Introduction
Polyphenolic compounds are a large family of natural compounds found in plants that offer benefits in numerous applications, such as pharmaceutical, food and cosmetic industries. In fact, it is an increasing interest in the search, extraction and isolation of natural compounds with biological activity from a huge number of plants [1]. The choice of the most appropriate extraction method, usually, takes into consideration the analyte-matrix interactions, the requirements of the analytical method, and the analyte concentration in the sample. Extraction step is always the bottleneck of analytical procedures, consuming the main part of the total analysis time and being a source of errors [2]. It must provide a quantitative extraction of the target analytes but, also, it must be taken into account other factors such as fastness, cost, global availability, and cross contamination.
Extraction of bioactive compounds from plants like legumes has been carried out by a wide range of traditional methodologies, like hydrodistillation, soxhlet and liquid-liquid extraction, which are based on the extracting power of different solvents and the application of heat and/or shaking systems [3] The main drawbacks of these traditional methods are related to the high amount of solvents to be used during the extraction and the slow diffusion and desorption of target analytes from sample matrix. Thus, high efficient extraction strategies as ultrasound-assisted extraction (UAE) [4], microwave-assisted extraction (MAE)[5], and pressurized solvent extraction (PSE) [6] have been incorporated in the last decades; because they usually require less solvent amount and short analysis time than traditional systems [7]. However, high acquisition cost and a lack in the global availability of equipment are important drawbacks of these methods.
Recently, the use of a hard cap espresso machines has been proposed for the efficient, rapid and low cost extraction of organic compounds from solid samples, such as: polycyclic aromatic hydrocarbons [8] and polychlorinated biphenyls in soil [9], bioactive compounds in vegetables and spices [10], and pesticides in PM10 filters [11]. In the former approaches extraction was performed at the mild conditions of 72 °C and 19 bars offered by the domestic available systems and really short extraction times were found of the order of few seconds. Thus, in this study it has been evaluated the extraction of polyphenolic compounds from pulses using a conventional and commercially available hard cap espresso machine, followed by the use of a spectrometric determination by the classic Folin-Ciocalteu method. The proposed method was validated in terms of sensitivity, trueness, and precision. Food samples were extracted by the proposed methodology and results were compared to those obtained by a reference method based in UAE.
Materials and Methods
Apparatus and Reagents
A Nespresso© Essenza Manual XN2003 Krups (Barcelona, Spain) coffee machine was obtained from a local appliance store. No modifications were carried out to the coffee machine, excluding the replacement of the original water reservoir by an increased 2 L volume glass bottle. Nespresso© compatible stainless steel refillable capsules (26*25*23 mm size, 8.8 mL internal volume) were obtained from Mycoffestar GAMMA (Zurich, Switzerland) and 47 mm borosilicate filters were obtained from Scharlau (Barcelona, Spain). A Spe-ed Matrix dispersing agent from Applied Separations (Allentown, PA, USA) was used to avoid sample compaction inside the capsules.
A Bransonic ultrasound water bath 2510E-MT from Sigma-Aldrich (Barcelona, Spain) was employed for the UAE reference procedure and an IKA (Staufen, Germany) MS1 S1 Vortex Shaker was used for the homogenization of the mixture of sample extracts and reagents before their spectrophotometric analysis.
A Hewlett Packard (Palo Alto, CA, USA) 8452A diode array spectrophotometer was used to perform the spectrometric analysis of polyphenols using a wavelength range of 190-820 nm, and a quartz 1 cm path length cuvette. A Hermle Benchtop Centrifuge was obtained from Labnet (Corning, NY, USA).
Gallic acid was employed as polyphenol standard and it was obtained from Sigma (St. Louis, MO, USA). Folin-Ciocalteu reagent was purchased from Scharlab (Barcelona, Spain). Sodium bicarbonate was purchased from Guinama (Valencia, Spain). Analytical reagent grade ethanol absolute (≥ 99.8 %) was obtained from VWR chemicals (Barcelona, Spain). Milli-Q pure water was obtained from a MILLI-Q PLUS purification system from Millipore Corporation (Burlington, MA, USA).
Fifteen raw pulse samples were purchased in local Spanish and Tunisian markets. Samples were ground using a Moulinex (Alençon, France) MC300132 coffee grinder, sieved to 250 μm and stored in closed canisters at room temperature till their extraction and analysis.
General procedure
Espresso machine was purged with 200 mL 50 % (v/v) ethanol in water to clean and pre-heat the system using stainless steel capsule, filled with dispersing agent plus a borosilicate filter. To do sample extraction 100 mg sample was homogenized with 2 g dispersing agent and introduced inside an empty capsule. Then, a borosilicate filter was placed on the top of the capsule and it was closed. Filled capsule was placed in the extraction compartment of the espresso machine and it was extracted with 150 mL 50% (v/v) ethanol in water, taken the extract in a graduated cylinder. Between samples a purge with 50 mL 50% (v/v) ethanol in water was carried out to avoid any memory effect. At the end of each extraction session, espresso machine system was purged with 200 mL deionised water in order to avoid possible damages of tubing. Fig. 1 shows the hard cap espresso machine used to do the extraction of polyphenolic compounds from samples. The extraction system was operated inside a vent chamber for safety conditions and the removable parts of the machine were opened to verify the state of the tubing.

Fig. 1 Hard cap espresso machine employed through this study (A) and the refillable stainless steel capsule used (B).
The total phenolic content of sample extracts was determined by the Folin-Ciocalteu method [12] as follow: 3 mL sample extract were added to 3 mL water and 100 μL Folin-Ciocalteu reagent, and the mixture was homogenized by vortex shaking. After 8 min, 200 μL 20 % (w/v) sodium carbonate was added to the solution and it was centrifuged 5 min at 4500 rpm. Afterwards, the absorbance at 764 nm was measured to calculate the polyphenol concentration as gallic acid equivalents.
UAE reference procedure
The reference procedure used for the extraction of phenolic compounds from pulses was based on UAE. 100 mg sieved sample were extracted at room temperature by sonication in closed 250 ml glass Erlenmeyer flask with 150 mL 50% (v/v) ethanol in water. After 30 min extraction time, the extract was transferred to a conical tube and centrifuged 5 min at 4500 rpm. The supernatant was analysed by the Folin-Ciocalteu method as aforementioned.
Results and Discussion
Polyphenol determination
Concentration of polyphenols in sample extracts was established by using the Folin-Ciocalteu method. It can be mentioned that Folin-Ciocalteu assay is not specific to phenolic compounds, because it can also provide signals from non-phenolic substances like reducing sugars, ascorbic acid or amino acids, which may interfere the analysis. Nevertheless, this assay is already widely employed and results obtained can be expressed as Folin-Ciocalteu reducing capacity that usually is quantified as gallic acid equivalents (GAEs) [13].
Linearity of the method was established using calibration curves, from 0.25 to 30 mg L-1 gallic acid prepared in 50 % (v/v) ethanol in water and analysed as aforementioned in the experimental part. The obtained coefficient of determination (R2) was 0.9990. Blanks were processed and measured as samples, following the whole extraction procedure. Limits of detection (LOD) and quantification were calculated as 3 and 10 times, respectively, the standard deviation of blank divided by the calibration slope. Precision was established as the relative standard deviation (RSD) of 6 independent measurements of a 1 mg L-1 gallic acid standard prepared in 50 % (v/v) ethanol in water. The aforementioned analytical features are summarized in Table 1.
Table 1 Analytical features of the proposed methodology for the determination of polyphenols in pulses and sorghum by Folin-Ciocalteu after hard cap espresso extraction.
| Parameter | Value |
|---|---|
| Slope | 0.041 |
| Intercept | 0.004 |
| Linearity range (mg L-1) | 0.8-30 |
| Coefficient of determination (R2) | 0.9990 |
| LOD (mg L-1) | 0.2 |
| LOQ (mg L-1) | 0.8 |
| RSD (%, n=6) | 14 |
| Sample LOD mg g-1 | 0.4 |
| Sample LOQ mg g-1 | 1.2 |
Extraction conditions
Hard cap espresso machines operate at fixed conditions of 72°C and 19 bars, when applied to the extraction of samples confined in 8.8 mL capsules. Thus, the single factor to be modified in order to obtain an efficient extraction recovery concerns: the organic solvent nature and its concentration, sample amount, and extractant volume. Folin-Ciocalteu method is usually carried out by using acetone, methanol or ethanol solvents; thus, ethanol was selected because it has a medium/high boiling point and was employed in previously espresso extraction studies without any damage of the system [10].
The most appropriate ethanol concentration as extracting solution was established by the extraction of 100 mg lentils sample extracted by using 400 mL (8 successive extractions with 50 mL) ethanol solution from 0 to 80 % (v/v). Extraction aliquots were analysed separately and results found can be seen in Fig. 2. Pure water provided a partial extraction of polyphenols (2.4 mg GAE g-1 equivalent to 45 % of present polyphenols) that increases on increasing the concentration of ethanol till a 50 % (v/v) ethanol in water, where a total concentration of 6.5 mg GAE g-1 was obtained. Increased percentages of ethanol provided a reduced efficiency in the obtained values of polyphenolic compounds. The obtained results are in good agreement with those reported in the literature, where solvent mixtures in water provided a better extracting power for polyphenolic compounds than pure solvents [14]. So, 50 % (v/v) ethanol in water was selected as working extractant.
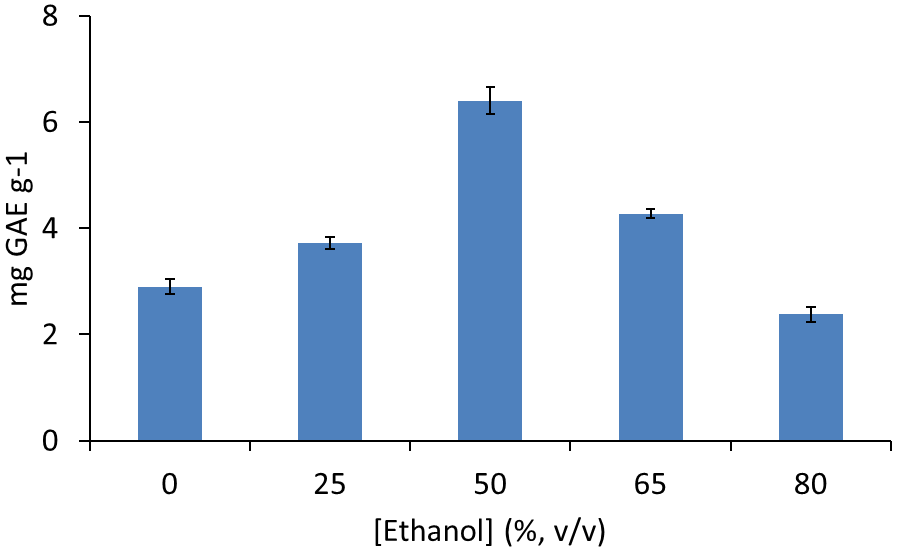
Fig. 2 Polyphenol extraction of lentil sample 1 by hard cap espresso extraction with 400 mL of ethanol: water solutions and analysis by Folin Ciocalteu method. Note: error bars correspond to 3 separate assays.
Extraction efficiency was also evaluated for different sample amounts. The most appropriate sample amount was assessed by the extraction of 0.1, 0.5 and 1.0 g lentils sample extracted by using a total of 400 mL 50 % (v/v) ethanol solution, by using successive extractions with 50 mL, and analysed by Folin-Ciocalteu assay. Quantitative recoveries were obtained for all sample amounts evaluated (see Fig. 3) with total recoveries of 87, 105 and 109 % to that obtained by the reference USE procedure, respectively. However, the lowest sample amount (100 mg) allowed a quantitative and quick extraction of polyphenols in less than 30 s using only 150 mL 50 % (v/v) ethanol in water, also reducing the solvent consumption. So, the use of 100 mg sample was proposed following the standards of green analytical chemistry to reduce the environmental impact of the methodology. The obtained LOD and LOQ values for the analysis of polyphenols from food samples, considering the dilution grade of the proposed procedure, were 0.4 and 1.2 mg GAE g-1, respectively.
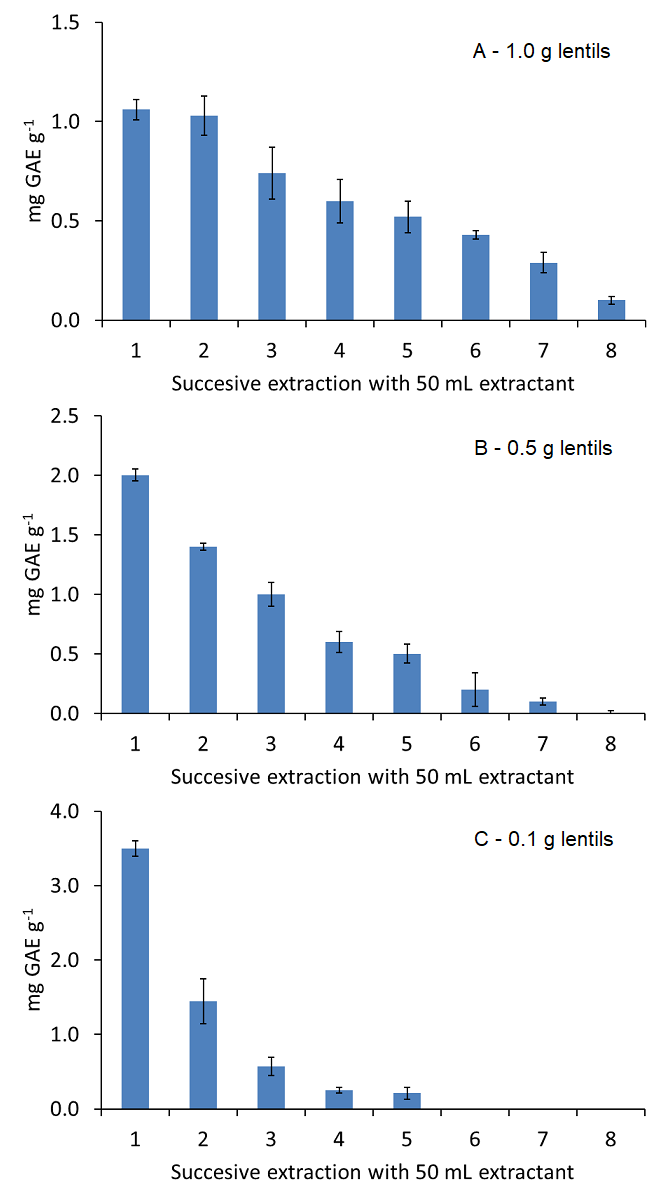
Fig. 3 Effect of sample mass on the extraction efficiency of hard cap assisted extraction for 1.0 (A), 0.5 (B) and 0.1 (C) g lentil 1 sample extracted by using 8 successive extractions with 50 mL 50 % (v/v) ethanol solution and analysed by Folin-Ciocalteu assay. Note: error bars correspond to 3 separate assays.
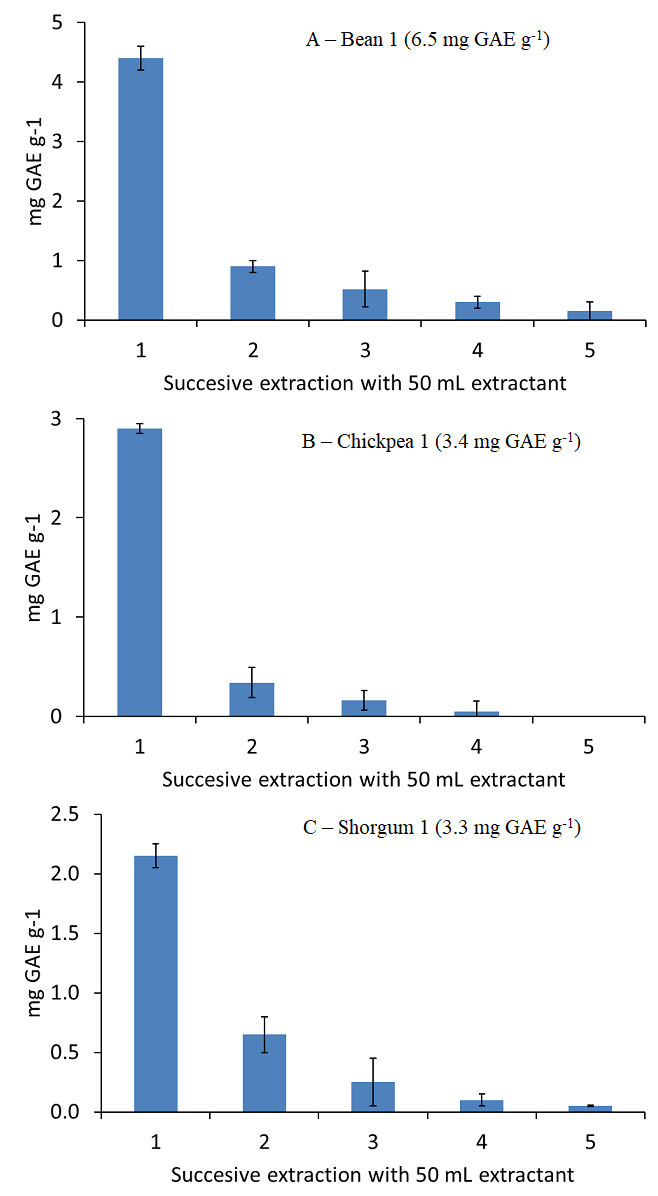
Extraction conditions were assessed only for the extraction of polyphenols from lentils. Additionally, 100 mg of beans, chickpeas and sorghum were extracted using 5 successive extractions with 50 mL 50 % (v/v) ethanol in water in order to check the polyphenol extraction efficiency in other pulse samples. Fig. 4 shows the polyphenols extracted after each 50 mL aliquote for each sample type. As it can be seen, an extraction with 150 mL 50 % (v/v) ethanol in water provided the quantitative extraction of polyphenols, 5.82 mg GAE g-1 for bean, 3.40 mg GAE g-1 for chickpea, and 3.10 mg GAE g-1 for sorghum, which correspond to a 91, 101 and 91 % of the UAE reference contents, respectively. Thus, the proposed method can be extended to the analysis of other pulses than lentils.
Analysis of field samples
Fifteen field pulses (3 lentils, 6 beans, 5 chickpea, and 1 sorghum) were analysed by using hard cap espresso extraction and Folin-Ciocalteu assay for the determination of polyphenolic compounds. Table 2 shows the obtained values, with polyphenol concentrations ranging from 4.62 to 5.51 mg GAE g-1 in lentils, from 3.41 to 5.80 mg GAE g-1 in beans, from 2.26 to 3.54 mg GAE g-1 in chickpeas, and 3.04 mg GAE g-1 in sorghum. These values were in accordance to those found in the literature, from 4.9 to 17.4 mg GAE g-1 for lentils, and from 0.67 to 7.8 mg GAE g-1 for beans [15,16].
Table 2 Polyphenol concentration found in the analysed pulses extracted by the proposed hard cap espresso extraction and by ultrasound-assisted extraction (UAE), and analysed in both cases by Folin-Ciocalteu method.
| Sample type | Sample | [Polyphenol] (mg GAE g -1 ± s, n=3) | ||
|---|---|---|---|---|
| hard cap espresso | UAE | Difference | ||
| Lentils | 1 | 5.51 ± 0.45 | 5.47 ± 0.30 | 0.0 |
| 2 | 4.62 ± 0.20 | 5.33 ± 0.39 | -0.7 | |
| 3 | 4.74 ± 0.53 | 5.32 ± 0.01 | -0.6 | |
| Beans | 1 | 5.80 ± 0.80 | 6.53 ± 0.29 | -0.7 |
| 2 | 3.41 ± 0.28 | 4.01 ± 0.12 | -0.6 | |
| 3 | 5.43 ± 0.12 | 5.31 ± 0.02 | 0.1 | |
| 4 | 5.74 ± 0.33 | 6.23 ± 0.54 | -0.5 | |
| 5 | 4.86 ± 0.05 | 4.90 ± 0.14 | 0.0 | |
| 6 | 3.49 ± 0.44 | 4.08 ± 0.09 | -0.6 | |
| Chickpeas | 1 | 3.37 ± 0.23 | 3.38 ± 0.07 | 0.0 |
| 2 | 2.26 ± 0.01 | 3.14 ± 0.05 | -0.9 | |
| 3 | 3.15 ± 0.13 | 3.01 ± 0.05 | 0.1 | |
| 4 | 3.54 ± 0.20 | 3.20 ± 0.14 | 0.3 | |
| 5 | 2.82 ± 0.04 | 2.53 ± 0.13 | 0.3 | |
| Sorghum | 1 | 3.04 ± 0.05 | 3.31 ± 0.10 | -0.3 |
Trueness of the proposed hard cap espresso procedure was evaluated by the comparison of the obtained results with those obtained using an UAE reference method. Table 2 also shows the polyphenol concentration values obtained by UAE. Results obtained by using both assayed methodologies deeply agree with differences between results of -0.3 ± 0.4 mg GAE g-1. The two methods were statistically comparable with a calculated t test value of 1.17, which is lower than the tabulated t-student value for 3 freedom degrees and α = 0.05 that is 3.18. Additionally, a comparison of the hard cap assisted extraction results with those obtained using UAE produced a least squares regression slope of 0.86 [0.69, 1.04] and an intercept of 0.3 [-0.5, 1.1]. Therefore, the obtained slope and intercept were statistically equal to 1 and 0, respectively. So, it could therefore be concluded that the proposed hard cap espresso extraction procedure provided results statistically comparable to the UAE reference method.
Conclusions and perspectives
Hard cap espresso machines have been confirmed as a real alternative to currently available extraction methods for the easy, fast and low cost analysis of polyphenols from relevant food matrices like legumes, obtaining concentration values in the same range of those reported in the literature. Polyphenolic compounds were quantitatively extracted with 150 ml 50 % (v/v) ethanol in water in a single and fast (30 s) extraction step and later analysed using Folin-Ciocalteu assay. In addition, the obtained results were statistically comparable with those obtained by an UAE reference procedure.











 text new page (beta)
text new page (beta)