Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.59 n.1 Ciudad de México Jan./Mar. 2015
Article
A Rapid, One-pot, Multi-component Route to 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols)
Asadollah Hassankhani
Department of New Materials Science, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, PO Box 76315-117, Kerman, Iran. hassankhani_a@yahoo.com
Received April 14th, 2014
Accepted July 30th, 2014
Abstract
A new, efficient and environmentally benign protocol for the one-pot, multicomponent synthesis of 4,4'-arylmethylene-bis(3-methyl1-phenyl-1H-pyrazol-5-ols) by condensation of aryl aldehydes, phenyl hydrazine and ethyl acetoacetate catalyzed by Ce(SO4)2.4H2O as an ecofriendly catalyst with high catalytic activity and reusability at 125 °C under solvent-free conditions is reported. The reaction proceeds to completion within 5-12 min in 81-98% yield. This new green methodology is of interest due to minimizing the cost of operational hazards and environmental pollution, its excellent yields, short reaction time and high catalyst activity and reusability.
Key words: Ce(SO4)2.4H2O, 4,4'-arylmethylene-bis(3-methyl-1-phenyl-1H-pyrazol-5-ols), Heterogeneous Catalyst, Environmentally Friendly, Solvent-free Conditions.
Resumen
Se describe un procedimiento eficiente y benigno al medio ambiente para la síntesis de 4,4'-arilmetilen-bis(3-metil-1-fenil-1H-pirazol-5oles) por condensación de multicomponentes en un solo paso de aril aldehídos, fenil hidracina y acetoacetato de etilo catalizada por Ce(SO4)2.4H2O como un catalizador con alta actividad catalítica y reusabilidad a125 °C bajo condiciones libres de disolvente. Las reacciones proceden eficientemente en tiempos de reacción de 5-12 min en rendimientos de 81-98%. Esta nueva metodología verde es interesante puesto que minimiza costos de operación y evita la contaminación ambiental.
Palabras clave: Ce(SO4)2.4H2O, 4,4'-arilmetilen-bis(3-metil-1-fenil-1H-pirazol-5-oles), catálisis heterogénea, amigable al medio ambiente, condiciones libres de disolvente.
Introduction
Green chemistry emphasizes the development of environmentally benign chemical processes and technologies [1]. According to the principle of safe and green chemistry, synthetic method should be designed to use substances that exhibit little or no toxicity to human health and the environment [1]. Recent endeavours have focused on limiting the use of organic solvents and replacing them with environmentally benign media. Multi-component reactions (MCRs) comply with the principles of green chemistry in terms of economy of steps as well as many of the stringent criteria of an ideal organic synthesis [2]. The rapid assembly of molecular diversity utilizing multicomponent reaction has received a great deal of importance in synthetic organic chemistry, due to their atom economy, selectivity, simplicity in the procedure and equipment, time, and cost saving [3, 4].
Nowadays, the pyrazolone derivatives were paid much attention for their various biological activities such as anti-tumor [5, 6], a selective COX-2 inhibitory [7] and cytokine inhibitors [8]. The compounds that contain two pyrazolone rings can be used as gastric secretion stimulatory [9], antidepressant [10] or antibacterial [11, 12]. Moreover, these compounds are applied as fungicides [13], pesticides [14], insecticides [15] and dyestuffs [16]. Due to the possible importance of these compounds and our interest in the development of heterocyclic drug-like compounds [17], in this study, I wish to report the synthesis of some heterocycle-based chromophores centered on pyrazolone.
Numerous synthetic methods have been reported for the preparation of 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol5-ols) under classical or modified conditions [18-36]. However, some of these methods suffer from expensive reagents, low yields, prolonged reaction times, use of toxic organic solvents, and tedious work-up procedures. Thus, a search for new reagents and the development of new methods are still of practical importance.
In this regard, I decided to explore the possibility of synthesizing 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) via a novel, one-pot, multi-component condensation of phenyl hydrazine (2 equiv), ethyl acetoacetate (2 equiv) and aromatic aldehydes (1 equiv) under solvent-free conditions using Ce(SO4)2.4H2O as a recyclable catalyst with high catalytic activity (Scheme 1).

Results and Discussion
This protocol offers flexibility in tuning the molecular complexity and diversity. The reactions proceeded to completion very rapidly, and pure product was obtained, without using any chromatographic techniques, simply by recrystallization from ethanol. Comparison of the reaction times and the yields of 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) with the reported conventional chemical approach in the literature was shown, this method is more efficient, requires less time and catalyst loading.
To develop optimum conditions, first, the effect of temperature on the rate of the reaction was studied for the preparation of 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols). At 125 °C, the reactions proceeded to completion very rapidly. A decrease in temperature leads to decreasing product yields and rate of reaction. It was observed that the reaction did not proceed at room temperature (Table 1).
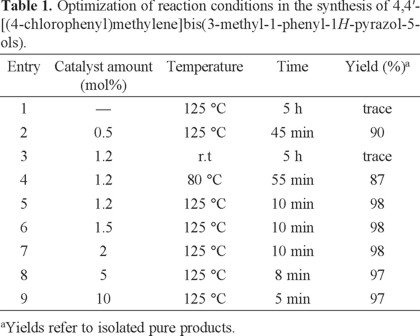
With these optimistic results in hand, further investigation was carried out for the catalytic evaluation of cerium (IV) sulfate for the optimum reaction conditions. The increase in the amount of cerium (IV) sulfate up to 10 mol% did not show much difference in terms of yield or reaction time. As summarized in Table 1, the best result was obtained by the application of 1.2 mol% of Ce(SO4)2.4H2O at 125 °C in neat conditions.
The generality of the reaction was investigated by using different aromatic aldehydes under thermal conditions (Table 2). Various aromatic aldehydes having electron-withdrawing groups such as nitro and halide groups or electron-donating groups such as hydroxyl and alkoxy groups undergo smooth condensation with excellent yields of products in high purity. Steric hindrance seems to have no significant effects on the yields.
A reasonable mechanism for the formation of 1-10 (Table 2) is proposed in Scheme 2. The reaction occurs via the initial formation of the phenylhydrazone A from condensation of ethyl acetoacetate 1 and phenylhydrazine2 which subsequently cyclizes to afford the desired compound 3. The second step involves the formation of benzylidene B by the nucleophilic addition of 1phenyl-3-methyl-5-pyrazolone 3 to aromatic aldehyde 4 followed by dehydration. Then, the second molecule of 1-phenyl-3-methyl5-pyrazolone 3 is added to intermediate B by the Michael addition fashion to give 4,4'-(arylmethylene)bis(3-methyl-1-phenylpyrazol-5-ols) 5.

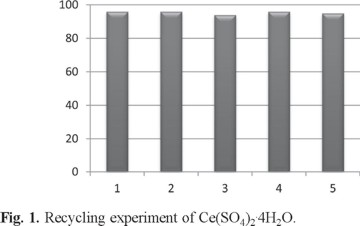
In addition, it was found that Ce(SO4)2.4H2O can be reused several times without any loss of its activity, simply by adding H2O to the reaction mixture followed by filtering the soluble catalyst from the solid products, distillation of solvent, washing with dichloromethane and drying at 60°C (Fig. 1).
Experimental
General
Melting points were recorded on a Gallenkamp melting point apparatus and are uncorrected. NMR spectra were recorded at 500 (1H) and 125 (13C) MHz on a Bruker DRX-500 Avance spectrometer, respectively. Chemical shifts (δ) are reported relative to TMS (1H) and DMSO-d6 (13C) as the internal standards. IR spectra were measured on a Mattson 1000 FT-IR spectrophotometer.
General procedure for preparation of 4,4'-[(4-chlorophenyl)methylene]bis(3-methyl-1-phenyl-pyrazol-5-ols (Table 2, entry 3)
4-Chlorobenzaldehyde (0.14 g, 1 mmol) was added to a mixture of phenylhydrazine (0.21 g, 2 mmol), ethyl acetoacetate (0.26 g, 2 mmol) and Ce(SO4)2.4H2O (10 mg, 1.2 mol%) in a test tube at 125 °C. The reaction was complete within 10 min (TLC). The reaction was cooled to 45 °C, then H2O (10 mL) was added and the mixture and stirred for 5 min. The solid residue was filtered and isolated from the soluble catalyst. The solid product was purified by recrystallization from aqueous ethanol (25%). All compounds were known in the literature [18-36] and the NMR and IR spectra of the products were in agreement with earlier data.
Spectral data for selected compounds
4,4'-[(phenylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 2, entry 1). Solid powder, Mp. 170-172 °C, IR (KBr, cm-1): 3361(OH), 2927, 1625, 1574(C = C), 1400,1101, 787, 755; 1H NMR (500 MHz, DMSO-d6, δ): 2.49 (s, 6H, CH3), 4.94 (s, 1H, CH), 7.15-7.70 (m, 14H, Ar-H), 12.44 (s, 1H, OH), 13.98 (s, 1H, OH); 13C NMR (125.17 MHz, DMSO-d6, δ): 11.6, 33.1, 100.5, 105.3, 120.5, 121.5, 125.4, 125. 8, 127.1, 128.1, 128.3, 142.8, 146.2.
4,4'-[(4-Bromophenyl)methylene]bis(3-methyl-1-phenyl-1Hpyrazol-5-ol) (Table 2, entry 2). Solid powder, Mp. 180-182 °C, IR (KBr, cm-1): 3086 (OH), 2957, 1591, 1577 (C = C), 1478, 1276, 1197, 1047, 808, 746; 1H NMR (500 MHz; DMSO-d6; δ): 2.30 (s, 6H, 2CH3), 5.07 (s, 1H, CH), 7.18-7.69 (m, 14H, Ar-H), 12.35 (s, 1H, OH), 13.83 (s, 1H, OH); 13C NMR (125.17 MHz, DMSO-d6, δ): 11.4, 33.3, 104.4, 121.7, 122.9, 125.8, 126.8, 129.3, 130.4, 131.0, 136.9, 145.3, 146.9.
4,4'-[(4-chlorophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 2, entry 3). Crystalline solid, Mp. 210-212 °C, IR (KBr, cm-1): 3463 (OH), 2927, 1625, 1574 (C = C), 1447, 1236, 776, 755; 1H NMR (500 MHz, DMSO-d6, δ): 2.30 (s, 6H, CH3), 4.95 (s, 1 H, CH), 7.22-7.70 (m, 14H, Ar-H), 12.50 (s, 1H, OH), 13.87 (s, 1H, OH); 13C NMR (125.17 MHz, DMSO-d6, δ): 11.5, 32.6, 105.7, 120.5, 125.5, 127.8, 128.8, 129.0, 129.6, 130.5, 137.3, 141.1, 146.1.
4,4'-[(2,4-dichlorophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (Table 2, entry 9). Crystalline solid, Mp. 227-229 °C, IR (KBr, cm-1): 3465 (OH), 3056, 2927, 1625, 1574 (C = C), 1444, 1221, 776, 755; 1H NMR (500 MHz, DMSO-d6, δ): 2.27 (s, 6H, CH3), 5.10 (s, 1 H, CH), 7.22- 7.77 (m, 14H, Ar-H), 12.50 (s, 1H, OH), 13.87 (s, 1H, OH); 13C NMR (125.17 MHz, DMSO-d6, δ): 11.7, 31.3, 103.2, 120.5, 125.5, 126.9, 128.7, 128.8, 131.4, 131.6, 132.8, 137.1, 138.4, 145.9.
Conclusion
In summary, an efficient protocol for the one-pot synthesis of 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) derivatives has been described under thermal solvent-free conditions using inexpensive starting materials. To the best of our knowledge, this new procedure represents the first example of an efficient synthetic method for these derivatives via a multi-component reaction.
Acknowledgment
The author thanks the Institute of Science and High Technology and Environmental Sciences, and the Graduate University of Advanced Technology, for partial financial support.
References
1. Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice. Oxford University Press: Oxford, 1998. [ Links ]
2. Mekheimer, R. A.; Abdelhameed, A. M.; Mohamed, S. M.; Sadek, K. U. Green Chem. Lett. Rev.2010, 3, 161-163. [ Links ]
3. Domling, A. Chem. Rev. 2006, 106, 17-89. [ Links ]
4. Burke, M. D.; Schreiber, S. L. Angew. Chem. Int. Ed.2004, 43, 46-58. [ Links ]
5. Park, H. J.; Lee, K.; Park, S. J.; Ahn, B.; Lee, J. C.; Cho, H. Y.; Lee, K. I. Bioorg. Med. Chem. Lett. 2005, 15, 3307-3312. [ Links ]
6. Clark, M. P.; Laughlin, S. K.; Laufersweiler, M. J.; Bookland, R. G.; Brugel, T. A.; Golebiowski, A.; Sabat, M. P.; Townes, J. A.; VanRens, J. C.; Djung, J. F.; Natchus, M. G.; De, B.; Hsieh, L. C.; Xu, S. C.; Walter, R. L.; Mekel, M. J.; Eitmeyer, S. A.; Brown, K. K.; Juergens, K.; Taiwo, Y. O.; Janusz, M. J. J. Med. Chem. 2004, 11, 2724-2727. [ Links ]
7. Cho, I.-H.; Noh, J.-Y.; Park, S.-W.; Ryu, H.-C.; Lim, J.-W.; Kim, J.-H.;Chae, M.-Y.; Kim, D.-H.; Jung, S.-H.; Park, H.-J.; Kim, Y.-H.; Min, I.-K. WO Patent 2004000206, 2003. [ Links ]
8. Clark, M. P.; Laughlin, S. K.; Golebiowski, A.; Brugel, T. A.; Sabat, M. WO Patent 2005047287, 2005. [ Links ]
9. Rosiere, C. E.; Grossman, M. I. Science. 1951, 113, 651-651. [ Links ]
10. Bailey, D. M.; Hansen, P. E.; Hlavac, A. G.; Baizman, E. R.; Pearl, J.; Defelice, A. F.; Feigenson, M. E. J. Med. Chem. 1985, 28, 256-260. [ Links ]
11. Mahajan, R. N.; Havaldar, F. H.; Fernandes, P. S. J. Indian Chem. Soc. 1991, 68, 245-246. [ Links ]
12. Chauhan, P. M. S.; Singh, S.; Chatterjee, R. K. Indian J. Chem. Sect. B: 1993, 32, 858-861. [ Links ]
13. Singh, D.; Singh, D. J. J. Indian Chem. Soc. 1991, 68, 165-167. [ Links ]
14. Londershausen, M. Pestic. Sci. 1996, 48, 269-292. [ Links ]
15. Lubs, H. A. The Chemistry of Synthetic Dyes and Pigments. American Chemical Society: Washington D.C., 1970. [ Links ]
16. (a) Uzoukwu, B. A.; Al-Juaid, S. S.; Hitchcock, P. B.; Smith, J. D. Polyhedron1993, 12, 2719-2724. [ Links ] (b) Maurya, R. C.; Verma, R. Indian J. Chem. Sect. A 1997, 36, 596-598. [ Links ] (c) Garnovskii, A. D.; Uraev, A. I.; Minkin, V. I. Arkivoc 2004, 3, 29-41. [ Links ]
17. (a) Hassankhani, A.; Mosaddegh, E.; Ebrahimipour, Y. Arab. J. Chem. in press. [ Links ] (b) Mosaddegh, E.; Hassankhani, A. Tetrahedron Lett. 2011, 52, 488-490. [ Links ] (c) Mosaddegh, E.; Hassankhani, A. Catal. Commun. 2013, 33, 70-75. [ Links ]
18. Stefan, S. L.; El-Behary, M.; Ramadan, A. A.; Mahmoud, S. A. J. Chem. Res. Miniprint 1992, 1951. [ Links ]
19. Ahluwalia, V. K.; Sharma, P.; Goyal, B. Indian J. Chem. Sect. B. 1997, 36, 1059. [ Links ]
20. (a) Hamama, W. S. Synth. Commun. 2001, 31, 1335-1345 (b) Li, [ Links ] X. L.; Wang, Y. M.; Tian, B.; Matsuura, T.; Meng, J. B. J. Heterocycl. Chem. 1998, 35, 129-134. [ Links ]
21. (a) Singh, D.; Singh, D. J. Chem. Eng. Data. 1984, 29, 355-356 (b) Mitra, [ Links ] A. S.; Rout, M. K. J. Indian Chem. Soc. 1961, 38, 893-895. [ Links ]
22. Pavlov, T.; Goleneva, A. F.; Lesnov, A. E.; Prokhorova, T. S. Pharm. Chem. J. 1998, 32, 370-372. [ Links ]
23. Buzykin, B. I.; Lonshchakova, T. I. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1971, 2340-2342. [ Links ]
24. Li, L.; Ma, H.-r; Wang, Y.-m.; Meng, J.-b. Chem. J. Chin. Univ. 1995, 12, 1903-1910. [ Links ]
25. Bai, Y.-j.; Lu, J.; Gan, H.-y.; Wang, Z.-j.; Ma, H.-r. Chin. J. Org. Chem. 2002, 22, 638-641. [ Links ]
26. Shi, D. Q.; Chen, J.; Wu, N.; Zhuang, Q. Y.; Wang, X-S. Chin. J. Org. Chem. 2005, 25, 405-408. [ Links ]
27. Wang, W.; Wang, S.-X.; Qin, X.-Y.; Li, J.-T. Synth. Commun. 2005, 35, 1263-1269. [ Links ]
28. Elinson, M. N.; Dorofeev, A. S.; Nasybullin, R. F.; Nikishin, G. I. Synthesis. 2008, 1933-1937. [ Links ]
29. Sujatha, K.; Shanthi, G.; Selvam, N. P.; Manoharan, S.; Perumal, P. T.; Rajendran, M. Bioorg. Med. Chem. Lett. 2009, 19, 4501-4503. [ Links ]
30. Moosavi-Zare, A. R.; Zolfigol, M. A.; Zarei, M.; Zare, A.; Khakyzadeh, V.; Hasaninejad, A. Appl. Catal. A: Gen, 2013,467, 61-68. [ Links ]
31. Niknam, K.; Saberi, D.; Sadegheyan, M.; Deris, A. Tetrahedron Lett. 2010, 51, 692-694. [ Links ]
32. Karimi-Jaberi, Z.; Pooladian, B.; Moradi, M.; Ghasemi, E. Chin. J. Catal. 2012, 33, 1945-1949. [ Links ]
33. Zang, H.; Su, Q.; Mo, Y.; Cheng, B. Ultrason. Sonochem. 2011, 18, 68-72. [ Links ]
34. Babaie, M.; Sheibani, H. Arab. J. Chem. 2011, 4, 159-162. [ Links ]
35. Sobhani, S.; Hasaninejad, A.-R.; Maleki, M.-F.; Mahdi, F.; Parizi, Z. P. Synth. Commun. 2012, 42, 2245-2255. [ Links ]
36. Sobhani, S.; Safaei, E.; Hasaninejad, A.-R..; Rezazadeh, S. J. Organomet. Chem. 2009, 694, 3027-3031. [ Links ]














