Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.58 n.1 Ciudad de México Jan./Mar. 2014
Article
Effect of Multiwalled Carbon Nanotubes on the Properties of PMMA/PEO Blends
Khalid Saeed* and Nasib Khan
Department if Chemistry, University if Malakand, Chakdara, Dir (Lower), Khyber Pakhtunkhwa, Pakistan. khalidkhalil2002@yahoo.com
Received June 10, 2013.
Accepted October 8, 2013.
Abstract
Poly (methyl methacrylate) (PMMA)/Poly ethylene oxide (PEO) (15 wt.% PMMA/85 wt.% PEO (PMMA/PEO-A) and 30 wt.% PMMA/70 wt.% PEO (PMMA/PEO-B)) blends, and functionalized multiwalled carbon nanotubes (F-MWNTs) filled PMMA/PEO-A and PMMA/PEO-B composites were prepared via solution casting technique. The morphological study showed the formation of cracks in F-MWNTs/PMMA/PEO blend. The mechanical properties of PMMA/PEO blend were decrease by incorporation of F-MWNTs, which might be due to less compatibility between F-MWNTs and PMMA/PEO blends. Neat PEO upon crystallization showed distinct sized spherulites, which decreased by the incorporation o F-MWNTs (nucleation effect of FMWNTs). The X-ray diffraction (XRD) analyses revealed that the intensity of peaks decreased by incorporation of nanotubes, which means that the PEO crystallinity was suppressed in the presence of F-MWNTs. The thermal stabilities of PMMA/PEO blend were improved by incorporation of F-MWNTs.
Keywords: Polymer blends, Multiwalled carbon nanotubes, Composites, spherulites.
Resumen
Poli (metacrilato de metilo) (PMMA)/óxido de etileno Poli (PEO) (15% en peso. PMMA/85% en peso PEO (PMMA/PEO-A) y 30 en peso PMMA/70% en peso.% PEO (PMMA/PEO-B)), mezclas y nanotubos de carbono de pared múltiple funcionalizados (F-MWNTs) PMMA/PEO-A y PMMA/PEO-B fueron preparados mediante la técnica de fundición solución. El estudio morfológico mostró la formación de grietas en la mezcla F-MWNTs/PMMA/PEO. Las propiedades mecánicas de mezcla PMMA/PEO se redujeron mediante la incorporación de F-MWNT, lo que podría deberse a una menor compatibilidad entre F-MWNT y de las mezclas de PMMA/PEO. PEO por cristalización mostró esferulitas de distintos tamaños, que disminuyeron en la incorporación de F-MWNT (efecto de nucleación de FMWNTs). La difracción de rayos X (DRX) revelaron que la intensidad de los picos disminuyó mediante la incorporación de nanotubos, lo que significa que la cristalinidad de PEO fue suprimida en presencia de F-MWNT. Las estabilidades térmicas de mezcla de PMMA/PEO se mejoraron mediante la incorporación de F-MWNT.
Palabras clave: Mezcla polimérica, nanotubos de carbono multicapas, compositos, esferulitas.
Introduction
Carbon nanotubes (CNTs) were discovered in 1991 by S. Iijema [1]. CNTs have unique thermal, mechanical, electrical, and magnetic properties, high flexibility, low density, large surface area per gram and high resistance toward chemicals. The thermal conductivity for both SWNTs and MWNTs is 3000 W m-1 K-1, which is twice as compared to diamond. CNTs have high elastic modulus (1 TPa for SWNTs and 0.3-1 TPa for MWNTs). The electric current capacity of CNTs is 1000 time greater than copper wire. The density of SWNTs and MWNTs is 0.8 and 1.80 g/cm3, respectively. Specific surface area of CNTs is 10-20m2/g [2, 3]. Due to these outstanding properties, CNTs are consider as important reinforcing materials in polymeric matrixes, actuators, hydrogen storage, chemical sensors, field emission displays, transistors, biomedical applications, advance materials for aerospace and nanoelectronic devices [47]. Although, CNTs have unique properties in various fields but the dispersion of CNTs is problem in various types of solvents and polymeric materials. To overcome this problem various approaches are used to get better dispersion of CNTs such as chemical functionalization, wrapping, electrospinning, and use of surfactants [8-11].
Polymers have different physical, thermal, electrical and mechanical properties, which depends upon the nature if the polymer. To achieve the required valuable properties, blending is suitable technique, in which two/more polymers uniformly mixed with each other.
The blending process is very attractive because of its versatility, simplicity, inexpensiveness and producing new polymeric materials with modified properties (morphological, thermal, mechanical, electrical or degradation behaviour can be change by a favourable choice of the second component of the blend) without having to synthesize totally new materials. Blending technology has important and valuable applications in various fields of science and engineering. Various research groups prepared polymer-polymer blend and reinforcing materials (CNTs, carbon fibers etc.) filled polymer blend. Wu and Shaw, prepared four different polymer blends and incorporated CNTs in these blends and found that CNTs-filled polymer blend show high electrical conductivity and high mechanical properties as compared to pure polymer blends [12]. Chen et al. prepared carbon fiber reinforced polyamide 66/polyphenylene sulfide blend composite, which significantly enhanced the mechanical properties of the polymer blend [13]. The polymer blend system can also be applied for preparation of solid polymer electrolyte by incorporating ionic liquid in suitable polymer matrix [14, 15].
In the present study, we used F-MWNTs as filler in PM-MA/PEO blends in order to study the effects of nanotubes on various properties of PMMA/PEO blends. The films of pure blend and F-MWNTs filled blend were prepared by solution casting technique. The morphology, thermal and mechanical properties of PMMA/PEO blends and F-MWNTs filled PM-MA/PEO composite were studied via scanning electron microscopy (SEM), POM, thermogravometric analyzer (TGA), universal testing machine (UTM).
Results and Discussion
Morphology of F-MWNTs/PMMA/PEO composite
Fig. 1 show the SEM micrograph of PMMA/PEO-A and PMMA/PEO-B blends and F-MWNTs (6wt %)/PMMA/PEO-A and F-MWNTs (6 wt %)/PMMA/PEO-B composite. The SEM images (Fig. 1a and c) (b, d) of pure PMMA/PEO blend show that there is no phase separation between PMMA and PEO, which indicates that PMMA is miscible with PEO. The miscibility of PMMA with PEO might be due to the interactions between the carbonyl carbon atoms of PMMA and the oxygen atoms of PEO (might be due to the transestrification) [16].
The SEM micrographs of F-MWNTs filled PMMA/PEO-A and PMMA/PEO-B nanocomposite are shown in Fig. 1b and d. The SEM images showed that the agglomeration/cracks are formed within the F-MWNTs filled PMMA/PEO-A and PM-MA/PEO-B nanocomposite films while such types of cracks Fig 1. are not observed within the pure polymer blends system. The agglomeration/cracks formation might be due to less compatibility between F-MWNTs and PMMA/PEO-A and PMMA/ PEO-B blends.
Thermal study of PMMA/PEO blend and F-MWNTs/PMMA/PEO nanocomposites
The TGA thermograms of PMMA/PEO-A and PMMA/PEO-B, F-MWNTs (3 and 6 % wt) filled PMMA/PEO-A and PMMA/ PEO-B blend are shown in Fig. 2 and 3. TGA curves (Fig. 2) shows that the PMMA/PEO-A blend remained stable at 250 oC. The weight loss is started at about 260 °C and decomposed completely at 520 oC. Fig. 2 also illustrate TG thermogram of F-MWNTs/PMMA/PEO-A composite, which showed higher thermal stability than pure PMMA/PEO-A blend. The F-MWNTs (3 % wt)/PMMA/PEO-A composite started weight loss at about 260 °C and decomposed at about 530 oC, which was about 10 oC higher than pure PMMA/PEO-A blend. The F-MWNTs (6 %wt)/PMMA/PEO-A composite started thermal degradation at about 310 oC and decomposed completely at about 540oC, which showed about 20 oC higher thermal degradation than pure PMMA/PEO-A blend.
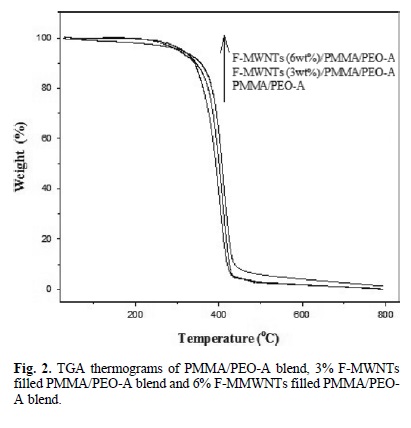
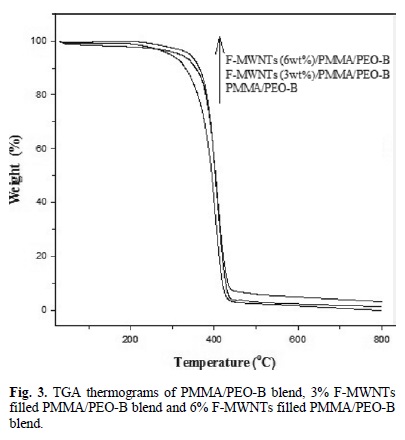
Fig. 3 shows the thermal degradation of PMMA/PEO-B blend and F-MWNTs (3 and 6 % wt)/PMMA/PEO-B nanocom-posite. The PMMA/PEO-B started weight loss at about 280 oC and decomposed completely at about 530 oC. The F-MWNTs (3 % wt)/PMMA/PEO-B composite started weight loss at about 340 °C and decomposed at about 540 oC. Similarly, the FMWNTs (6 % wt)/PMMA/PEO-B composite started weight loss at about 340 °C and decomposed at about 550 oC. The TGA thermograms showed that the incorporation of F-MWNTs enhanced the thermal stability of the PMMA/PEO-B blend. The residual amounts, which represent the F-MWNTs in the composites, are remained at higher temperature.
Mechanical properties of PMMA/PEO-A, PMMA/PEO-B, and F-MWNTs-filled PMMA/PEO-A and PMMA/PEO-B composites
The mechanical properties of PMMA/PEO-A, PMMA/PEO-B and F-MWNTs filled PMMA/PEO-A and PMMA/PEO-B blend are listed in tables 1 and 2. Table 1 show that the tensile strength, modulus and elongation at break of pure PMMA/ PEO-A blend was 12.9 N/mm2, 385.50 N/mm2 and 58.3 %, respectively. Table 1 also shows that the mechanical properties of the PMMA/PEO-A were decreased when F-MWNTs were added to the blend system. The tensile strength and moduli of F-MWNTs filled PMMA/PEO-A blend was decreased inthe range of 5-6 N/mm2 and 73-144 N/mm2, respectively. The elongation at break (show the flexibility) was also decreased in the case of F-MWNTs filled PMMA/PEO-A (4-37 %) than pure PMMA/PEO-A.
Table 2 shows the mechanical properties of PMMA/PEO- B and F-MWNTs filled PMMA/PEO-B, which also presented that the mechanical properties of pure PMMA/PEO-B blend was higher than F-MWNTs filled PMMA/PEO-B blends. The lower mechanical properties of the F-MWNTs filled PMMA/ PEO-A and PMMA/PEO-B blends was due to the presence of cracks within the samples, which was not observed in the case of pure PMMA/PEO-A and PMMA/PEO-B blends (Fig. 1a and b). The other reason for the decreased of mechanical properties is the less compatibly of CNTs with PMMA/PEO blend. Wuand Shaw were also observed the decrease in mechanical properties of CNTs-filled PET/PVDF blends. They reported that the cracks formation is responsible for the suppressing of the mechanical properties of CNTs-filled PET/PVDF blends [17].
POM study PMMA/PEO-A, PMMA/PEO-B, and F-MWNTs filled nanocomposites of PMMA/PEO-A and PMMA/PEO-B
The effect of PMMA and F-MWNTs on the spherulites of PEO is shown in Fig. 4. The Fig. 4(a) presented that pure PEO upon cooling showed distinct sized crystalline spherulites. The size of spherulites in pure PEO film is in the range of 20-50 urn and has similar arrangement. The POM images also showed that the size of spherulites were decreased in both PMMA/PEO-A and PMMA/PEO-B blends gradually when PMMA (amorphous polymer) was incorporated in PEO (Fig. 4b and e). The decrease in size of spherulites is due to inter molecular interaction on crystallization behaviour of two polymers [18].
It was also found that the size of spherulites are decreased at regular pattern in 3 and 6% wt F-MWNTs/PMMA/PEO-A composites and 3 and 6% wt F-MWNTs/PMMA/PEO-B nano-composites, which might be due to nucleation role of F-MWNTs in F-MWNTs/PMMA/PEO-A and F-MWNTs/PMMA/PEO-B nanocomposites. The decreased in size of spherulites was also observed by Saeed et al. [19] in case of MWNTs/PCL.
XRD Study
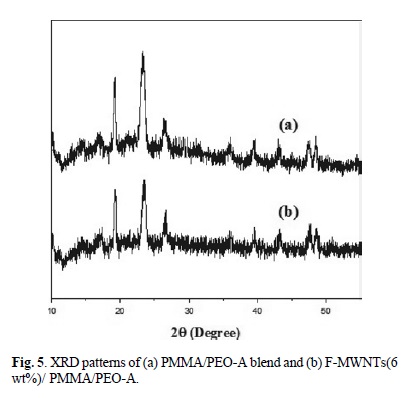
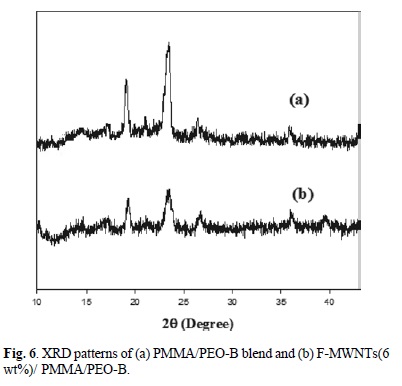
The XRD patterns of PMMA/PEO-A and PMMA/PEO-B, F-MWNTs (3 and 6 % wt) filled PMMA/PEO-A and PMMA/ PEO-B are shown in Fig. 5 and 6. The PMMA/PEO-A and PMMA/PEO-B demonstrates two prominent diffraction peaks with diffraction angles 29 close to 19.2o and 23.5o, which are the characteristics of crystalline PEO. The XRD patterns presented that the characteristic peaks of PEO are preserved in the case of the blend as well as in F-MWNTs-filled blend. However, the intensity of diffraction peaks decreased by addition of nanotubes, which means that the PEO crystallinity was suppressed in the presence of F-MWNTs. The suppressed crystallinity of PEO is in complete agreement with that of POM results. Similarly, Ghelichi et al. [20] reported similar XRD patterns for pure PMMA/PEO blend. They were also found that the intensity of the diffraction peaks decreased when lithium salt was incorporated in the blend system.
Experimental
Materials
The PEO (average molecular weight 6,000,000) and PMMA were purchased from Aldrich. The chloroform was purchased from BDH and was used as received. The MWNTs were provided by Iljin Nanotech and prepared by thermal chemical vapour deposition (CVD). Diameter and length of the CVD MWNT were 10-20 nm and 10-50 μm respectively, and its purity was higher than 97 wt %. The MWNTs were functionalized by the same method as discussed somewhere else [21].
Preparation of PMMA/PEO blend
The PMMA/PEO-A blend solution was prepared in chloroform at room temperature. The solution was stirred for 4 h in order to get homogeneous solution. The film of PMMA/PEO-A was obtained after the complete evaporation of solvent. The same method was used for the preparation of PMMA/PEO-B blends.
Preparation of F-MWNTs/PMMA/PEO composite
The known amount of F-MWNTs was first dispersed in 10 mL chloroform in the presence of minute quantity of Poly ethylene glycol (PEG) via stirring (15 min) and then sonicated (Power Sonic 405, Hwashin, Technology Co.) for 40 min. The homogenous dispersed F-MWNTs were introduced in known amountof PMMA/PEO solution. The F-MWNTs/PMMA/PEO solution was stirred for 5 min and then sonicated for 1 h. The 1wt % F-MWNTs/PMMA/PEO-A composite films were obtained by complete evaporation of solvent at room temperature. The same procedure was used to prepared 3 and 6 wt % F-MWNTs/ PMMA/PEO-A composite films. Similarly, 1, 3 and 6 wt % F-MWNTs/PMMA/PEO-B composite films were also prepared by the same method.
Instrumentation
The morphological study of gold-coated fracture surfaces of PMMA/PEO blend and F-MWNTs/PMMA/PEO composites (broken in nitrogen atmosphere) were analyzed using JEOL, JSM-5910 Scanning Electron Microscope. The TG thermograms of PMMA/PEO blends and their F-MWNTs/PMMA/PEO composites were obtained in nitrogen atmosphere at heating rate of 20 °C/min from room temperature to 800 °C using TGA (TG/DTA, Perkin Elmer). The mechanical properties of PMMA/PEO blends and F-MWNTs/PMMA/PEO composites were analyzed using UTM (Model 100-500 KN, Iestomeric Inc. UK). The study was performed at room temperature with a crosshead speed of 50 mm/min. The dimensions of sample were 70.9 (gauge length) x 27 (width)x 0.1 (thickness) mm. The POM study of pure blend and F-MWNTs/PMMA/PEO was performed using Optika B-600 POL POM. The sample was melt on heater and squeeze between two glass slides for 10 min.
Conclusion
In this study PMMA/PEO blends and F-MWNTs/PMMA/PEO composites were prepared via solution casting technique. The Khalid Saeed and Nasib Khan morphological study presented that F-MWNTs were embedded in PMMA/PEO-A and PMMA/PEO-B blends. The SEM images of F-MWNTs filled PMMA/PEO-A and PMMA/PEO-B showed cracks formation in these blend systems, which might be less compatibility between F-MWNTs with PMMA/PEO-A and PMMA/PEO-B blends. The POM study showed that the pure PEO having spherulites size from 20-50 urn The sizes of these spherulites were decreased by the addition of PMMA and F-MWNTs in PEO. The decreased in the size of spherulites might be due to the nucleation role of F-MWNTs. It was also found that the thermal stabilities of PMMA/PEO-A and PMMA/PEO-B blends were increased by the incorporation of F-MWNTs while the mechanical properties were decreased. The decrease in mechanical properties might be due to the less compatibility between F-MWNTs and PMMA/PEO-A and PMMA/PEO-B polymer blends.
References
1. Iijima, S. Nature 1991, 354, 56-58. [ Links ]
2. Saeed, K.; J. Chem. Soci. Pak. 2010, 32, 559-564. [ Links ]
3. Zhang, X. X.; Meng, Q. J.; Wang, X. C.; Bai, S. H. J. Mater. Sci. 2011, 46, 923-930. [ Links ]
4. Sheehan, P. E.; Lieber, C. M. Science 1997,277, 1971-1975. [ Links ]
5. Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. J. Anal. Chem. 2002, 74, 1993-1997. [ Links ]
6. Saeed, K.; Park, S. Y. J. Polym. Res. 2010, 17, 535-540. [ Links ]
7. Tans, S.J. Nature 1998, 393, 49-52. [ Links ]
8. Carabineiro, S. A. C.; Pereira, M. F. R.; Pereira, J.N.; Caparros, C.; Sencadas. V.; Mendez, S. L. Nanoscale. Res.Lett. 2011, 6, 302-306. [ Links ]
9. Hong, K.; Kim, S. H.; Yang, C.; Yun, W. M.; Nam, S.; Jang, J.; Park, C.; Park, C. E. American. Chem. Soci. 2011, 3, 74-79. [ Links ]
10. Miyauchi, M.; Miao, J.; Simmons, T. J.; Lee, J. W.; Doherty, T. V.; Dordick, J. S.; Linhardt, R. J. Biomacromolecules, 2010, 11, 2440-2445. [ Links ]
11. Vaisman, L.; Wagner, H. D.; Marom, G. Adv.Colloid. Interface. Sci. 2006, 128-130, 37-46. [ Links ]
12. Wu, M.; Shaw, L. J. Appl. Polym. Sci. 2006, 99, 477-488. [ Links ]
13. Chen, Z.; Liu, X.; Lu, R.; Li, T. J. Appl. Polym. Sci.2007, 105, 602-608. [ Links ]
14. Awasthi, S. K.; Bajpai, S. K.; Dubey, A. Polym. Plast. Technol. Eng. 2012, 51, 160-163. [ Links ]
15. Tang, Z.; Qi, L.; Gao, G. Polym. Adv. Technol. 2010, 21, 153-157. [ Links ]
16. Straka, J.; Schmidt, P.; Dybal, J.; Schneider, B.; Spevacek, J. Polymer 1995, 36, 1147- 1155. [ Links ]
17. Wu, M.; Shaw, L.; J. Power. Sources. 2004, 136, 37-44. [ Links ]
18. Ma, D.; Zhang, J.; Wang, M.; Ma, J.; Luo, X. Macromol. Chem. Phys. 2001, 202, 961-966. [ Links ]
19. Saeed,K.; Park, S. Y. J. Appl. Polym. Sci. 2007, 104, 1957-1963. [ Links ]
20. Ghelichi, M.; Qazvini, N. T.; Jafari, S. H.; Khonakdar, H. A.; Farajollahi, Y.; Scheffler, C. J. Appl. Polym. Sci. DOI; 10.1002/ APP.38897 (2012). [ Links ]
21. Saeed, K.; Park, S. Y.; Haider, S.; Baek, J. B. Nanoscale. Res. Lett. 2009, 4, 39-46. [ Links ]














