Introduction
Coronavirus disease (COVID-19) is a deadly disease caused by a novel severe acute respiratory syndrome coronavirus (SARS-Cov-2) that first appeared in Wuhan province in China [1, 2]. Coronaviruses (CoVs) are single-stranded positive sense RNA viruses belong to the Coronaviridae family. The genome is 26-32 kb in size and has 6-11 open reading frames (ORFs) that code for 9680 amino acid polyproteins [3]. The virus primarily affects the respiratory system, resulting in flu-like symptoms such as coughing, fever, and, in severe cases, difficulty breathing [4].
Based on genomic organization and phylogenetic relationship, coronaviruses have been classified into the subfamily Coronavirinae that consists of four genera, i.e., Alphacoronavirus (αCoV), Betacoronavirus (βCoV), Gammacoronavirus (γCoV), and Deltacoronavirus (δCoV) [5]. The surface viral protein spike, membrane, and envelope of coronavirus are embedded in the host membrane-derived lipid bilayer encapsulating the helical nucleocapsid comprising viral RNA [6]. Coronaviruses have four proteins that enclose the viral genomic RNA: membrane glycoprotein (M), nucleocapsid protein (N), envelope protein (E), and spike glycoprotein (S) [7]. After proteolytic cleavage, the S protein forms two subunits, S1 and S2, which are important in the virus entrance pathway to host cells [8]. S1 promotes receptor binding, whereas S2 helps membrane fusion; both are essential for virus entrance into the endocytic pathway. The endocytic pathway is mainly employed as the primary viral entry mechanism. It is reported that SARSCoV-2 exploits the receptor that is angiotensin converting enzyme II (ACE2) for entry into the host cells [9]. The major sites of ACE2 protein expression are epithelial cells of the human lung and small intestine [10]. Since SARS-CoV-2 also interacts with ACE2 receptor [11] and is also susceptible to inhibitors so it was assumed that it follows the same endocytic pathway for infection [12]. Entry of CoVs into the host cells is mainly mediated by the endocytic pathway, meanwhile the autophagy has also been implicated in the viral replication in the cells, a process partly related to the formation of double-membrane vesicles in the host cells. As a result, several groups of inhibitors including the lysosomotropic agents such as CQ and inhibitors for clathrin-mediated endocytosis such as chlorpromazine have been proposed to have therapeutic efficacy against CoVs-induced diseases including COVID-19 [12]. Several evidences reported that Clathrin-mediated endocytosis is the key mechanism for coronaviruses for entry into the host cell [13-15].
The paper highlights the potential antiviral activity of plant compounds as effective and reliable agents against viral infections. Various antiviral mechanisms shown by crude plant extracts and plant-derived bioactive compounds. The understanding of the action mechanisms of complex plant extract and isolated plant-derived compounds will help pave the way towards the combat of this life-threatening disease [16]. Ashwagandha (Withania somnifera) mentioned in Ayurveda belongs to the family Solanaceae. All parts of this plant are utilized in the prevention and cure of a wide range of ailments as it contains alkaloids, flavonoids and steroidal lactones. The root of Ashwagandha has been applied as a tonic, stimulant, astringent, aphrodisiac, anthelmintic, diuretic, thermogenic and narcotic [17]. The natural phytochemicals of Ashwagandha has distinct effects on the viral RBD (receptor-binding domain) and host ACE2 (angiotensin-converting enzyme 2) receptor complex. Natural phytochemicals may be a viable option in managing host cells for COVID-19 entry. The antiviral potential of Ashwagandha may project as potential one in fighting for management of COVID-19 infections and other virus infection because of its tremendous immuno boosting properties [18-19]. Harsingar (Nyctanthes arbor-tristis Linn.) belongs to the family Oleaceae. The plant has been screened for anti-malarial anti-histaminic, anti-arthritis activities, local anaesthetic, anti-hypnotic, analgesic, anti-ulcer, anti-pyretic, anti-depressant, anti-cancer, anti-larvicidal, anti-allergic, antiviral, immune modulatory, anti-helminthic, antioxidant, antidiuretic, antioxidant, and CNS modulatory properties [20-21]. Harsingar possesses antiviral activity against enveloped virus (V) [22]. Murraya koenigii (also known as the ‘curry tree’, meethi neem) is an important medicinal plant. Several parts of this plant constitute the vital ingredients of many Ayurvedic formulations such as fresh leaves, fruits, bark, and roots are used to treat several health disorders such as diabetes, bronchial disorders, vomiting and skin ailments [23]. Through various research and pharmacological evaluation, it has been shown that the plant extracts possess antiviral, anti-inflammatory, antioxidant, antidiabetic, antidiarrhoeal, antileishmanial, and antitumor activity [24]. Tulsi (Ocimum sanctum Linn.) belongs to the family Lamiaceae. This aromatic shrub has been reported for antidiabetic, wound healing, antioxidant, radiation protective, immunomodulatory, antifertility, anti-inflammatory, antimicrobial, antistress and anti-cancer activities [25-26]. Hydro-alcoholic extract ofOcimum sanctuminhibited intracellular multiplication of virus. It also inhibits non-specific interference with virus-cell interactions in H9N2 viruses [27-28]. Tulsi have been revealed to target reverse transcriptase activity and have shown inhibitory actions towards HIV proteases [29].
Molecular docking plays an important role in predicting the binding modes and binding abilities of small molecules towards the targeted proteins, which is crucial in designing potential drugs [30]. In view of the above, the present investigation was undertaken to identify the active constituents of Ashwagandha, Harsingar, Meethi neem and Tulsi extracts through LC-QTOF-MS/MS analysis and an in-silico study was carried out to predict the molecular interaction between the clathrin and the ligands that were identified in Ashwagandha, Harsingar, Meethi neem and Tulsi extracts.
Experimental
Materials and methods
Plant material collection
Meristematic leaf of Harsingar, Meethi neem, Tulsi was collected from Motilal Nehru National Institute of Technology Allahabad, Prayagraj and dry root of Ashwagandha was collected from the shop in Teliarganj near Motilal Nehru National Institute of Technology Allahabad. The plant material was cleaned with deionized water, followed by distilled water wash, and cut into small pieces.
Preparation of Extract
About 5 g of plant material was taken and added into 100 ml of distilled water and boiled for 3 min. After boiling, the extract was filtered with Whatman filter paper and keep in the oven for drying at 50 °C for 48 hrs. After drying, the extract was dissolved in DMSO.
HR-LCMS analysis of LCME
The bioactive components of Harsingar, Meethi neem, Tulsi and Ashwagandha extract were analyzed by High Resolution Liquid Chromatograph Mass Spectrometer (HR-LCMS) G6550A system (Agilent technologies). The method used for Chroma tography was 30 mins ± ESI 11012021_MSMS.m. The Gas temperature used for analysis was 250 °C and theoretical mass of protonated com pound was used for identification. HR-LC-MS analysis of Harsingar, Meethi neem, Tulsi and Ashwagandha extracts was performed at Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology, Mumbai, India. The compounds were identified by comparison with their retention time (RT) and mass with stored metlin library available with IIT, Bombay [31]. The compounds containing a variety of bioactive constituents, such as alkaloids, phenolic compounds, saponins, flavonoids which make them a suitable treatment option against viral infections were selected [32].
Molecular Docking
Clathrin protein structure
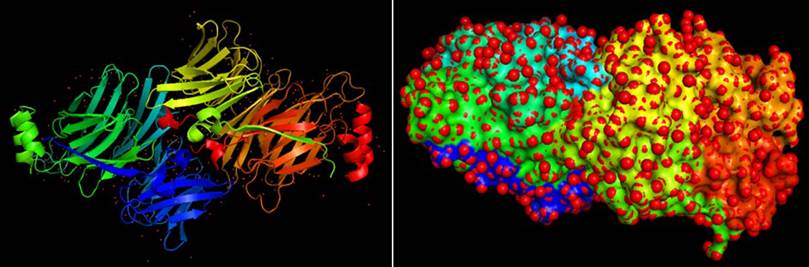
The 3D structure of clathrin protein (PDB ID:1UTC) was downloaded from PDB (Protein Data Bank) by using URL http://www.rcsb.org/PDB in pdb format (Figure 1). The PDB structure of the clathrin protein was generated by eliminating water molecules, adding hydrogen atoms and Kollman charges. The protein file was saved in PDBQT format for future analysis, and then AutoDock Tools 1.5.6 was used to optimize the file for docking.
Ligands
Ligands were selected from the results of HR-LCMS and they were downloaded from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). SMILES was used to design the structures of all compounds, and 3-D optimization was done using chemsktech software and save them in MOL format. Following that, the Open Babel tool was used to convert the compounds from MOL to PDB format and save them [33]. The ligand files were saved in PDBQT format and then optimized for docking using AutoDock Tools 1.5.6 for further research.
Docking of Protein and Ligand
The molecular docking of the screened ligands with clathrin protein was performed using AutoDock 1.5.6 [34]. In this process, the grid was setup onto the clathrin protein and grid parameter file was saved. The grid map x-y-z-dimension was 47.3 Angstroms. Then target proteins were docked with screened ligands and binding energy was calculated. During the docking procedure, the various conformations for ligand were generated, but the best conformations with minimal energy were considered as output. Using the Autodock tool results were analyzed and the binding energies of all 55 ligands with clathrin protein along with H-bond formation were estimated.
ADME and Toxicity analysis
The online tool ALOGPS 2.1 (http:/www.vcclab.org/lab/alogps/) [35] was used to conduct the ADME analysis of the selected ligands and to estimate their logP and logS values. The molecular structure of compounds was submitted to ADMET-SAR server (http://lmmd.ecust.edu.cn/admetsar1/home/) [36] to analyzed their blood brain barrier penetration, carcinogenicity, subcellular localization, LD50 and category of acute oral toxicity.
Results and discussion
HR-LCMS analysis
HR-LCMS analysis of LCME resulted in the presence of phytoconstitu ents from Ashwagandha, Harsingar, Meethi neem and Tulsi. Among them, there are 55 compounds from Ashwagandha, Harsingar, Meethi neem and Tulsi (Table 1) were known for antiviral and steroidal properties. Medicinal plant extracts potentially improve the inherent antiviral defense mechanisms of the human body, which involve a complicated system and might use several concurrent pathways [37].
Table 1 The phytoconstitu ents from Ashwagandha, Harsingar, Meethi neem and Tulsi with their molecular formula.
| S.No. | Compounds | Extracted from plant | Molecular Formula |
|---|---|---|---|
| 1. | Ammothamnine | Ashwagandha | C15 H24 N2 O2 |
| 2. | Petasinine | Ashwagandha | C13 H21 N O3 |
| 3. | Fusicoccin H | Ashwagandha | C26 H42 O8 |
| 4. | Stanolone benzoate | Ashwagandha | C26 H34 O3 |
| 5. | 16alpha,17alpha-Dihydroxyprogesterone acetophenide | Ashwagandha, Harsingar, Meethi neem, Tulsi | C29 H36 O4 |
| 6. | Neohesperidin | Ashwagandha, Meethi neem | C28 H34 O15 |
| 7. | Eugenol O-[3,4,5-Trihydroxybenzoyl-(->6)-b-D-glucopyranoside] | Ashwagandha, Meethi neem | C23 H26 O11 |
| 8. | 3beta-Hydroxy-16-phosphonopregn-5-en-20-one monoethyl ester | Ashwagandha, Harsingar, Meethi neem | C23 H37 O5 P |
| 9. | Norethindrone acetate | Ashwagandha | C22 H28 O3 |
| 10. | 3-Hydroxycoumarin | Harsingar, Meethi neem, Tulsi | C9 H6 O3 |
| 11. | Quercetin | Harsingar | C15 H10 O7 |
| 12. | Kaempferol 3-rhamnoside 7-galacturonide | Harsingar | C27 H28 O16 |
| 13. | Peganine | Harsingar, Meethi neem, Tulsi | C11 H12 N2 O |
| 14. | Bufotalin | Harsingar | C26 H36 O6 |
| 15. | Polyporusterone D | Harsingar | C28 H44 O5 |
| 16. | 6''-Malonylastragalin | Harsingar | C24 H22 O14 |
| 17. | (3S,5R,6R,7E)-3,5,6-Trihydroxy-7-megastigmen-9-one | Harsingar | C13 H22 O4 |
| 18. | Aromadendrin 4'-methyl ether 7-rhamnoside | Harsingar | C22 H24 O10 |
| 19. | Geranyl acetoacetate | Harsingar | C14 H22 O3 |
| 20. | 7-Dehydrologanin tetraacetate | Harsingar | C25 H32 O14 |
| 21. | Piceatannol 4'-galloylglucoside | Harsingar | C27 H26 O13 |
| 22. | L-Menthyl acetoacetate | Harsingar | C14 H24 O3 |
| 23. | Silandrin | Harsingar | C25 H22 O9 |
| 24. | Maritimetin | Meethi neem, Tulsi | C15 H10 O6 |
| 25. | 6-C-Galactosylluteolin | Meethi neem, Tulsi | C21 H20 O11 |
| 26. | 8-Epideoxyloganin tetraacetate | Meethi neem | C25 H34 O13 |
| 27. | Demethyloleuropein | Meethi neem | C24 H30 O13 |
| 28. | 8-Hydroxypinoresinol 8-glucoside | Meethi neem | C26 H32 O12 |
| 29. | 10-Acetoxyligustroside | Meethi neem | C27 H34 O14 |
| 30. | trans-Caffeic acid [apiosyl-(1->6)-glucosyl] ester | Meethi neem | C20 H26 O13 |
| 31. | Pimentol | Meethi neem | C23 H26 O12 |
| 32. | Hesperidin | Meethi neem | C28 H34 O15 |
| 33. | Cynaroside | Meethi neem | C21 H20 O11 |
| 34. | Pedaliin | Meethi neem | C22 H22 O12 |
| 35. | Tectoridin | Meethi neem | C22 H22 O11 |
| 36. | Irisolidone 7-O-glucuronide | Meethi neem | C23 H22 O12 |
| 37. | Solanocapsine | Tulsi | C27 H46 N2 O2 |
| 38. | Apigenin 7-glucoside | Tulsi | C21 H20 O10 |
| 39. | 3-Methylellagic acid 8-rhamnoside | Tulsi | C21 H18 O12 |
| 40. | Butin | Tulsi | C15 H12 O5 |
| 41. | Fluticasone propionate | Tulsi | C25 H31 F3 O5 S |
| 42. | Caffeic acid | Tulsi | C9 H8 O4 |
| 43. | Sulochrin | Tulsi | C17 H16 O7 |
| 44. | Quercetin 7-glucuronide 3-rhamnoside | Tulsi | C27 H28 O17 |
| 45. | Ginkgetin | Tulsi | C32 H22 O10 |
| 46. | Hesperetin 3',7-O-diglucuronide | Tulsi | C30 H34 O16 |
| 47. | Neoastilbin | Tulsi | C21 H22 O11 |
| 48. | Sagerinic acid | Tulsi | C36 H32 O16 |
| 49. | Chrysosplenol D | Tulsi | C18 H16 O8 |
| 50. | Epicatechin-(4beta->8)-epigallocatechin 3-O-gallate | Tulsi | C37 H30 O17 |
| 51. | Baicalin | Tulsi | C21 H18 O11 |
| 52. | 6-Methoxyaromadendrin 3-O-acetate | Tulsi | C18 H16 O8 |
| 53. | Phytolaccoside E | Tulsi | C42 H66 O16 |
| 54. | Flavonol 7-O-beta-D-glucoside | Tulsi | C21 H20 O9 |
| 55. | 6-Hydroxy-2-(4-hydroxyphenyl)-5,7-dimethoxy-4H-1-benzopyran-4-one | Tulsi | C17 H14 O6 |
Molecular Docking
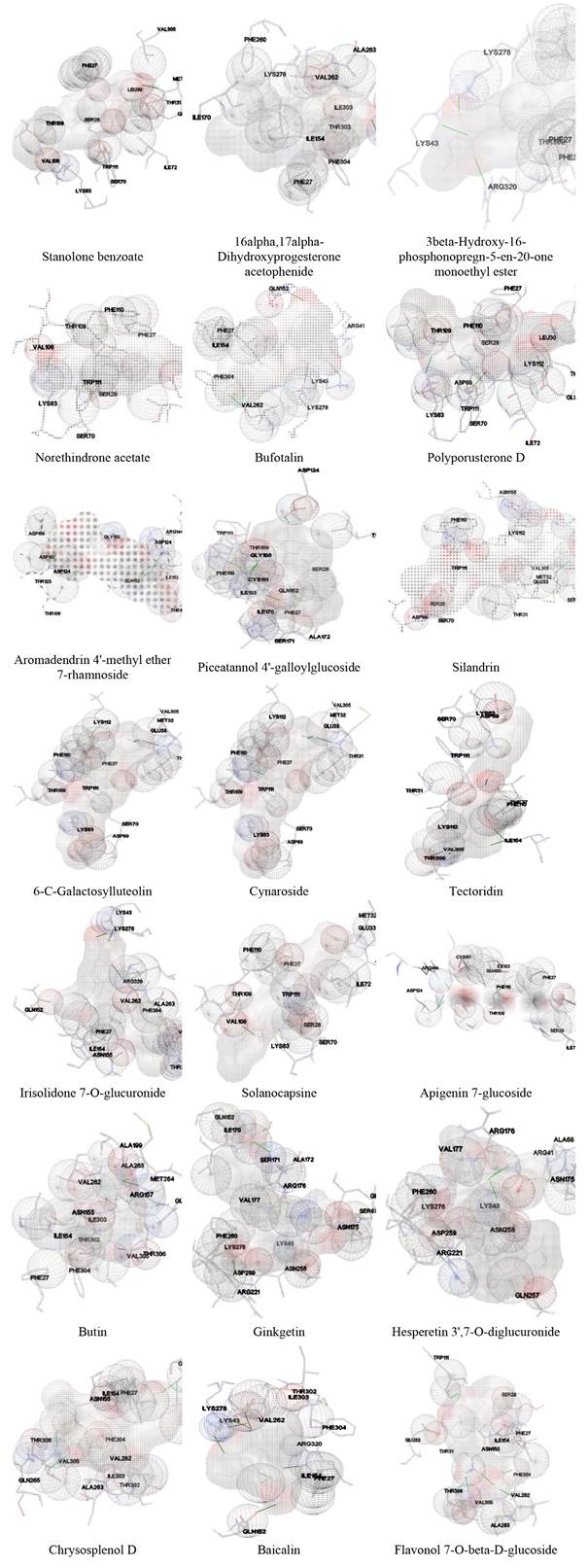
Molecular binding studies of the clathrin protein with selected ligands were performed and estimate binding energies using AutoDock 4.2. Docking studies have been used to evaluate numerous natural or synthetic compounds as drugs against various diseases in a relatively fast and reliable manner. The lowest binding energy indicates the most significant interaction between ligand and protein [38]. Different binding positions and binding energies, as well as the bond length of H-bonds, are depicted in Table2 and Fig. 2. From the Table 2 it is evident that, minimum binding energy was shown by the extracted compounds with Solanocapsine i.e., -11.34 Kcal/mol majorly followed by 16alpha,17alpha-Dihydroxyprogesterone acetophenide, Polyporusterone D, Flavonol 7-O-beta-D-glucoside, Stanolone benzoate, Ginkgetin, Norethindrone acetate, Silandrin, 6-C-Galactosylluteolin, Irisolidone 7-O-glucuronide, 3beta-Hydroxy-16-phosphonopregn-5-en-20-one monoethyl ester, Aromadendrin 4'-methyl ether 7-rhamnoside, Tectoridin, Bufotalin, Chrysosplenol D, Apigenin 7-glucoside, Butin, Baicalin, Piceatannol 4'-galloylglucoside, Hesperetin 3',7-O-diglucuronide and Cynaroside having -10.35, -9.65, -9.62, -9.61, -9.56, -9.51, -9.37, -9.25, -9.13, -9.04, -8.93, -8.83, -8.72, -8.38, -8.25, -8.24, -8.24, -8.21, -8.17 and -8.05.
Table 2 Docking results of selected ligands with clathrin protein.
| S.No. | Compound name | Binding Energy (kcal/mol) | Bond length (Å) | Chain | Residue |
|---|---|---|---|---|---|
| 1. | Ammothamnine | -7.38 | 2.098 | A | LYS112 |
| 2. | Petasinine | -6.27 | 1.753 | A | THR306 |
| 3. | Fusicoccin H | -5.69 | 1.971 | A | ALA48 |
| 4. | Stanolone benzoate | -9.61 | 2.125 | B | MET32 |
| 5. | 16alpha,17alpha-Dihydroxyprogesterone acetophenide | -10.35 | 2.09 | B | THR306 |
| 6. | Neohesperidin | -7.04 | 2.179 | B | LYS83 |
| 7. | Eugenol O-[3,4,5-Trihydroxybenzoyl-(->6)-b-D-glucopyranoside] | -7.11 | 2.139 | A | ASN155 |
| 8. | 3beta-Hydroxy-16-phosphonopregn-5-en-20-one monoethyl ester | -9.04 | 2.157 1.749 2.162 | A B B | LYS43 LYS278 ARG320 |
| 9. | Norethindrone acetate | -9.51 | 1.766 | B | LYS83 |
| 10. | 3-Hydroxycoumarin | -6.5 | 2.011 1.756 1.823 | B | MET264 ALA199 ARG157 |
| 11. | Quercetin | -7.63 | 1.937 | B | THR306 |
| 12. | Kaempferol 3-rhamnoside 7-galacturonide | -6.87 | 2.179 1.993 1.852 1.753 2.095 | A B A A A | ARG41 LYS278 TRP111 LYS43 ILE153 |
| 13. | Peganine | -6.32 | 1.889 | B | THR306 |
| 14. | Bufotalin | -8.72 | 1.982 2.175 2.032 | A A B | GLN152 LYS278 LYS43 |
| 15. | Polyporusterone D | -9.65 | 2.146 | B | LYS83 |
| 16. | 6''-Malonylastragalin | -7.92 | 1.797 | B | SER67 |
| 17. | (3S,5R,6R,7E)-3,5,6-Trihydroxy-7-megastigmen-9-one | -6.72 | 2.138 1.955 | A | TRP111 LYS83 |
| 18. | Aromadendrin 4'-methyl ether 7-rhamnoside | -8.93 | 1.974 1.721 | A B | ASP124 ARG144 |
| 19. | Geranyl acetoacetate | -6.18 | 2.238 2.219 | A | ASN155 GLN265 |
| 20. | 7-Dehydrologanin tetraacetate | -6.99 | 2.195 2.071 1.921 1.99 | A B B B | ARG41 PHE27 ARG320 ARG320 |
| 21. | Piceatannol 4'-galloylglucoside | -8.21 | 1.843 2.134 1.882 | A | TRP111 GLN152 ILE153 |
| 22. | L-Menthyl acetoacetate | -6.86 | 1.764 | B | ALA71 |
| 23. | Silandrin | -9.37 | 1.839 1.814 1.816 | B | TRP111 ASN155 GLN265 |
| 24. | Maritimetin | -7.53 | 2.058 | A | TRP111 |
| 25. | 6-C-Galactosylluteolin | -9.25 | 1.823 2.056 | B | MET32 TRP111 |
| 26. | 8-Epideoxyloganin tetraacetate | -6.86 | 1.865 1.98 2.039 | A A B | ARG144 ILE153 LYS83 |
| 27. | Demethyloleuropein | -4.59 | 1.656 2.057 1.981 2.108 1.734 | A B B B B | LYS278 ARG41 LYS43 LYS43 ALA48 |
| 28. | 8-Hydroxypinoresinol 8-glucoside | -6.76 | 1.987 | B | ARG320 |
| 29. | 10-Acetoxyligustroside | -6.31 | 1.58 | A | ILE153 |
| 30. | trans-Caffeic acid [apiosyl-(1->6)-glucosyl] ester | -5.98 | 2.065 2.073 2.048 2.003 | B | LYS278 THR302 THR306 ARG320 |
| 31. | Pimentol | -6.21 | 2.232 | B | ILE153 |
| 32. | Hesperidin | -7.57 | 1.721 1.838 | A | LYS83 ILE153 |
| 33. | Cynaroside | -8.05 | 2.216 | A | THR306 |
| 34. | Pedaliin | -7.57 | 2.125 | A | TRP111 |
| 35. | Tectoridin | -8.83 | 2.117 2.154 | A | TRP111 THR306 |
| 36. | Irisolidone 7-O-glucuronide | -9.13 | 1.888 1.97 2.114 | A B B | LYS43 LYS278 ARG320 |
| 37. | Solanocapsine | -11.34 | 2.129 | B | LYS83 |
| 38. | Apigenin 7-glucoside | -8.25 | 1.695 2.1 | B | ARG144 ILE153 |
| 39. | 3-Methylellagic acid 8-rhamnoside | -7.03 | 1.879 2.038 2.084 | A B B | ALA172 SER70 TRP111 |
| 40. | Butin | -8.24 | 1.823 2.045 1.82 | B | ARG157 MET264 THR306 |
| 41. | Fluticasone propionate | -7.78 | 2.046 | B | THR306 |
| 42. | Caffeic acid | -7.05 | 1.839 1.828 1.997 | A A B | ARG41 LYS43 LYS278 |
| 43. | Sulochrin | -6.12 | 1.732 1.756 | A | SER70 LYS83 |
| 44. | Quercetin 7-glucuronide 3-rhamnoside | -6.42 | 2.117 2.056 1.992 1.646 1.718 1.931 | A A A B B B | GLN152 GLN152 ALA172 ARG144 GLY150 GLN152 |
| 45. | Ginkgetin | -9.56 | 2.191 | A | ALA172 |
| 46. | Hesperetin 3',7-O-diglucuronide | -8.17 | 2.126 2.119 2.056 | B | ARG41 ARG41 LYS43 |
| 47. | Neoastilbin | -7.83 | 1.961 2.151 | B | ALA199 THR306 |
| 48. | Sagerinic acid | -5.64 | 2.165 1.988 1.702 1.998 | B | SER70 TRP111 ARG144 ILE153 |
| 49. | Chrysosplenol D | -8.38 | 2.134 | A | THR306 |
| 50. | Epicatechin-(4beta->8)-epigallocatechin 3-O-gallate | -7.89 | 2.004 1.8 | A | ARG144 GLY150 |
| 51. | Baicalin | -8.24 | 1.789 2.03 1.881 1.978 1.609 | A A B B B | LYS43 LYS43 LYS278 THR306 ARG320 |
| 52. | 6-Methoxyaromadendrin 3-O-acetate | -7.6 | 2.085 2.197 | B | GLN265 THR306 |
| 53. | Phytolaccoside E | -7.88 | 2.091 2.194 1.932 | A | ARG41 LYS83 ILE153 |
| 54. | Flavonol 7-O-beta-D-glucoside | -9.62 | 2.213 2.025 | A | ASN155 THR306 |
| 55. | 6-Hydroxy-2-(4-hydroxyphenyl)-5,7-dimethoxy-4H-1-benzopyran-4-one | -7.66 | 2.081 | B | THR306 |
The bond length of the formed H-bonds was measured thus showing the effective H-bonding between the receptor and ligand molecules. Solanocapsine formed one hydrogen bonds with LYS83 of B chain having bond lengths 2.129 Å. 16alpha,17alpha-Dihydroxyprogesterone acetophenide formed one hydrogen bonds with THR306 of chain B having bond lengths 2.09 Å. Polyporusterone D formed one hydrogen bonds with LYS83 of B chain having bond lengths 2.146 Å. Flavonol 7-O-beta-D-glucoside formed two hydrogen bonds with ASN155 and THR306 of chain A having bond lengths 2.213 and 2.025 Å respectively. Stanolone benzoate formed one hydrogen bonds with MET32 of B chain having bond lengths 2.125 Å. Ginkgetin formed one hydrogen bonds ALA172 of A chain having bond lengths 2.191 Å. Norethindrone acetate formed one hydrogen bonds with LYS83 of B chain having bond lengths 1.766 Å. Silandrin formed three hydrogen bonds with TRP111, ASN155 and GLN265 of chain B having bond lengths 1.839, 1.814 and 1.816 Å respectively. 6-C-Galactosylluteolin formed two hydrogen bonds with MET32 and TRP111 of B chain having bond lengths 1.823 and 2.056 Å respectively. Irisolidone 7-O-glucuronide formed three hydrogen bonds with LYS43 of A chain, LYS278 of B chain and ARG320 of chain B having bond lengths 1.888, 1.97 and 2.114 Å respectively. 3beta-Hydroxy-16-phosphonopregn-5-en-20-one monoethyl ester formed three hydrogen bonds LYS43 of A chain, LYS278 of B chain and ARG320 of B chain having bond lengths 2.157, 1.749 and 2.162 Å respectively. Aromadendrin 4'-methyl ether 7-rhamnoside formed two hydrogen bonds ASP124 of A chain and ARG144 of B chain having bond lengths 1.974 and 1.721 Å respectively. Tectoridin formed two hydrogen bonds with TRP111 and THR306 of chain A having bond lengths 2.117 and 2.154 Å respectively. Bufotalin formed three hydrogen bonds with GLN152 of A chain, LYS278 of A chain and LYS43 of chain B having bond lengths 1.982, 2.175 and 2.032 Å respectively. Chrysosplenol D formed one hydrogen bonds THR306 of A chain having bond lengths 2.134 Å. Apigenin 7-glucoside formed two hydrogen bonds with ARG144 and ILE153 of chain B having bond lengths 1.695 and 2.1 Å respectively. Butin formed three hydrogen bonds with ARG172, SER70 and TRP111 of B chain having bond lengths 1.823, 2.045 and 1.82 Å respectively. Baicalin formed five hydrogen bonds with LYS43 of A chain, LYS43 of A chain, LYS278 of B chain, THR306 of B chain and ARG320 of chain B having bond lengths 1.789, 2.03, 1.881, 1.978 and 1.609 Å respectively. Piceatannol 4'-galloylglucoside formed three hydrogen bonds with TRP111, GLN152 and ILE153 of chain A having bond lengths 1.843, 2.134 and 1.882 Å respectively. Hesperetin 3',7-O-diglucuronide formed three hydrogen bonds with ARG41, ARG41 and LYS43 of B chain having bond lengths 2.126, 2.119 and 2.056 Å respectively. Cynaroside formed one hydrogen bonds THR306 of A chain having bond lengths 2.216 Å. Rest of the ligands having higher binding energy show lower binding affinity with clathrin protein.
ADME and Toxicity analysis
The ADME analysis was done via ALOGPS tool and the logP represents lipophilicity of drug defining the drug partition in water and octane at equilibrium. Hence this parameter provides the significance of the drug into the cells. According to Lipinski’s rule, the safe and permissible range of logP value is between 0-5 [39]. These values indicate that the compounds can quickly diffuse across the cell membranes due to their high organic (lipid) permeability. It is evident from Table 3 that, except Ammothamnine, Neohesperidin, trans-Caffeic acid [apiosyl-(1->6)-glucosyl] ester and Hesperidin, all other ligands exhibit positive logP values. However, all the compounds except 16alpha,17alpha-Dihydroxyprogesterone acetophenide exhibited logP values lower than 5.
Table 3 ADME and Toxicity analysis of selected phytocompounds.
| ADME | TOXICITY | |||||||
|---|---|---|---|---|---|---|---|---|
| S.NO. | Extracted compounds | LogP | LogS | Subcellular localization | Carcinogenicity | Acute Oral Toxicity | BBB | LD50 |
| 1. | Ammothamnine | -0.33 | -2.24 | Mitochondria | No | III | + | 2.5699 |
| 2. | Petasinine | 1.12 | -0.60 | Mitochondria | No | III | + | 2.5538 |
| 3. | Fusicoccin H | 0.86 | -2.80 | Mitochondria | No | III | + | 3.1485 |
| 4. | Stanolone benzoate | 4.91 | -6.38 | Mitochondria | No | III | + | 1.7166 |
| 5. | 16alpha,17alpha-Dihydroxyprogesterone acetophenide | 5.20 | -6.10 | Mitochondria | No | III | + | 2.9411 |
| 6. | Neohesperidin | -0.27 | -2.26 | Mitochondria | No | III | - | 2.4045 |
| 7. | Eugenol O-[3,4,5-Trihydroxybenzoyl-(->6)-b-D-glucopyranoside] | 1.79 | -2.84 | Mitochondria | No | III | - | 2.6006 |
| 8. | 3beta-Hydroxy-16-phosphonopregn-5-en-20-one monoethyl ester | 2.95 | -4.32 | Mitochondria | No | III | + | 2.4148 |
| 9. | Norethindrone acetate | 3.58 | -4.80 | Mitochondria | No | III | + | 1.8958 |
| 10. | 3-Hydroxycoumarin | 1.09 | -1.86 | Mitochondria | No | II | + | 2.5276 |
| 11. | Quercetin | 1.81 | -3.06 | Mitochondria | No | II | - | 3.0200 |
| 12. | Kaempferol 3-rhamnoside 7-galacturonide | 0.36 | -2.30 | Mitochondria | No | III | - | 2.5907 |
| 13. | Peganine | 0.74 | -1.79 | Mitochondria | No | III | + | 2.7329 |
| 14. | Bufotalin | 2.84 | -4.52 | Mitochondria | No | I | + | 4.4313 |
| 15. | Polyporusterone D | 2.78 | -3.73 | Mitochondria | No | III | + | 2.9867 |
| 16. | 6''-Malonylastragalin | 1.21 | -2.81 | Mitochondria | No | III | - | 2.7823 |
| 17. | (3S,5R,6R,7E)-3,5,6-Trihydroxy-7-megastigmen-9-one | 0.58 | -1.57 | Mitochondria | No | III | + | 2.6300 |
| 18. | Aromadendrin 4'-methyl ether 7-rhamnoside | 0.63 | -2.41 | Mitochondria | No | III | - | 2.6861 |
| 19. | Geranyl acetoacetate | 3.70 | -3.34 | Mitochondria | No | III | + | 1.4700 |
| 20. | 7-Dehydrologanin tetraacetate | 1.01 | -2.66 | Mitochondria | No | III | + | 3.1883 |
| 21. | Piceatannol 4'-galloylglucoside | 2.11 | -3.34 | Mitochondria | No | III | + | 2.6315 |
| 22. | L-Menthyl acetoacetate | 3.18 | -3.79 | Mitochondria | No | III | + | 1.7131 |
| 23. | Silandrin | 3.08 | -4.44 | Mitochondria | No | III | - | 2.5289 |
| 24. | Maritimetin | 2.70 | -3.34 | Mitochondria | No | II | + | 2.4653 |
| 25. | 6-C-Galactosylluteolin | 0.39 | -2.34 | Mitochondria | No | IV | - | 2.3664 |
| 26. | 8-Epideoxyloganin tetraacetate | 1.98 | -3.08 | Mitochondria | No | III | + | 2.9306 |
| 27. | Demethyloleuropein | 0.53 | -2.70 | Mitochondria | No | III | - | 2.3730 |
| 28. | 8-Hydroxypinoresinol 8-glucoside | 0.61 | -2.74 | Mitochondria | No | III | - | 3.1133 |
| 29. | 10-Acetoxyligustroside | 0.51 | -2.96 | Mitochondria | No | III | - | 2.7323 |
| 30. | trans-Caffeic acid [apiosyl-(1->6)-glucosyl] ester | -1.18 | -1.88 | Mitochondria | No | III | + | 2.4940 |
| 31. | Pimentol | 1.61 | -2.70 | Mitochondria | No | III | - | 2.6006 |
| 32. | Hesperidin | -0.27 | -2.36 | Mitochondria | No | III | - | 2.6228 |
| 33. | Cynaroside | 0.58 | -2.62 | Mitochondria | No | III | - | 2.3869 |
| 34. | Pedaliin | 0.66 | -2.66 | Mitochondria | No | III | - | 2.2498 |
| 35. | Tectoridin | 0.62 | -2.71 | Mitochondria | No | III | - | 2.2498 |
| 36. | Irisolidone 7-O-glucuronide | 1.18 | -2.73 | Mitochondria | No | III | - | 2.7471 |
| 37. | Solanocapsine | 3.63 | -6.10 | Lysosome | No | III | + | 2.6209 |
| 38. | Apigenin 7-glucoside | 0.68 | -2.65 | Mitochondria | No | III | - | 2.3755 |
| 39. | 3-Methylellagic acid 8-rhamnoside | 1.05 | -2.28 | Mitochondria | No | III | - | 2.6315 |
| 40. | Butin | 2.55 | -3.16 | Mitochondria | No | II | - | 3.0230 |
| 41. | Fluticasone propionate | 3.69 | -4.64 | Mitochondria | No | III | + | 2.3753 |
| 42. | Caffeic acid | 1.67 | -2.05 | Mitochondria | No | IV | - | 1.4041 |
| 43. | Sulochrin | 2.51 | -3.72 | Mitochondria | No | II | + | 2.9889 |
| 44. | Quercetin 7-glucuronide 3-rhamnoside | 0.50 | -2.15 | Mitochondria | No | III | - | 2.5907 |
| 45. | Ginkgetin | 4.96 | -5.30 | Mitochondria | No | III | - | 2.8985 |
| 46. | Hesperetin 3',7-O-diglucuronide | 0.06 | -2.33 | Mitochondria | No | III | - | 3.0497 |
| 47. | Neoastilbin | 0.79 | -2.09 | Mitochondria | No | III | - | 2.5458 |
| 48. | Sagerinic acid | 3.91 | -3.79 | Mitochondria | No | III | - | 2.6462 |
| 49. | Chrysosplenol D | 2.88 | -3.70 | Mitochondria | No | II | - | 2.9468 |
| 50. | Epicatechin-(4beta->8)-epigallocatechin 3-O-gallate | 3.20 | -3.83 | Mitochondria | No | IV | - | 2.4797 |
| 51. | Baicalin | 1.27 | -2.41 | Mitochondria | No | II | - | 2.7357 |
| 52. | 6-Methoxyaromadendrin 3-O-acetate | 2.62 | -3.28 | Mitochondria | No | III | - | 3.1632 |
| 53. | Phytolaccoside E | 1.38 | -3.37 | Mitochondria | No | III | - | 3.4672 |
| 54. | Flavonol 7-O-beta-D-glucoside | 0.68 | -2.61 | Mitochondria | No | III | - | 2.3869 |
| 55. | 6-Hydroxy-2-(4 hydroxyphenyl)-5,7 dimethoxy-4H-1-benzopyran-4-one | 3.22 | -3.88 | Mitochondria | No | III | - | 3.0584 |
LogS measures the aqueous solubility, which is significant physical property related to ADME profile of a compound. The logS values of most of these compounds are more than -5 [40], except Stanolone benzoate, 16alpha,17alpha-Dihydroxyprogesterone acetophenide, Solanocapsine and Ginkgetin (Table 3).
The toxicity of the selected phytocompounds was predicted using admetSAR. The parameters including blood-brain barrier (BBB) penetration, subcellular localization, LD50 and rat acute oral toxicity were estimated and are represented in Table 3 [36]. From the results, it is evident that none of the compounds exhibited carcinogenicity.
Compounds are classified into four categories based on their acute oral toxicity (ADMET prediction profile). The majority of the ligands showed acute oral toxicity category III, indicating that their LD50 values are in the range of 500 mg/kg to 5000 mg/kg body weight. Bufotalin was shown to have a category I acute oral toxicity, indicating that its LD50 values were less than or equal to 50 mg/kg. 3-Hydroxycoumarin, Quercetin, Maritimetin, Butin, Sulochrin, Chrysosplenol D, and Baicalin are among the compounds in Category II, having LD50 values more than 50 mg/kg but less than 500 mg/kg. 6-C-Galactosylluteolin, Caffeic acid and Epicatechin-(4beta->8)-epigallocatechin 3-O-gallate have LD50 values more than 5000 mg/kg were classified as Category IV.
On the basis of Molecular docking, the potential compound suggested was Solanocapsine, 16alpha,17alpha-Dihydroxyprogesterone acetophenide, Polyporusterone D, Flavonol 7-O-beta-D-glucoside, Stanolone benzoate, Ginkgetin, Norethindrone acetate, Silandrin, 6-C-Galactosylluteolin, Irisolidone 7-O-glucuronide, 3beta-Hydroxy-16-phosphonopregn-5-en-20-one monoethyl ester. On the basis of ADME analysis, the compound suggested was Solanocapsine, Polyporusterone D, Flavonol 7-O-beta-D-glucoside, Stanolone benzoate, Ginkgetin, Norethindrone acetate, Silandrin, 6-C-Galactosylluteolin, Irisolidone 7-O-glucuronide, 3beta-Hydroxy-16-phosphonopregn-5-en-20-one monoethyl ester. The toxicity results suggested that Solanocapsine is the most potential candidate predicted to be found in the Lysosome according to Table 3 and as well as the strongest binding affinity to Clathrin protein.
Conclusion
SARS-CoV-2 and COVID-19 have emerged as a health threat worldwide in a recent situation. The evidence from different studies have already shown that clathrin mediated endocytic pathway is a key factor in mediating viral infection. In this study, we have tried to search and identify the drugs for preventing the corona virus infection via in silico method. Ashwagandha, Harsingar, Meethi neem and Tulsi is an important medicinal plant widely used in traditional medicine. The findings of this research study demonstrate that the leaves of Harsingar, Meethi neem and Tulsi and root of Ashwagandha possess a strong antiviral capacity. LC-MS-QTOF analysis, as well as molecular docking studies, led to the tentative identification of the compounds that are likely to be responsible for the antiviral effect of these plant. After the screening Solanocapsine is the only compound predicted to have one of the strongest binding affinity with Clathrin protein and the ADMET results exhibit their drugability. Further research is needed to obtain such bioactive compounds in pure form for complete pharmacological evaluations.











 text new page (beta)
text new page (beta)