Introduction
Roselle (Hibiscus sabdariffa L.) is an annual shrub which belongs to family Malvaceae and grows in regions with dry tropical weather, countries like China, Egypt, Sudan, Thailand, Senegal, Tanzania and Mexico. It is known by different synonyms and vernacular names around the world and its cultivation has diverse purposes, but the most important one is to produce infusions from the calyx of their flowers [1]. Nevertheless, because of its brilliant red colour and unique flavor, its calyxes are also used to make jellies, jams, sauces, wines and beverages [2-4].
Recently there has been increasing interest in this due to their beneficial health effects and there are studies about its antihyperlipidemic, antihypertensive, antypyretic, apoptotic and antioxidant activity [5, 6]. Thus, roselle could not only be used as nutrient or colorant but also as a functional food ingredient [7]. The responsible compounds for the brilliant colour and some of mentioned activities are anthocyanins.
Anthocyanins constitute a major polyphenolic family of compounds widespread in the plant kingdom; they are of interest for the food industry as potential replacements for banned dyes [8]. However, their application has been limited because of their low stability to several factors [9-16]. Consequently, diverse research groups have been searching for new anthocyanin-based natural colorants that have better colour and stability properties [17].
In 1996, Cameira dos Santos et al . [18] isolated a class of anthocyanin-derived pigment. After that, Fulcrand et al . [19] presented a structure with an additional pyrano ring between C-4 and the hydroxyl group at C-5 of a malvidin core, demonstrating that this compound was the result of the reaction of malvidin-3-glucoside and 4-vinylphenol [20]. This pyranoanthocyanin family has been named hydroxyphenyl-pyranoanthocyanins, and their formation happens due to both hydroxycinnamic acids and 4-vinylphenols reacting with free anthocyanins. The family is also known as pinotins [21] since they were firstly isolated from Pinotage wines [22]. Later, those compounds were discovered in other varieties of wine [23-25] and in some fruits juices [26]. Relating to the characteristics of the pinotins family, they show less polarity and a hypsochromic shift in relation to the original anthocyanins [19], plus, a higher stability against several factors such as pH [27].
On the other hand, Lu and Foo [28] observed the formation of new compounds when blackcurrant anthocyanins were extracted with aqueous acetone, proposing that this compounds were the result of the nucleophilic reaction with acetone. These derivatives named methylpyranoanthocyanins, show a yellow-orangish colour as a result of their hypsocromic effect.
Therefore, pyranoanthocyanins could be formed by the reaction of anthocyanins and small molecules [29, 30]. Their importance lies in the fact that they have shown to have a greater colour stability against pH changes and bleaching by sulfur dioxide than the original anthocyanins [31] and higher colour at similar concentration. Thus, since their discovery, pyranoanthocyanins have received much attention. Nevertheless, isolation and characterization of such pigments has proved to be difficult because of their concentration in food substrates is much lower than original anthocyanin. For this reason, they have been synthesized in model solutions for being characterized [32, 33].
The aim of the present work was to obtain new anthocyanin derived compounds which could expand the colorant range of food colorants since they exhibit different colors than starting material. For that reason, we synthesize and characterize six new pyranoanthocyanins related to the families of hydroxyphenyl-pyranoanthocyanins and methylpyranoanthocyanins. They were obtained as a result of the reaction of anthocyanins extracted from roselle (Hibiscus sabdariffa L.) calyxes with 4-vinylphenol, acetone and 2-butanone.
Results and discussion
Extraction of anthocyanins from roselle calyxes
Usually, roselle extracts contain four anthocyanins [34]: delphinidin-3-sambubioside (1), delphinidin-3-glucoside, cyanidin-3-sambubioside (2) and cyanidin-3-glucoside, but the majority ones are the two sambubiosides, while the concentration of glucosides is less than 7% [35]. In this study, the roselle extract which has been purified using Amberlite XAD-7, showed only two of the four common anthocyanins in roselle extracts. This mixture of delphinidin-3-sambubioside (1) and cyanidin-3-sambubioside (2) (Fig. 1) was used for the synthesis of pyranoanthocyanins. Under the HPLC conditions used, delphinidin-3-sambubioside (1) showed an absorption peak at 526 nm, while cyanidin-3-sambubioside (2), at 518 nm (Fig. 2a).
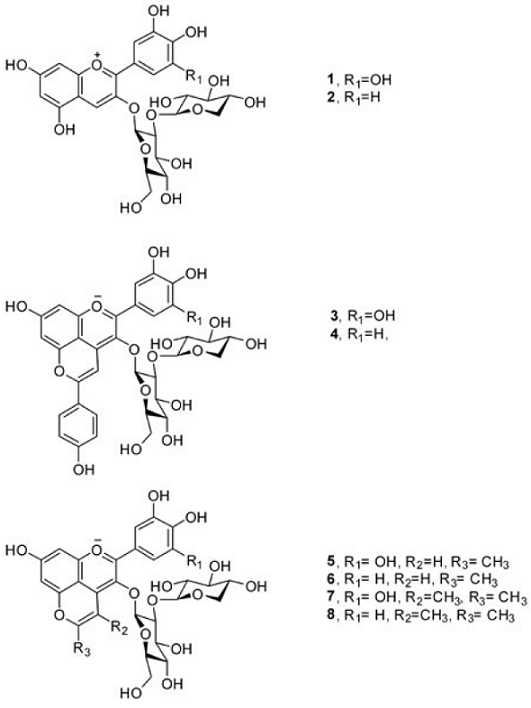
Fig. 1 Structures of the anthocyanins present in roselle (Hibiscus sabdariffa L. ) calyxes extract and their derived pyranoanthocyanins.
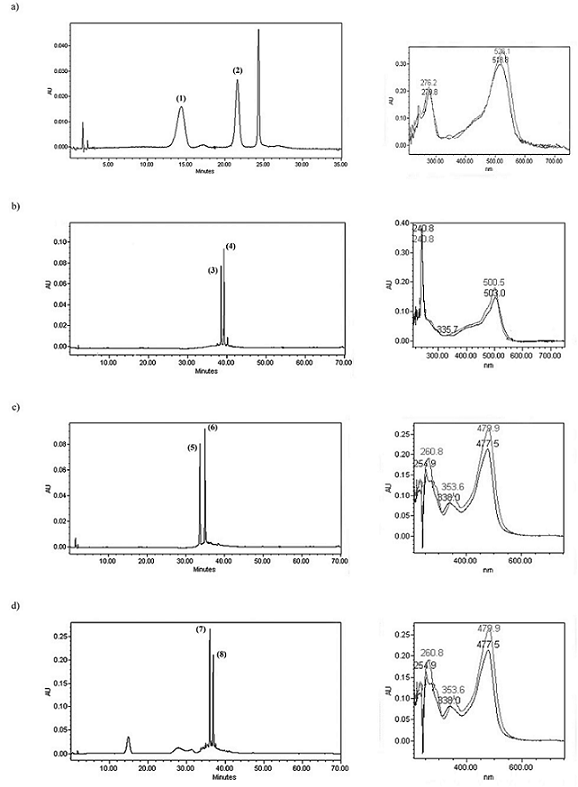
Fig. 2 HPLC chromatograms which evidence the presence of anthocyanins (520 nm) and pyranoanthocyanins (480 nm). UV-spectra for a) mixture of anthocyanins (dephinidin-3-sambubioside (1) and cyanidin-3-sambubioside (2)); b) hydroxyphenyl-pyranoanthocyanins (3) and (4); c) methylpyranoanthocyanins (5) and (6); d) methylpyranoanthocyanins (7) and (8).
Synthesis of hydroxyphenyl-pyranoanthocyanins (3) and (4)
The formation of these products was achieved using the conditions proposed by Fulcrand et al . [19], which consist of reacting anthocyanin mixture with 4-vinylphenol in acid-aqueous media. Reaction was performed during 24 h, and passing that time, the presence of anthocyanins was not observed. Products were confirmed by HPLC-TOF-MS. It was observed the [M]+ ion located in 713.3 m/z for (3) and in 697.3 m/z for (4), which match with the molecular weight for the proposed structure. Likewise the fragmentation pattern of anthocyanins, these pinotins showed [M-294] ion due to loss of sambubioside unit: 419.3 m/z for (3) and 403.2 m/z for (4).
The newly synthesized pyranoanthocyanins show different spectroscopic characteristics, compared with their anthocyanic counterparts. It was observed a hypsochromic shift, in which the pinotin (3) shows a peak of absorption at 503 nm, while pinotin (4) at 501 nm, therefore they present a hypsochromic shift of 23 and 17 nm, for (3) and (4), respectively (Fig. 2b). Table 1 summarizes the data for anthocyanins and pyranoanthocyanins.
Table 1 Spectroscopic and chromatographic data from roselle anthocyanins and their derivated pyranoanthocyanins.
Synthesis of methylpyranoanthocyanins (5) and (6)
Two members of the family of pyranoantocyanins were synthesized through the nucleophilic addition of acetone, when reacted with the methanolic mixture of anthocyanins from roselle calyxes. The reaction was monitored by HPLC during 120 h. It is worth mentioning that the ratio acetone:anthocyanin was an important factor for yield, reaching better yields with a 30:1 ratio (volume).
The resulting compounds were purified using a column Sephadex LH-20 and analyzed by HPLC and HPLC-TOF-MS. The molecular weight obtained, coincided with proposed structure and the presence of the ions [M]+ and [M-294] was exhibited, demonstrating the characteristic fragmentation pattern of anthocyanins.
Methylpyranoanthocyanins show structural similarities with vitisins. It has been reported that vitisin A and B have hypsochromic shifts of 18-19 nm and 36-39 nm, respectively, depending on solvent. Bakker and Timberlake [36] proposed that hypsochromic shift was probably caused by the delocalization of the positive charge on C ring, which could be caused by resonance and for its partial residence on the oxygen atom of the newly formed D ring. They also proposed that the unusual shoulder observed in the UV spectra of vitisin is due to C-4 substitution. In the same way, the methylpyranoanthocyanins (5) and (6) have absorption peaks in 478 and 475 nm (Fig. 2c), showing a hypsochromic shift related to the original anthocyanins, but higher than that observed on vitisins (43-48 nm). Also these compounds show an unusual band in 354 and 340 nm, which is not present in anthocyanins and nor in pinotins.
Synthesis of methylpyranoanthocyanins (7) and (8)
The reaction products were certainly identified as methylpyranoanthocyanins-derived since they had similar retention times (Table 1) and UV spectrum than methylpyranoanthocyanins (5) and (6) (Fig. 2d). Furthermore, molecular weight of methylpyranoanthocyanins (7) and (8) is consistent with the insertion of 2-butanone molecule. According to mechanism proposed by Lu and Foo [28], there is a dehydration (-18 units) and oxidation (-2 units) plus molecular weight of 2-butanone (+72.1 units); thus, there is a difference among molecular weight of anthocyanins (1) and (2) and methylpyranoanthocyanins (7) and (8) of 52 units. Besides, these compounds show the same characteristics of fragmentation pattern than other pyranoanthocyanins, that is, the presence of the ions [M]+ and [M-294].
It is important to notice that any pyranoanthocyanin have a retention time close to anthocyanins (Table 1), indicating different polarity due to the formation of the new ring. Nevertheless, despite the retention times are close, they are longer for pyranoanthocyanins (Table 1; 3-8) respect to precedent anthocyanins (Table 1; 1 and 2). All pyranoanthocyanins synthetized in this work had a hypsochromic shift that made them more orange in comparison with the red hue of the original anthocyanins. On the other hand, the differences between delphinidin and cyanindin-derivatives of the same compound are lesser than the difference between anthocyanins; in this case, the B-ring pattern could not be a determinant factor for wavelength of maximum absorbance.
Experimental
Chemicals
Delphinidin-3-sambubioside (1) and cyanidin-3-sambubioside (2) were isolated from sun-dried roselle calyxes (Hibiscus sabdariffa L.) grown in Chiautla de Tapia, Puebla (980 m.a.s.l.).
Amberlite XAD-7 and Sephadex LH-20 were purchased from Sigma-Aldrich. Water and acetonitrile used in chromatography assays were HPLC-grade and purchased from Merck. Formic acid >88%, acetone reactive grade, clorhidric acid, 2-butanone and 4-vinylphenol were purchased from Sigma-Aldrich. Hexane, ethyl acetate and methanol used on columns were industrial grade double distilled.
Anthocyanin extraction
Roselle calyxes (Hibiscus sabdariffa L.) were previously grounded and then macerated using methanol for 2 h, using a ratio 40 g /200 mL. The extract obtained was filtrated, vacuum-dried, and adsorbed in Amberlite XAD-7 (64 g). The adsorbed resin was packed on a column (3.2 x 45 cm), then eluted with acid water (2 L, 0.01% HCl), hexane (1 L), ethyl acetate (1 L), and acid methanol (800 mL, 0.01% HCl). The water, hexane and ethyl acetate fractions were discarded; meanwhile, the methanol fraction was vacuum-distilled for obtaining a mixture composed by delphinidin-3-sambubioside (1) and cyanidin-3-sambubioside (2).
Chromatographic profile
HPLC of Anthocyanins. The mixture obtained above, was analyzed by HPLC Waters 600 equipped with 20 μL loop, using a reverse phase column C18 LiChroCART (25 x 0.4 cm, 5 μm). A gradient was established using acetonitrile (A) and 4.5% formic acid (B): isocratic 9:91 (A:B) for 25 min, 26-28 min, 100:0 (A:B) and 28-30 min, 9:91 (A:B) at a flow rate of 1.5 mL/min. Detection was carried out using a Waters 996 PDA detector at 520 nm.
HPLC of pyranoanthocyanins. Products obtained were analyzed using the same column and flow rate described earlier, but the gradient was changed: isocratic 9:91 (A:B) for 20 min, 20-25 min, 5:95 (A:B); 25-65 min 100:0 (A:B), 65-70 min, 9:91 (A:B). Detection was carried out at 480 nm.
HPLC-TOF-MS. Pyranoanthocyanins were analyzed by HPLC Agilent 1200 series coupled to Agilent 6220 series time-of-flight mass spectrometer (TOF-MS) system. Mass spectra were acquired using an electrospray source in positive mode (ESI+), from 200-1200 m/z. A gradient was established using acetonitrile (A) and 4.5% formic acid (B) using a C18 column with a flow rate of 0.8 min/mL in the following conditions: 9:91 (A:B) at 0 min, 0-30 min, 60:40 (A:B), 30-32 min, 0:100 (A:B), isocratic 0:100 (A:B) for 5 min, 37-39 min 9:91 (A:B).
Synthesis of hydroxyphenyl-pyranoanthocyanins (3) and (4)
A mixture (150 mg) of the extracted anthocyanins was dissolved in 40 mL of a solution of aqueous 0.001M HCl, using a flask provided with a stirrer. Then 1 mL of 4-vinylphenol was added. The mixture was stirred for 24 h. After this time, the mixture was filtrated and was adsorbed in a Sephadex LH-20 column (1 x 9 cm), then was eluted using water. The resulting fraction was lyophilized.
Synthesis of methylpyranoanthocyanins (5) and (6)
Using a flask provided with a stirrer, 200 mg of mixture of anthocyanins were dissolved in 6 mL of methanol and 14 mL of acetone. Reaction mixture was stirred for 120 h; then the solvent was vacuum-distilled and absorbed in a Sephadex LH-20 (1 x 9 cm) which was eluted using water. The resulting fraction was lyophilized.
Synthesis of methylpyranoanthocyanins (7) and (8)
Using a stirred-flask, 200 mg of mixture of anthocyanins were dissolved in 6 mL of methanol and 14 mL of 2-butanone. The reaction and purification were developed under the same conditions than those described for the synthesis of methylpyranoanthocyanins (5) and (6).
Conclusions
Nucleophilic reactions between roselle anthocyanins and 4-vinylphenol, and some ketones were achieved. These reactions generate products with different spectral and chromatographic properties than those from original anthocyanins.
Roselle anthocyanins react with 4-vinylphenol to generate hydroxyphenyl-pyranoanthocyanins derivatives. This pyranoanthocyanin family can be synthesized faster than other pyranoanthocyanins, and they might be used as pigments in medium- and low-acid food.
On the other hand, the anthocyanin reaction with acetone produces methylpyranoanthocyanins (5) and (6), which are relatively fast synthesized. We observed that the relation anthocyanin-acetone-solvent is an important factor to obtain them, as confirmed from preliminary ratios assayed for the reaction (10:1, 20:1, 30:1, 40:1, 50:1, and 80:1, acetone:anthocyanin), and the best was selected in terms of HPLC signal intensity. These compounds had a more pronounced hypsocromical effect showing a yellow-orange colour; in this way, they expanded the colour range in their application as food colorants.
Methylpyranoanthocyanins (7) and (8) were synthesized in the same manner and they had similar spectral properties, nevertheless further investigations are necessary to determine steric hindrance on pyranoanthocyanin formation when ketone chain is increased.
Pyranoanthocyanins are no just more stable than anthocyanins but also they display more colours depending on their structure, we proposed that methodologies used here could expand the structural variety of pyranoanthocyanins.











 text new page (beta)
text new page (beta)


