Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.57 n.2 Ciudad de México Apr./Jun. 2013
Article
1,4-Dioxane Degradation Using Persulfate Ion and Ag(I) Ion
Rosa María Felix-Navarro,1* Shui Wai Lin,1 Arturo Zizumbo-López,1 Sergio Pérez-Sicairos,1 Edgar Alonso Reynoso-Soto,1 and José Heriberto Espinoza-Gómez2
1 Centro de Graduados e Investigación, Instituto Tecnológico de Tijuana A.P. 1166, C.P. 22000, Tijuana, B. C. México. rmfelix2003@yahoo.com.mx
2 Facultad de Ciencias Químicas e Ingeniería, Universidad Autónoma de Baja California, Calzada Universidad 14418, Parque Industrial Internacional, CP. 22390, Tijuana, B.C.
Received September 24, 2012
Accepted April 22, 2013
Abstract
The kinetics of oxidation of 1,4-dioxane by persulfate ion with Ag+ in aqueous solutions at various temperatures and concentrations of S2O82−, Ag+ and H2SO4, were investigated. Experimental results indicate that 1,4-dioxane degradation follows a pseudo-first-order decay model and that reaction rate significantly accelerate by increasing temperature and concentration of oxidant, sulfuric acid and Ag+ ions up to 0.46 mM; for the range from 0.46-0.70 mM of Ag+ ions the reaction rate remains constant and at higher concentrations the reaction rate decreases. It was possible to degrade approximately 100% of 1,4-dioxane in less than one hour with the following conditions: [Ag+] = 0.46 mM, [H2SO4] = 0.30 M and from a concentration of 25 mM Na2SO4.
Keywords: 1,4-dioxane, degradation, kinetics, persulfate ion, advanced oxidation.
Resumen
En este trabajo se investigó la cinética de oxidación de 1,4-dioxano mediante iones persulfato con iones Ag+, en soluciones acuosas a diferentes temperaturas y concentraciones de S2O82−, Ag+ y H2SO4. La degradación de 1,4-dioxano presenta un decaimiento de pseudo-primer orden y la velocidad de reacción aumenta al incrementar la temperatura y concentración del oxidante, del H2SO4 y de Ag+ hasta 0.46 mM; en el intervalo de 0.46 a 0.70 mM de Ag+, la velocidad de reacción se mantiene constante y a concentraciones mayores, la velocidad de reacción disminuye. Fue posible degradar aproximadamente el 100% de 1,4-dioxano en menos de una hora con las siguientes condiciones: [Ag+] =0.46 mM, [H2SO4] =0.30 M y a partir de una concentración de Na2SO4 de 25 mM.
Palabras clave: 1,4-dioxano, degradación, cinética, ion persulfato, oxidación avanzada.
Introduction
The 1,4-dioxane is a cyclic ether and a problematic water pollutant that has major impacts on the human health and the environment. It is used widely in industry as a solvent for many organic and inorganic compounds. It is also produced as byproducts in many industrial processes such as ethylene glycol, ethylene oxide, and polyethylene terephthalate manufacturing. Moreover, 1,4-dioxane is a known carcinogen to animals and a suspected carcinogen to human, and hence, is classified as a hazardous compound and a priority pollutant [1]. If it is not removed from industrial wastewater effluent, 1,4-dioxane appears as a xenobiotic constituent of groundwater and drinking water [2].
Conventional water and wastewater treatment processes [3] may include chemical treatment, air stripping, carbon adsorption, and biological treatment; however these treatment processes are generally ineffective for eliminating 1,4-dioxane because of its high aqueous solubility and resistance to biodegradation. There is currently very little data on the removal of 1,4-dioxane by conventional biological wastewater treatment plants. However, the available information on biodegradation, sorption and air stripping suggest that removal efficiencies for 1,4-dioxane are very low by these methods. The advanced oxidation processes (AOP), which use the hydroxyl radical as the oxidant, can achieve substantial reductions in 1,4-dioxane. Choi et al. [4] used boron-doped diamond electrodes for the anodic oxidation of 1,4-dioxane, in their study the removal of 1,4-dioxane was monitored by chemical oxygen demand (COD), they reported that the anodic oxidation of 1,4-dioxane by boron-doped diamond electrodes were both an economical and an efficient process. Yanagida et al. [5], prepared stainless mesh with TiO2 coating using electrophoretic deposition (EPD), and used this stainless coated mesh as electrode to examine the synergy effect on photocatalysis of both 1,4-dioxane and ethylene glycol diformate (EGDF), a main intermediate of the photocatalysis of 1,4-dioxane. They reported that the photocatalytic decomposition rate of 1,4-dioxane depends on applying voltage and that the voltage swing provides high-efficiency photocatalysis of 1,4-dioxane while suppressed the EGDF formation.
Maurino et al. [6] found that sodium persulfate combined with UV light was more effective in degrading 1,4-dioxane than UV light with H2O2. The effect of pH on TiO2 photo-catalysis reactor systems used to degrade the 1,4-dioxane were investigated by Vescovi et al. [7], their experimental results indicate that at neutral pH the degradation of 1,4-dioxane was found to be most effective. Kinetic study on the degradation of 1,4-dioxane by zero-valent iron (Fe0)/UV light system was studied by Son et al. [8]; they determined that the increase supply of HO• radicals induced by the photolysis of Fe0 and H2O was responsible for the greatly increase of the degradation rate of 1,4-dioxane. Coleman et al. [9] studied the photocatalytic degradation of 1,4-dioxane using TiO2 and the H2O2/UV process. For the photocatalytic process, this group used magnetic photocatalyst as TiO2 particles (P25) as well as suspended and fixed arrangement. They concluded that mineralization of 1,4-dioxane, by the photocatalysis (with TiO2 and magnetic photocatalyst) was complete.
Some byproducts reported from 1,4-dioxane degradation are: ethylene glycol diformate, methoxyacetic acid, formic acid, glycolic acid and formaldehyde [10, 11] and acetic and oxalic acids [11].
An advanced oxidation process for water treatment that combines ozonation with electrolysis (ozone-electrolysis) was reported by Kishimoto et al. [12]. They reported some advantages of this process; as some reagents such as H2O2 or ferrous salts are unnecessary, less influence from chromaticity and the electric power was only required for operation.
The reactions of persulfate ions (also known as peroxydisulfate and peroxodisulfate ion) with various chemicals compounds have been extensively studied since many decades ago [13, 14]. In order to increase the rate of oxidation significantly, persulfate ion oxidation is generally conducted under heat, photo or metal-catalyzed conditions. Highly reactive species such as sulfate radicals (SO4−•) and hydroxyl radicals (HO•) are generated as a result of photolysis or heat decomposition of persulfate ions in aqueous phases [15].
The silver ion catalyzes the reaction of organic compound oxidation by S2O82−; according to Bacon et al. [16], the reactions of silver catalysis may be represented as the following;
S2O82− + Ag+ → SO42− + •SO4− + Ag2+ (1)
•SO4− + Ag2+ → SO42− + Ag3+ (2)
or
S2O82− + Ag2+ → SO42− + •SO4− + Ag3+ (3)
Ag2+ and Ag3+ ions can degrade organic compound like 1,4-dioxane. In this study sodium persulfate (Na2S2O8) with addition of Ag+ ions is evaluated as a potential oxidant alternative for the treatment of wastewater contaminated with 1,4-dioxane, according to references, this process has all the characteristics to achieve our purpose. Factors that influence the 1,4-dioxane degradation by S2O82−/Ag+ were studied, experiments at different pH were carried out with H2SO4 in order to minimize interferences due to different ions during the reaction. Kinetics parameters including rate constant, activation energy (Ea) and parameters of transition state such as enthalpy (∆H≠), energy (∆G≠) and entropy (∆S≠) were calculated.
Results and discussion
The rate law for the degradation of 1,4-dioxane by persulfate ion is expressed as equation (4):
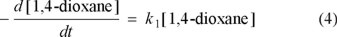
where k = f(T, [Na2S2O8] and [Ag+]) is the pseudo-first-order rate constant that represent a combined rate of 1,4-dioxane degradation by all oxidizing agents (e.g. S2O82− and Ag2+, Ag3+).
The results for each set of experiments for kinetics study under various conditions (i.e., different concentration in Na2S2O8, Ag+ and H2SO4 and temperature) are presented in Table 1, Figures 1-6 (2, 3, 4, 5). The discussions of the effects of degradation of 1,4dioxane under these experimental conditions are summarized below.
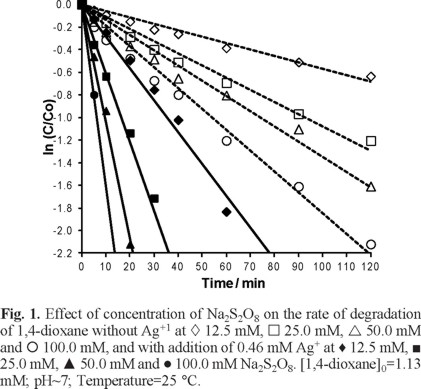
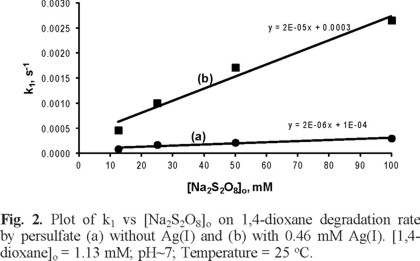
Effect of Na2S2O8 concentration on degradation of 1,4-dioxane by S2O82− with and without Ag+
The reaction rate of degradation of 1,4-dioxane against time at various initial concentrations of the oxidant (S2O82−) with and without addition of Ag+ ions are shown in Figure 1. The experimental results clearly indicate that 1,4-dioxane was degraded more rapidly at higher S2O82− concentration both with and without addition of Ag+ in the test solution. However, degradation of 1,4-dioxane was observed to be faster in presence of Ag+ ions, this may due to the fact that silver might catalyze persulfate ion oxidation of organic compound by Ag2+ and Ag3+ ions generated via reactions 1-3 shown above. The CO2 generated from degradation of 1,4-dioxane was determined qualitatively by bubbling the gas outlet into a 1.0 M Ba(OH)2 solution, where BaCO3 was precipitated from the reaction of CO2 and Ba(OH)2.
As shown in Table 1, the values of rate constant for degradation of 1,4- dioxane adding Ag+ are considerably higher than that of the correspondent rate constants for the degradation by S2O82− without addition of Ag+. This clearly demonstrated that the addition of Ag+ in the reaction solution catalyzed the degradation of 1,4-dioxane.
In addition, plotting k1 against initial Na2S2O8 concentration yield a slopes of 2 × 10−6 for degradation of 1,4-dioxane by S2O82− without Ag+, and of 2 × 10−5 with Ag+ (Figure 2), this reveals that degradation rate of 1,4-dioxane was directly proportional to the initial Na2S2O8 concentration both with or without addition of Ag+.
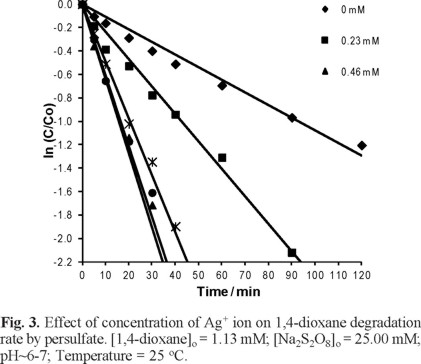
Effect of Ag+ ions concentration on degradation of 1,4-dioxane by S2O82
The Figure 3 shows the degradation of 1,4-dioxane versus time at five different Ag+ concentrations of 0.00, 0.23, 0.46, 0.70 and 0.93 mM. The 1,4-dioxane degradation rate was observed to be increased as the concentration of Ag+ was increased from 0.00 to 0.46 mM and there were no change for the Ag+ concentration from 0.46 to 0.70 mM. For concentration of Ag+ higher than 0.70 mM, the degradation rate was decreased. This decreasing in degradation rate at concentration of Ag+ greater than 0.70 mM can be due to the fact that after few minutes the test solution became obscure; this can be attributed to the CO2 generation in aqueous medium and subsequent Ag2CO3(s) production, which is poorly soluble in aqueous media, as shown in reactions (5) to (7) according to [17].
CO2 + H2O → HCO3− + H+ (5)
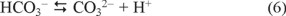
CO32− + 2Ag+ → Ag2CO3 (7)
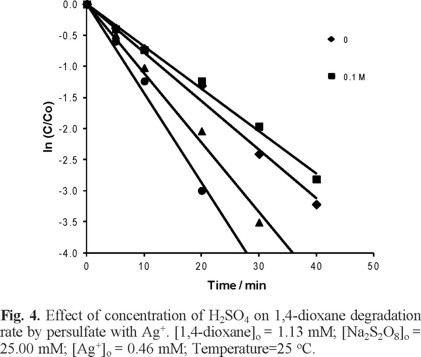
Effect of H2SO4 concentration on degradation of 1,4-dioxane by S2O82− with Ag+
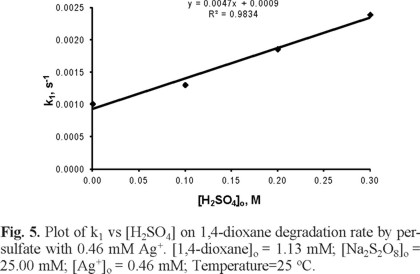
The degradation of 1,4-dioxane by persulfate ions with Ag+ ions were carried out at four concentrations of H2SO4, namely 0.0, 0.1, 0.2 and 0.3 M. As shown in Figure 4, the degradation of 1,4-dioxane was observed to be faster as the H2SO4 concentration was increased or as pH of the solution was lowered; this result was expected because decomposition of persulfate ion and generation of radicals occurred in neutral and alkaline aqueous solutions as reported by Singh and Venkatakao [18]. It was also in agreement with the findings, reported by Xu et al. [19] and Huang et al. [20] that in alkaline solutions the carbon dioxide, formed from complete oxidation of 1,4-dioxane, could lead to the formation of bicarbonate and carbonate ions that may inhibit the organic compound oxidation.
Plotting K1 against the initial concentration of H2SO4 (Figure 5) yielded a straight line with a slope of 0.0047 and R2 = 0.98; this indicates the dependency of reaction rate on H2SO4 concentration.
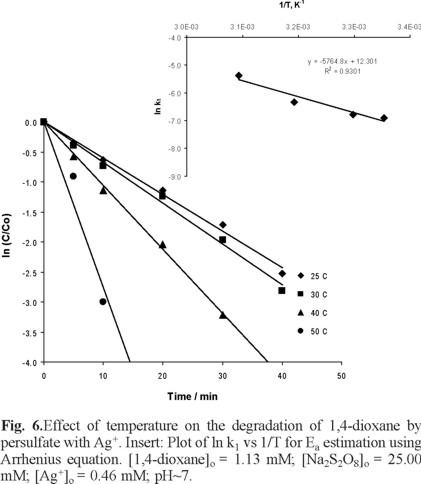
Effect of temperature on degradation of 1,4-dioxane by S2O82− with Ag+
The rate of degradation of 1,4-dioxane by S2O82− with Ag+ was observed to be significantly influenced by the temperature of the test solution (Figure 6); at higher temperature, the 1,4-dioxane was faster degraded. As shown in Table 1, the pseudo-first-order rate constant at 50 oC (46.05 × 10−4 s−1) for 1,4-dioxane degradation by persulfate ions with Ag+ is higher than that of by H2O2/UV oxidation process (20.00 × 10−4 s−1) and comparable with the degradation by TiO2/UV oxidation at pH 5.5 (48.3 × 10−4 s−1), but slightly smaller than the degradation with S2O82−/UV (73.00 × 10−4 s−1) [6].
Figure 6 shows the plot of ln (k1) versus 1/T which was used to estimate the activation energy of the degradation of 1,4-dioxane by S2O82− with Ag+. The activation energy was determined by the Arrhenius equation:

Where "A" is the frequency factor, Ea is the activation energy, R is the universal gas constant and T is the absolute temperature. From Figure 6, the plot of ln (k) vs 1/T yielded a slope of −5764.8 and an activation energy Ea= 11.45 kcal/mol for the degradation of 1,4-dioxane by persulfate ions with Ag+ ions, which is smaller than 21.0 kcal/mol, Ea value reported for the degradation of 1,4-dioxane by persulfate ions [21]. This smaller Ea value suggests a faster degradation reaction.
The thermodynamics parameters such as enthalpy (∆H≠), energy (∆G≠) and entropy (∆S≠) were computed with the following equations [22]:
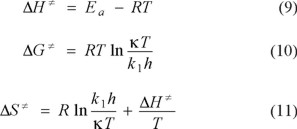
Where T is the ambient temperature, k1 is the rate constant at ambient temperature, kis the Boltzmann constant (1.381 × 10−23 J/K) and h is the Plank constant (6.626 × 10−34 J.s). The results for the parameters of the transition state are: ∆H≠ = 10.86 kcal/mol, ∆G≠ = 21.53 kcal/mol and ∆S≠ = −35.82 cal/mol-K, indicating that the reaction is endothermic and not a spontaneous reaction.
Conclusion
The degradation of 1,4-dioxane by persulfate and Ag+ ions was investigated. The 1-4-dioxane degradation was found to be of pseudo-first order respect to 1,4-dioxane concentration.
The presence of Ag+ ions increased the rate of 1,4-dioxane degradation reaction by persulfate.
The 1,4-dioxane degradation kinetics was affected favorably by the concentrations of oxidant (S2O82−) and H2SO4. It is also important not to perform the degradation of 1,4-dioxane at a Ag+ concentration greater than 0.70 mM because at higher values of this concentration the degradation rate decreases. Under the experimental conditions of 1.13 mM for 1,4-dioxane, 25.0 mM Na2S2O8 and 0.46 mM Ag+, the reaction has an activation energy of 11.45 kcal/mol at temperature range 25-50 oC.
Experimental
ACS-grade sodium persulfate (Na2S2O8), nitrate silver (AgNO3), 1,4-dioxane (C4H4O2), barium hydroxide (Ba(OH)2.8H2O) and acetonitrile, HPLC grade were supplied by Aldrich. All solutions were prepared with deionized (DI) water from SYBORN/Barnstead purification system model 02610.
The experiments were carried out in a reaction chamber (500 mL) surrounded by a glass-jacket filled with recirculation water for controlling the temperature of the solution during the reaction, also the reaction chamber was equipped with a stop-cork through which a thermometer, gas outlet and tube for withdrawing sample from test solution were installed. The gas outlet tube from the reaction chamber was bubbled into a 1.0 M Ba(OH)2 solution for detecting the formation of CO2 during the course of the degradation of 1,4-dioxane. The stirring rate and the temperature of the reacting solution were kept constant throughout the experiment. Temperature was controlled with VWR heated/refrigerated circulators, model 1166. Sample solutions, taken from the reactor at reaction time of 0, 5, 10, 20, 30 40, 60, 90 and 120 minutes, were immediately analyzed for determining the 1,4-dioxane concentration. The determination of 1,4-dioxane was done by High Performance Liquid Chromatography (HPLC); Hewlett Packard, model 1090 with spectrophotometric detection at 200 nm [23]. Acetonitrile-water (50% v/v) was used as the eluent at 1 mL/min through a C18 reversed-phase column (Altech, 10 cm), each sample injection was set at 10 µL.
Experiments were performed to study the effect of several parameters on the degradation of 1,4-dioxane; the parameters studied were concentration of oxidant (Na2S2O8), Ag+, H2SO4 and the temperature of the test solution. Effects of changing Na2S2O8 concentration and the presence of Ag+ ions on the degradation of 1,4-dioxane were carried out at 12.5, 25.0, 50.0 and 100.0 mM in Na2S2O8 for test solution containing 1.13 mM of 1,4-dioxane at 25 oC and at 0.46 mM of Ag+ ions, for the experiments where Ag+ were required. The initial pH of the test solution was adjusted to 5-6. When the effect of changing the concentration of Ag+ ions was studied; 0.00, 0.23, 0.46, 0.70 and 0.93 mM of Ag+ ions were added to each test solution containing 1.13 mM of 1,4-dioxane and 25.00 mM of Na2S2O8 at 25 oC. For the effect of the concentration of H2SO4 (0.0, 0.1, 0.2 and 0.3 M) on the degradation of 1,4-dioxane, experiments were carried out at conditions of 1.13 mM in 1,4-dioxane, 25.00 mM in Na2S2O8, 0.46 in mM Ag+ and at 25 °C. For the determination of the activation energy, experiments were run at 25, 30, 40 and 50 °C using 1.13 mM of 1,4-dioxane, 25.00 mM of Na2S2O8 and 0.46 of mM Ag+ ions.
Acknowledgements
The authors would like to take this opportunity to thank DGEST of Mexico for the financial support of this investigation (Grant No. 3602.10-P).
References
1. Stefan, M. I.; Bolton, J. R. Environ. Sci. Technol. 1998, 32, 1588-1595. [ Links ]
2. Adams, C. D.; Scanlan, P. A.; Secrist, N. D. Environ. Sci. Technol. 1994, 28, 1812-1818. [ Links ]
3. Zenker, M. J.; Borden, R. C.; Barlaz, M. A. Environ. Eng. Sci. 2003, 20, 423-432. [ Links ]
4. Choi, J. Y.; Lee, Y. J.; Shin, J.; Yang, J. W. J. Hazard. Mater. 2010, 179, 762-768. [ Links ]
5. Yanagida, S.; Nakajima, A.; Kameshima, Y.; Okada, K. Cat. Commun. 2006, 7, 1042-1046. [ Links ]
6. Maurino, V.; Calza, P.; Minero, C.; Pelizzetti, E.; Vicenti, M. Chemosphere 1997, 35, 2675-2688. [ Links ]
7. Vescovi, T.; Coleman, H. M.; Ama, R. J. Hazard Mater. 2010, 182, 75-79. [ Links ]
8. Son, H-S.; Im, J-K.; Zoh, K-D. Water Research 2009, 43, 1457-1463. [ Links ]
9. Coleman, H. M.; Vimonses, V.; Leslie, G.; Amal, R. J. Hazard. Mater. 2007, 146, 496-501. [ Links ]
10. Beckett, M. A.; Hua, I. Environ. Sci. Technol. 2000, 34, 3944-3953. [ Links ]
11. Stefan, M. I.; Bolton, J. R. Environ. Sci. Technol. 1998, 32, 1588-1595. [ Links ]
12. Kishimoto, N.; Nakagawa, T.; Asano, M.; Abe, M.; Yamada, M.; Ono, Y. Water Res. 2008, 42, 379-385. [ Links ]
13. Kolthoff, I. M.; Miller, J. K. J. Am. Chem. Soc. 1951, 73, 3055-3059. [ Links ]
14. House, D. A. Chem. Rev. 1962, 62, 185-200. [ Links ]
15. Tanner, D. D.; Osman, S. A. J. Org. Chem. 1987, 52, 4689-4693. [ Links ]
16. Bacon, R. G.; Grime, R.; Munro, D. J. J. Chem. Soc. 1954, 2275-2280. [ Links ]
17. Ponce de Leon, C.; Pletcher, D. J. Appl. Electrochem. 1995, 25, 307-314. [ Links ]
18. Sing, U. C.; Venkatarao, K. J. Inorg. Nucl. Chem. 1976, 38, 541-543. [ Links ]
19. Xu, S. C.; Zhou, H.; Wei, X.; Jun, L. Ozone Sci. Eng. 1989, 11, 281-296. [ Links ]
20. Huang. K. C.; Couttenye. R. A.; Hoag. G. E. Chemosphere 2002, 49, 423-420. [ Links ]
21. Félix-Navarro, R. M.; Lin-Ho, S. W.; Barrera-Díaz, N.; Pérez-Sicairos, S. J. Mex. Chem. Soc. 2007, 51, 67-71. [ Links ]
22. Espenson, J. H. Chemical Kinetics and Reaction Mechanisms, 2nd ed. McGraw-Hill, Inc., New York, 1995. 156-160 pp. [ Links ]
23. Scalia, S.; Guarneri, M.; Menegatti, E. Analyst 1990, 115, 929-931. [ Links ]














