal medicines are either taken singly or in combination with other plant species and give access to inherent biologically effective components for the therapeutic effect [3]. The extracts of Z. officinale, M. charantia and S. cumini have been reported to decrease hyperglycemia, oxidative stress, lipid peroxidation and activity of carbohydrate metabolism enzymes [4]. According to Arya et al. [5] Parthenium hysterophorus has a hypoglycemic effect.
Aside from antioxidant, antimicrobial, hypoglycemic and numerous activities of natural products, limited recognition regarding diverse therapeutic attributes of medicinal plants such as Momordica charantia, Syzygium cumini, Zingiber officinale and Parthenium hysterophorus exist. Additionally, valid experimental evidence outweighing their toxic properties is limited. In view of the given valuable properties of these plants and in the quest for the development of new strategies based on natural products to mitigate the numerous pathophysiological manifestations, the current study was designed to explore the enzyme inhibitory (alpha glucosidase and acetylcholinesterase) and cytotoxicity capacities of solvent fractions of these indigenous plants.
Experimental
Sample collection
Momordica charantia L. (fruit), Syzygium cumini L. (fruit) and Zingiber officinale L. (bulb) were collected from the local retail market, while Parthenium hysterophorus L. (leaves) was collected from the rose garden of the Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan. Plant samples were authenticated by Dr. Mansoor Hameed, Associate Professor, Department of Botany, University of Agriculture, Faisalabad, Pakistan and voucher specimens were deposited in the Botany department herbarium.
Preparation and fractionation of extracts
Plant parts were shade dried and ground into fine powder after washing with the tap water and then preserved in the airtight containers for further usage. Extracts were prepared by keeping 100 g powder of each sample in the 1L solvent i.e. methanol (CH3OH) at room temperature for seventy-two hours. Filtrates were dried completely in a water bath. Later, distilled water was utilized to dissolve these extracts and separating funnel was used for fractionation of a few polarity-based solvents viz. ethyl acetate, chloroform, n-hexane, methanol, n-butanol and ethanol along with water at 1:10:10 ratio (extract: distilled water: solvent) and then for further use were preserved at 4 ºC [6].
Enzyme inhibition assays
Alpha glucosidase inhibitory assay
Alpha glucosidase inhibitory assay of selected medicinal plants was conducted as explained by Alu’datt et al. [7]. Briefly; p-nitrophenyl-α-D-glucopyranoside substrate (5 mM) solution was prepared in the 0.1M phosphate buffer having a pH of 6.9. Alpha glucosidase enzyme (1.0 U/mL) solution (G5003-100UN, from S. cerevisiae, Sigma Aldrich, USA) was formed in the 0.1M phosphate buffer having a pH of 6.9. Enzyme solution (500 µL) along with 100 µL of plant extracts was added into the test tube and then incubated for ten minutes at 25 °C. After that 500 µL of substrate solution was added and then again kept at room temperature for five minutes. Then absorbance of all samples was recorded using a spectrophotometer at 405 nm. Extraction solvent was used to replace the extract in the negative control sample, whereas acarbose was used as a positive control. Following equation (1) was used to calculate percentage inhibition:
Acetylcholinesterase inhibitory assay
Inhibitory assay of acetylcholinesterase was accomplished as mentioned by Rahman et al. [8]. In two separate beakers, (15 g) Na2HPO4.2H2O was dissolved in (750 mL) distilled water and their pH was set at 8 and 7. Then volume was made up to 1L by adding more distilled water and these solutions were kept at 4 °C. Buffer solutions having pH of 8 and 7 are known as phosphate buffer-I and -II, which were used for enzyme solution, test sample preparation, and Ellman’s reagent respectively. For enzyme solution, 0.0025 U/mL acetylcholinesterase (C3389-2KU, from E. electricus, Sigma Aldrich, USA) was dissolved in the phosphate buffer-1 and later placed in the iced water bath at the 5 °C. Acetylcholine iodide (108.35 mg per 5 mL distilled water) was the substrate preserved at 4 °C. In 10 mL solution of phosphate buffer-II, (15 mg) NaHCO3 and (36.9 g) Ellman’s reagent were dissolved and this mixture was also kept at 4°C. Following is the brief protocol: In a test tube, plant extract (30 µL) + Ellman’s reagent (100 µL) + phosphate buffer-I (2.8 mL) + enzyme solution (30 µL) were added and incubated for 10 minutes at 25 °C. Then (30 µL) substrate solution was added. Absorbance was checked at 412 nm using a spectrophotometer. Extraction solvent was used to replace the extract and was used for samples of control, whereas amigra (physostigmine) was used as a positive control. Percentage inhibition was assessed by using the equation 1f.
Toxicological analysis - Mutagenicity Assay
Ames test was established on a validated bacterial reverse mutation experiment. The experimental setup is given in Table 1. The liquid culture was used to conduct this assay [9]. K2HPO4 (1.12 g), KH2PO4 (0.48 g), (NH4)2SO4 (0.65 g), MgSO4 (0.76 g) and trisodium phosphate (0.08 g) were dissolved in 160 mL distilled water to prepare Davis-Mingioli salt (reagent mixture). Then D-glucose (14.34 mL), Bromocresol purple (16 mL), D-biotin (8.6 mL) and L-Histidine (0.1 mL) were added to 151 mL of Davis-Mingioli salt aseptically in a sterile bottle. The following procedure was adopted: TA98 and TA100, mutant strains of S. typhimurium were maintained at 3 ± 1 ºC on the nutrient agar. Beforethe test, strains of bacteria were incubated at 37 ºC for 1 day following the inoculation on nutrient broth. Standard mutagens were K2Cr2O7 (potassium dichromate) and sodium azide (NaN3) for TA 98 and TA 100 strains respectively. Preparation of methanolic extracts of all plants was done by their reconstitution in dimethyl sulfoxide (DMSO) (10 mg/mL). Methanolic extracts of plants along with reagent mixture, standard mutagen and deionized water were mixed in numerous sterilized bottles as indicated in Table 1 and then inoculation of these mixtures was done with both strains of S. typhimurium. After inoculation, each bottle solution was poured into 96 well microtiter plate which was then incubated at 37ºC for about four days. Results interpreted visually as yellow/turbid wells and purple wells were counted as positive and negative wells showing reverse- and no reverse mutation respectively. If yellow wells numbers were significantly greater in the test plate treated with plant extracts and standard mutagen than the numbers of positive wells in the background plate which was neither treated with plant extract nor with standard mutagen that extract was thought to be mutagenic. Plant extracts were supposed to be toxic to test strains if all wells in the test plate exhibited purple color.
Results and Discussion
Methanolic extracts of Z. officinale, S. cumini, M. charantia and P. hysterophorus were used for the preparation of fractionations in solvents i.e., ethyl acetate, chloroform, n-hexane, methanol, n-butanol and ethanol. The effects of inhibitions by these fractions on alpha glucosidase as well as acetylcholinesterase activities were investigated, and their percentage inhibition values are presented in Tables 2 and 3.
Table 2 Alpha glucosidase inhibition assay.
| Extracts | Plants | ||||
| Acarbose | P. hysterophorus | Z. officinale | S. cumini | M. charantia | |
| Water | 79.91 ± 0.77a | 28.30 ± 0.70l | 25.37 ± 0.77lm | 24.55 ± 0.11mn | 50.44 ± 0.72h |
| Chloroform | - | 21.25 ± 0.25no | 39.41 ± 0.77j | 35.15 ± 0.59k | 61.00 ± 0.94ef |
| Methanol | - | 33.25 ± 0.43k | 50.33 ± 0.99h | 73.91 ± 1.05b | 72.30 ± 1.17b |
| Ethyl acetate | - | 8.16 ± 0.04rs | 13.35 ± 0.02pq | 61.50 ± 0.85e | 68.46 ± 1.19c |
| n-butanol | - | 11.27 ± 0.15qr | 41.40 ± 0.53j | 66.05 ± 0.53cd | 64.51 ± 1.04de |
| Ethanol | - | 15.44 ± 0.19p | 5.30 ± 0.16s | 57.53 ± 0.71fg | 45.48 ± 0.48i |
| n-hexane | - | 9.24 ± 0.15r | 20.52 ± 0.21o | 16.35 ± 0.16p | 56.35 ± 0.98g |
Data presented as mean percentage ± S.E. Means sharing similar letters either in a column or in a row are statistically non-significant (P>0.05).
Table 3 Acetylcholinesterase inhibition assay.
| Extracts | Plants | ||||
| Acarbose | P. hysterophorus | Z. officinale | S. cumini | M. charantia | |
| Water | 81.52 ±1.01a | 3.34 ± 0.16opq | 14.68 ± 0.13ij | 4.21 ± 0.15n-q | 1.03 ± 0.09q |
| Chloroform | - | 4.95 ± 0.40n-q | 13.04 ± 0.11ijk | 5.22 ± 0.12n-q | 12.19 ± 0.23i-m |
| Methanol | - | 11.40 ± 0.26j-m | 44.05 ± 0.76ef | 71.55 ± 0.80b | 50.12 ± 0.82d |
| Ethyl acetate | - | 8.45 ± 0.68k-o | 14.37 ± 0.22ij | 12.76 ± 0.12i-l | 5.18 ± 0.11n-q |
| n-butanol | - | 2.55 ± 0.23pq | 40.45 ± 0.70f | 7.65 ± 0.16l-p | 17.29 ± 0.10i |
| Ethanol | - | 1.84 ± 0.09q | 25.20 ± 0.52h | 29.31 ± 0.14gh | 32.14 ± 0.64g |
| n-hexane | - | 0.23 ± 0.14q | 32.52 ± 2.51g | 48.04 ± 4.12de | 9.03 ± 1.18k-n |
Data is represented as mean percentage ± S.E. Means sharing similar letters either in a column or in a row are statistically non-significant (P>0.05). (P is p).
Alpha glucosidase inhibition assay
Table 2 represents the inhibition of alpha glucosidase by the various fractionations of botanical extracts. All the samples had an inhibitory effect on alpha glucosidase but methanolic fractionations of each plant exhibited greater inhibitory efficacy against enzyme action as compared to other fractionations. Except for the methanolic extract of Parthenium hysterophorus (33.25 ± 0.43), all other studied plants viz. Zingiber officinale (50.33 ± 0.99), S. cumini (73.91 ± 1.05) and Momordica charantia (72.30 ± 1.17) indicated more than 50% alpha glucosidase inhibitory potentials. The same plants with effective inhibition of alpha glucosidase have already been demonstrated to possess hypoglycemic potential, as α-glucosidases inhibition has become one of the major strategies to cure diabetes mellitus [10].
Although acarbose (positive control) exhibited maximum alpha glucosidase inhibition i.e. 79.91 ± 0.77 % (Table 2), side effects of this as well as other antidiabetic drugs encourage the consumption of the medicinal plants as safer natural substitutes. Although one investigation has reported 82 % alpha glucosidase inhibitory efficacy of acarbose, such results can be justified by the variations in experimental layout [11].
In the current study, ethanolic extract of M. charantia revealed greater enzyme inhibition following the methanolic fraction. Among all the fractionations of studied plants, minimum activity (8.16 ± 0.04) was revealed by the ethyl acetate fraction of P. hysterophorus and percentage inhibition of other fractionations was in the range of 33.25 ± 0.43 to 9.24 ± 0.15. While Z. officinale fractions exhibited the following trend in ascending order: chloroform < ethyl acetate < water < ethanol < n-hexane < n-butanol. Alu’datt et al. [7] reported that individual doses of methanolic extract and combined extract mixture (water and acetone) of ginger exhibited greater inhibitions of 82.33 % and 86.15 % respectively against α-glucosidase.
Regarding M. charantia, the least enzyme inhibition activity was given by ethanolic extract (45.48 ± 0.48), while the trend in ascending order for other fractions was as follows: ethyl acetate < n-hexane < chloroform < n- butanol. According to Sallau et al. [12], terpenoid-rich extract of bitter gourd had the highest IC50 value (1.60 mg/mL) against glucosidase enzyme. Earlier, Nhiem et al. [13] also documented moderate antidiabetic efficacy in terms of inhibition of alpha glucosidase in crude extracts as well as purified constituents from M. charantia.
On the other hand, water extracts of both S. cumini and Z. officinale exhibited less percentage inhibitions i.e. 24.55 ± 0.11 and 25.37 ± 0.77 respectively. S. cumini fractions followed the trend in descending order as: n-hexane > ethanol > ethyl acetate > n- butanol > chloroform. Previously, Alagesan et al. [14] also confirmed that phenolic compounds present in S. cumini play a vital role in the management of diabetes mellitus through alpha glucosidase inhibition. Shinde et al. [15] reported the inhibitory potential of 1-butanol, acetone, ethyl acetate and ethanol fractions of Syzygium cumini seeds against α-glucosidase in the range of 2.8 ± 0.1 to 24.6 ± 0.7 µg/mL.
Laoufi et al. [16] stated that a direct relationship exists between plants phenolic or flavonoid components and their ability to block or decrease α-amylase and α-glucosidase activities. Because a wide number of bioactive constituents are distributed in the polar fraction, it is believed that phenolic components and quercetin as polar compounds are responsible for the inhibition of alpha glucosidase and hence participate in the management of hyperglycemia. Phenolics influence the function of glucose and insulin receptors by augmenting the GLUT2 expression in the beta cells of the pancreas and also translocate the GLUT4 via AMP-activated protein kinase and AKT pathways. Among all the efficacies revealed by phenolics, the best is the inhibition of alpha glucosidase [17].
Bhatia et al. [18] also studied the inhibitory effect of plants against α-glucosidase. It was suggested that plants can be used for efficient normalization of hyperglycemia with the least side effects in contrast to acarbose as the majority of flavonoids were illustrated as competitive inhibitors which compete with the substrate in binding to the enzyme’s active site. Plants are a rich source of inhibitors of alpha glucosidase and therefore comparison and contrast among natural inhibitors and synthetic inhibitors are the focus of recent scientific research [17].
Acetylcholinesterase inhibition assay
Neurodegenerative diseases are mostly caused by the deficiency of acetylcholine. Acetylcholinesterase (AChE) terminates the action of neurotransmitters by hydrolyzing it into acetic acid and choline and influences cholinergic dysfunction coupled with Alzheimer’s disease (AD), ultimately activity of enzymes is raised in patients of AD as it is the regulator of cholinergic neurotransmission. Hence, AChE is targeted for AD therapy. Likewise, higher efficiency of AChE is detrimental to AD patients and free radicals in diabetes mellitus results in neural damage and AD. Persistent hyperglycemia interacts with receptors of acetylcholine, affecting binding affinities, leading to a rise in acetylcholinesterase levels and decomposition of neurotransmitters [19].
Donepenzil, tacrine, galantamine and rivastigmine are the recommended acetylcholinesterase inhibitors [20] but few studies have warned that these drugs have extreme side effects i.e. hepatotoxicity and tremor are caused by tacrine and galantamine as well as weight loss, vomiting and diarrhea [21]. In this context, antiamnesic efficacy (in terms of percentage inhibition) of selected medicinal plants i.e. P. hysterophorus, Z. officinale, S. cumini and M. charantia was performed and results of different fractionations were in the range of 0.23 ± 0.14 to 11.40 ± 0.26, 13.04 ± 0.11 to 44.05 ± 0.76, 4.21 ± 0.15 to 71.55 ± 0.80 and 1.03 ± 0.09 to 50.12 ± 0.82 respectively (Table 3). Among different fractionations, methanolic extract of all the tested plants demonstrated a higher percentage of inhibitory activity viz. 11.40 ± 0.26 (P. hysterophorus), 44.05 ± 0.76 (Z. officinale), 71.55 ± 0.80 (S. cumini) and 50.12 ± 0.82 (M. charantia) against AChE which recommended that this organic solvent extract has greater number of bioactive constituents with maximum AChE inhibition potentials. Physostigmine showed a percentage inhibition of 81.52 ± 1.01.
According to Al-Snafi [22], numerous medicinal plants have been reported to exert pharmacological effects on the central nervous system because of the presence of phytochemicals but further exploration is still required. Phytoconstituents such as terpineol and alpha-terpinene, members of the class monoterpene possessed anti-amnesic activity. Tyloside and quercetin are also used in the management of typical dementia associated with AD. Flavonoids because of their antioxidant and anti-amyloidogenic potentials can slow the neurodegenerative processes in AD [23].
In a previous study, Oboh et al. [24] stated that water extract of Z. officinale in a dose-dependent manner significantly inhibited the AChE efficacy which was further confirmed by El-Akabawy and El-Kholy [25] that expression of AChE is down-regulated by intake of Z. officinale in diabetic rats. Nagarani et al. [26] stated 77.6 % AChE inhibition by Momordica charantia. Furthermore, in an earlier study, it was declared that terpenoids are effective AChE inhibitors and Kuguacin J is among those terpenoids isolated from leaves of M. charantia and is believed to possess efficacy against AChE [27], whereas flavonoid derivatives also act as antiamnesic agents [28].
Darusman et al. [29] stated that different fractionations of S. cumini i.e. methanol, n-hexane and ethyl acetate showed AChE inhibition with IC50 value above 50 µg/mL. A study done by Alikatte et al. [30] confirmed that methanolic extract of S. cumini significantly functioned as an anti-amnesic agent for the management of spatial memory impairments induced by scopolamine in rats. However, Ajiboye et al. [19] demonstrated that polyphenols isolated from leaves of S. cumini showed inhibitory efficacy of AChE in a dose-dependent manner and hence indicated that extract can serve as a neurotransmitter booster.
The outcomes of our research work verified the therapeutic roles of tested plants in neurodegenerative diseases and underlying mechanisms are proposed by several studies. Khan et al. [31] and Kong et al. [32] suggested that phytoconstituents exhibit synergism during inhibition of AChE and reinforced these results by molecular docking simulations for the mechanism of enzyme action. Secondary metabolites either interfere with the substrate binding step by shielding the active site or interfere with the release of product [33]. However, further additional analysis in this regard is required.
Toxicological evaluation
An imperative prerequisite for the assessment of medicinal plants as curative agents in the toxicological screening. As methanolic fractions of Zingiber officinale, S. cumini, Momordica charantia and Parthenium hysterophorus were most potent during enzyme inhibition assays, therefore, these were used in further analysis.
Mutagenic assay/ Ames test
Several techniques are used for the evaluation of plant extracts as mutagenic or non-mutagenic. Ames test is one such method that uses bacteria to assess whether a chemical can cause mutations in the DNA of the organism. This biological assay is mostly employed to determine the toxicological effects of phytochemicals [34].
Mutagenicity of studied medicinal plants i.e. Zingiber officinale, S. cumini, Momordica charantia and Parthenium hysterophorus was assessed utilizing the two strains of S. typhimurium i.e. TA 98 and TA 100. Firstly, purple color was noticed in all the wells of the blank plate demonstrating no change and consequently indicating no contamination (Fig. 1(a)). Standard mutagens and all tested plates were seen visually, and numbers of negative and positive plates were counted very carefully. While considerable mutagenicity with greater numbers 94/96 and 87/96 of yellow (positive) wells were noticed in standard mutagen plates of TA 98 (Fig. 1(b)) and TA 100 (Fig. 1(c)) respectively. Results of the tested plant were compared with background plates TA 98 and TA 100 which exhibited 14/96 (Fig. 1(d)) and 28/96 (Fig. 6(e)) positive wells respectively. Purple coloration in all tested plates confirmed that plant extract is toxic to the strain. If yellow wells in the tested plate are two folds greater than the background plate, medicinal plants are thought to be mutagenic [35].
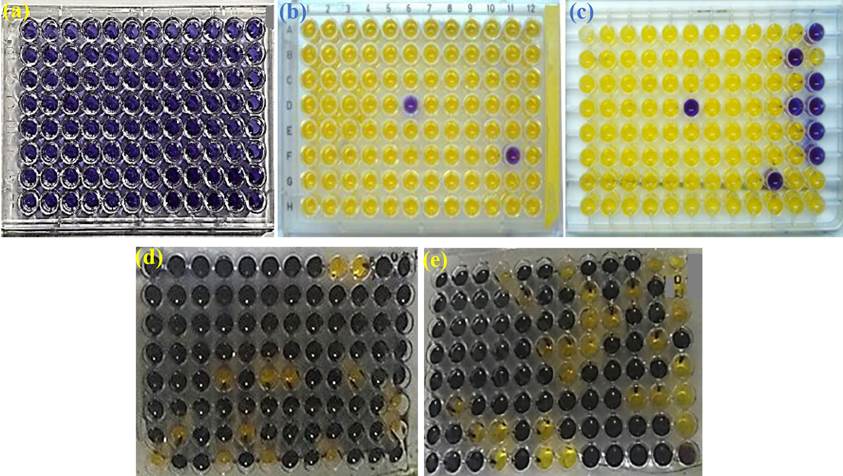
Fig. 1 (a) Blank plate, (b) Standard mutagen TA 98, (c) Standard mutagen TA 100, (d) Background plate TA 98, (e) Background plate TA 100.
Data presented in Table 4 demonstrated that among studied plants, Parthenium hysterophorus was toxic and mutagenic. Earlier, Roy and Shaik [36] also confirmed that in TA98 strain crude extract of P. hysterophorus showed mutagenicity. Being a noxious weed, the habitat of Parthenium hysterophorus is Asia, Australia, Africa and America and it causes dermatitis, respiratory problems, allergies, as well as mutagenicity in livestock and human [37].
Table 4 Mutagenicity assay.
| Plants | Number of +ve wells /total number of wells for TA 98 | Number of +ve wells /total number of wells for TA 100 | ||
| +ve/total | Result | +ve/total | Result | |
| Standard | 94/96 | + | 87/96 | + |
| Background | 14/96 | 28/96 | ||
| Z. officinale | 18/96 | - | 6/96 | - |
| S. cumini | 23/96 | - | 11/96 | - |
| M. charantia | 20/96 | - | 26/96 | - |
| P. hysterophorus | 74/96 | + | 90/96 | + |
Standard for TA98: Potassium dichromate, Standard for TA100: Sodium azide
+ = Mutagenic
‒ = Non-mutagenic
Investigations have illustrated that several plants used in traditional medicine or food possessed mutagenic as well as carcinogenic and toxic properties. Mutagenic constituents i.e. tannins, anthraquinones and furocoumarins are present in many plants [38]. That’s why screening medicinal plants is crucial to check their mutagenicity. Plants with mutagenic potentials should be deemed unsafe and consequently, further testing is mandatory before their consumption. According to Gautam et al. [39], the Ames test identified 50-70 % of known carcinogens. Mutations viz. frameshift mutations as well as the substitution in base pair are detected by two species of S. typhimurium TA 98 and TA 100 respectively.
Earlier in vivo and in vitro reports demonstrated that fruit and seeds of M. charantia have auspicious effects on the treatment of tumors and antimutagenic behavior. While momordin, a protein of M. charantia, has also shown anticancer efficacy [40].
Abudayyak et al. [41] demonstrated that methanolic and chloroform extracts of Zingiber officinale are non-mutagenic for TA 98 and TA 100 strains in the presence and absence of S9 metabolic activation, while water extract showed mutagenic and non-mutagenic efficacy against TA98 in presence and absence of S9 metabolic activation respectively. Numerous in vivo and in vitro studies investigated the antimutagenic properties of Z. officinale [42, 43].
According to Bekoe et al. [44] M. charantia showed non-mutagenicity against TA 98 and TA 100 and such a result is in accordance with the aforementioned results of current research. Previously, Islam and Jalaluddin [45] demonstrated that three varieties (white, dark green and light green) of M. charantia revealed lower antimutagenic potential against sodium azide with Salmonella typhimurium TA 100. A study done by Adewumi et al. [46] revealed that extract of M. charantia can modify the immune response in cancer patients by decreasing the IL-7 secretion from the intestine, hence decreasing the lymphocyte number as well as raising the population of NK-cells and T-helper cells. Phenolic constituents of M. charantia have exhibited anticancer activities [47]. Earlier another report demonstrated that triterpenoids and MAP30 isolated from fruit and seeds of M. charantia have shown auspicious effects for the treatment of tumors.
Ramos et al. [48] evaluated the mutagenic activity of the crude extract of P. hysterophorus against five strains of Salmonella i.e. TA 1535, TA 98, TA 102, TA1537 and TA 100. However, it did not show mutagenic potential, while opposed results are noticed in the current study. Khan et al. [49] investigated the antimutagenic efficacy of the ethyl acetate extract of S. cumini against TA 100 at different concentrations and findings revealed non-toxicity. Various studies demonstrated that the antimutagenic potential of plant extracts might be due to a considerable number of flavonoids and phenolics [34].
In the end, it can be concluded that antimutagenic efficacies of phenolics and flavonoids enrich plant extracts are mainly attributed to their ability to hinder mitochondrial enzymes P450 which is significantly responsible for physiological activation of the indirect mutagens. Oxidative intermediates which are responsible for DNA damage are neutralized by these phytoconstituents and are considered a protection mechanism based on the scavenging of free radicals. Furthermore, a complex of flavonoids and DNA via either non-covalent or covalent interaction is also believed to be responsible for protection against DNA damage.
Conclusion
The extracts exhibited significant enzyme inhibitory potentials against alpha glucosidase and acetylcholinesterase activities. Among all tested fractions, methanolic extracts of all plants showed the highest bioactivities. Regarding mutagenicity assays, Momordica charantia, Syzygium cumini, and Zingiber officinale were non-mutagenic. Further assessment of bioactive efficacies can offer authentic natural products for the management of numerous pathological conditions.











 text new page (beta)
text new page (beta)


