Services on Demand
Journal
Article
Indicators
Related links
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.56 n.4 Ciudad de México Oct./Dec. 2012
Article
Phenolic Characterization, Melanoidins, and Antioxidant Activity of Some Commercial Coffees from Coffea arabica and Coffea canephora
Lucía Margarita Pérez-Hernández,1 Karla Chávez-Quiroz,3 Luis Ángel Medina-Juárez,2 and Nohemí Gámez Meza2*
1 Posgrado en Biociencias de la Universidad de Sonora, Blvd. Colosio s/n, entre Sahuaripa y Reforma, Colonia Centro. C.P. 83000. Hermosillo, Sonora, México.
2 Departamento de Investigaciones Científicas y Tecnológicas de la Universidad de Sonora. Blvd. Colosio s/n, entre Sahuaripa y Reforma Colonia Centro. C.P. 83000. Hermosillo, Sonora, México. ngamez@guayacan.uson.mx.
3 Café del Pacífico S.A. de C.V. Investigación y Desarrollo. Hermosillo, Sonora, México.
Received August 09, 2012;
Accepted October 10, 2012.
Abstract
Coffee phenols were identified, quantified and correlated with antioxidant activity determined by the ABTS•+ (2,2’-azinobis(3-ethylbenzothiazoline-6-sulphonic acid)) and DPPH• (2,2-diphenyl -1-picrylhydrazyl radical) assays. Melanoidins (Maillard reaction products) were determined for green and processed coffee. For green Arabica beans and its products, results suggest that roasting decreases antioxidantactivity. However, torrefacto roasted coffee showed greater antioxidant activity than Caracoli beans. Instant coffee showed greater antioxidant activity than green Robusta beans by both assays. A high correlation was found between antioxidant activity and melanoidins, total phenols, caffeic acid, and caffeine.
Key words: Coffee, Coffee Processing, Chlorogenic Acid, Caffeine, Maillard Reaction Products.
Resumen. Se evaluaron dos cafés procesados de granos Arábica, café torrefacto de grano Arábica y café soluble de grano Robusta. Los fenoles fueron identificados, cuantificados y correlacionados con la actividad antioxidante determinada por ABTS•+ (ácido 2,2-azino-bis (3-etilbenzotiazolina-6-sulfónico)) y DPPH• (2,2-difenil-1-picrilhidracilo). Fueron determinadas las melanoidinas para todas las muestras. Para los granos Arabica y sus productos, los resultados sugieren que el tostado disminuye la actividad antioxidante. Sin embargo, el café torrefacto mostró una actividad antioxidante mayor que el Caracol verde. El café instantáneo mostró una actividad antioxidante mayor que los granos verdes Robusta. Se encontró una alta correlación entre la actividad antioxidante y el contenido de melanoidinas, fenoles totales, ácido cafeico y cafeína.
Palabras clave: Café, procesamiento de café, ácido clorogénico, cafeína, productos de la reacción de Maillard.
Introduction
Coffee is one of the most widely consumed beverages in the world [1]. Mexico is one of the main coffee exporters and is the ninth coffee producer in the world [2]. Two species have the highest commercial importance: Coffea arabica (commonly known as Arabica coffee) and Coffea canephora (commonly known as Robusta coffee) [1]. Recently, scientific studies have pointed out the effect of coffee on human health. The beverage also stands out as a dietary source of potential antioxidants, such as caffeine, chlorogenic acid, hydroxycinnamic acids, and Maillard reaction products, such as melanoidins. Thus, the antioxidant capacity of coffee is related to the presence of both natural constituents and compounds formed during its processing [5].
Roasting markedly affects the composition of coffee. Changes in the antioxidative capacity of coffee upon roasting are associated with the degradation of chlorogenic acid. Many authors attribute the antioxidative effectiveness of roasted coffee to Maillard reaction products [23].
There are numerous published methods measuring total antioxidant capacity in vitro, which can be classified into two types: assays based on hydrogen atom transfer (HAT) and assays based onsingle electrontransfer(SET).SET-basedassays include the total phenols assay by Folin-Ciocalteau reagent, DPPH• and ABTS•+ radical scavenging capacity assays [12]. The ABTS•+ assay measures the relative ability of antioxidant to scavenge the ABTS•+ generated in aqueous phase, as compared with a Trolox (water soluble vitamin E analogue) standard. The method is rapid and can be used over a wide range of pH values, in both aqueous and organic solvent systems. It also has good repeatability and is simple to perform; hence, it is widely reported. The DPPH• is a stable free radical with an absorption band at 515 nm. It loses this absorption when reduced by an antioxidant or a free radical species. The DPPH• method is widely used to determine antiradical/antioxidant activity of purified phenolic compounds as well as natural plant extracts [13].
The aim of the present investigation was to determine the total phenolic contents and antioxidant activity (determined by ABTS•+ and DPPH•) found in Arabica, Caracoli and Robusta green coffee beans and in four commercially available coffee types (italian roast, french roast, torrefacto and instant coffee).
Results and discussion
Phenols and caffeine content
Total phenolics and individual phenolic contents were significantly higher (p < 0.05) in green Arabica beans than in italian roasta and french roast coffee. The total phenolic content found was 20-25% lower than in green Arabicabeans. Significant differences (p < 0.05) were found between the italian and french roast coffee. Perez-Martinez et. al. [18], reported that the total phenolic compounds found in different types of coffees ranged from 37 to 55 mg/g. Italian and french coffees are roasted at a temperature of 280 °C for 20 and 18 minutes, respectively. Hence, the decrease of phenolic compounds in french and italian coffee may be attributed to the roasting procedure [4, 6, 8]. This behavior was not observed for torrefacto coffee, which could be due to the higher levels of melanoidins formed in torrefacto coffee. No significant difference (p > 0.05) was found between total phenolic in torrefacto coffee and green Caracoli coffee (Table 1). The coffee beans used for the preparation of torrefacto coffee, are pre-roasted for 15 minutes. After that, caramelized sugar is added (20%) to the pre-roasted coffee beans. Finally, coffee beans are roasted for 17 minutes with the caramelized sugar at a temperature of 280 °C. According to Lopez-Galilea et al. [19], the addition of sugar stimulates the formation of melanoidins, which can react with the Folin-Ciocalteu reagent increasing the value of total phenols [18]. Budryn et al. [14] reported higher values of total phenols in Arabica coffee than the ones found in this study (165.60 mg/g of chlorogenic acid for green coffee, and values that ranged from 20.62 to 70.20 mg/g for medium roasted and dark roasted coffee). Literature about phenol composition of green Caracoli Arabica beans is scarce.
It was observed that the amount of chlorogenic acid was lower (p < 0.05) in the samples that underwent a more intense roasting procedure (french and italian roast coffee). Caracoli beans contained the highest amount of chlorogenic acid (p < 0.05). It has been observed that during coffee roasting, chlorogenic acid and their thermal degradation products are involved in the formation of melanoidins, along with other compounds, such as polysaccharides (galactomannans and arabinogalactans) and proteins [20]. Literature about phenol composition of green Caracoli Arabica beans is scarce.
Caffeine content was significantly lower (p < 0.05) in green Arabica beans than in green Caracoli beans and processed coffees (Table 2).
Instant coffee presented a significantly higher (p < 0.05) amount of total phenols, caffeine and phenolic compounds (determined by HPLC) than green Robusta beans (except for coumaric acid and rutin, which were only found in green Robustabeans).Theamountoftotalphenoliccompoundsfoundin instant coffee increased threefold (p < 0.05) in comparison of the green beans (Table 1). Inordertoproduceinstantcoffee,an additional step of extraction follows the roasting and grinding operations is carried out. That step consists in the evaporation or freeze concentration, of soluble components. Freeze-drying and spray drying are the most frequently used methods to produce instant coffee [21]. The total phenolic compounds comprised 14.08% of the total content of soluble coffee. Vignoli et al. [5], reported values of total phenolic compounds for instant coffee from 14.58% to 15.14% and a caffeine content from 39.80 to 58.20 mg/g the total dry matter.
Melanoidin content
Roasted coffees presented a higher concentration of melanoidins than green coffee beans (p < 0.05) (Table 1). Bekedam et al. [22] found that in roasted coffee, melanoidins comprise around 25 g/100 g. However, Tagliazucchi et al. [16] reported 37.87 g of melanoidins per 100 g for roasted coffee which is higher than the concentration found in this study. Many complex physical and chemical changes take place during roasting, including the obvious change in color from green to brown. The major compositional changes occurring are decreases in protein, amino acids, arabinogalactan, reducing sugars, trigonelline, chlorogenic acid, sucrose, and water; and by the other side, the formation of melanoidins. Many of the changes are due to the Maillard reaction[8]. Hence, the amount of melanoidins found in roasted coffee was greater than the melanoidins found in green coffee beans. Torrefacto coffee presented a greater concentration of melanoidins compared to italian and french roast coffee (p < 0.05), mainlyas are sult of the addition of sugar prior to roasting of coffee beans [19].
Instant coffee showed a higher concentration of melanoidins (p <0.05)as compared tothe found in italian roast,french roast and torrefacto coffee (Table 1). That is due to the process, which concentrates the components found in roasted coffee [21].
Antioxidant activity of brewed coffee by ABTS•+ method
No significant differences (p > 0.05) were observed between the antioxidant activity of green Arabica beans and italian roast coffee, nor with antioxidant activity of green Caracoli beans and torrefacto coffee (Table 1). That means that in these cases, the roasting process did not affect the antioxidant activity. Although compounds with antioxidant properties are lost during roasting of coffee beans, the overall antioxidant properties of coffee brews can be maintained, or even enhanced, by the development of compounds with antioxidant activity, including Maillard reaction products [8]. Perez-Martínez et al. [18] reported ABTS•+ values of 296-445 µmol trolox per g of Arabica roasted coffee, which are slightly higher than the obtained in our study.
Instant coffee presented a greater antioxidant activity (p < 0.05) than the green Robusta beans (Table 1). This behavior could be attributed to the fact that the soluble components of Robusta roasted coffee are concentrated during the manufacture process of soluble coffee, including components that possess antioxidant activity such as phenols and melanoidins.
Antioxidant activity of brewed coffee samples measured by DPPH• method
Green Arabica coffee beans presented a greater (p < 0.05) antioxidant activity than its roasted products (french and italian roast coffee) (Table 1). The antioxidative effectiveness of coffee beans is due to the presence of polyphenols, which main component is chlorogenic acid. As it can be observed in Table 2, the chlorogenic acid content was lower in italian and french coffee in comparison with green Arabica beans.Changes in the antioxidative capacity of coffee upon roasting are associated with the degradation of chlorogenic acid [23]. Torrefacto coffee presented a significantly greater (p < 0.05) antioxidant activity than green Caracoli beans, french and italian roast coffee. Torrefacto coffee is different from the rest of the conventionally roasted coffees. The manufacture process of torrefacto coffee implicates the addition of sugar prior to roasting. Therefore, the high antioxidant capacity observed in torrefacto coffee could be due to the fact that the presence of sugar stimulates the formation of Maillard reaction products, such as melanoidins, which exhibit antioxidant capacity [19].
Some differences were observed between the results obtained with the ABTS•+. ABTS•+ and DPPH• assays are usually classified as SET [24]. The ABTS•+ radical scavenging assay is applicable for both lipophilic and hydrophilic antioxidants [25]. Ozgen et al. [13] suggest that the DPPH• assay is more suited for samples with lipophilic antioxidants or those having a high lipid content. Phenols are hydrophilic, and it is probable that these differences observed in the results of antioxidant activity of coffee samples, could be due to the solubility characteristics of phenols.
In the case of instant coffee and green Robusta beans, the antioxidant activity of the first was significantly greater (p < 0.05).Asithasbeenmentionedbefore,themanufactureprocess of instant coffee beans concentrates the soluble components of Robusta roasted coffee beans, including those with antioxidant activity. As a result, the antioxidant activity is enhanced.

Correlation between ABTS•+and DPPH• values and phenols, caffeine, and melanoidin content
Correlations suggest that the components mainly involved in the antioxidant activity are melanoidins, caffeic acid, and caffeine. Contrarily, the correlation with chlorogenic acid and ferulic acid was poor, which may suggest that the phenolic compound that exerts a greater antioxidant activity is caffeic acid. A high correlation was observed between total phenols and antioxidant activity (Table 3).

Conclusions
Results show that intensive roasting decreases antioxidant activity, however melanoidin formation could improve coffee antioxidant activity. During the manufacture process of instant coffee,phenols,melanoidins,andcaffeineareconcentrated,and as a
Materials and methods
Samples
In this study, seven coffee types were analyzed. Three belonged to Arabica coffee beans (green beans and two dark roast, french and italian); two from Caracoli Arabica beans (green and torrefacto or medium roasted with sugar); and two from Robusta coffee beans (green and instant coffee). Coffee samples came from Huatusco, Veracruz, Mexico (19°08’48" latitude, 096°57’00" longitude, 1344 m altitude) and were obtained from Cafe del Pacífico (Mexico).
6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox) was purchased from Aldrich (Milwaukee, WI.). 2,2’azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS•+), 2,2-diphenyl-1-picrylhydrazylradical(DPPH•),potassium persulfate, Folin-Ciocalteau reactive and the HPLC standards were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents were analytical grade.
Sample and coffee extracts preparation
All of the coffee beans were ground with a mill (Thoma sWiley Laboratory Mill Model 4) to obtain a particle size of 20 µm. The ground samples were kept at -20 °C until analysis. The extracts were prepared according to the method described by Budrynet. al. [14]. Samples of green and roasted ground coffee were extracted with hot water (75 °C) at a coffee: solvent ratio of 1:100. Afterwards, a homogenization was performed for 5 min by using ultrasonic movements produced by a sonicator (Branson sonicator 1510). Next, the sample was centrifuged at 7900 g (IECL31 Thermo electron) for 15min. The coffee infusions were later filtered with Whatman No. 2 filter paper. The extraction was repeated twice for each simple using the method described previously.Theextracts werestoredat atemperature of -20 °C until analysis.
Quantification and identification of phenols and caffeine
The concentration of phenolic compounds was measured according to the method of Molina et. al. [15] and calculated using chlorogenic acid as a standard. Coffe eextract (50µL) was mixed with 3 mL of deionized water and 250 µL of the Folin Ciocalteu reactive (1 N) .After 5min, 750 µL of Na2CO3 (20%) and 950 µL of deionized water were added to the mixture, which was then left for 30 min. The phenolic compounds were spectrophotometrically determined at 765 nm. The content of total phenols was expressed as chlorogenic acid equivalents (CAE)/g. The procedure for the identification of total phenols was done by HPLC following the method described by Molina et. al. [15]. Initially, 20 μL of the purified coffee extracts were introduced to a SupelcosilTMLC18 (30 × 0.4 cm × 5 m particle size, Supelco, Bellefonte, PA, USA) column. The chromatographic HPLC equipment (Varian, model ProStar 230) used was equipped with an ultraviolet light detector (Varian, model 9050). The solvents used were water plus 5% acetic acid (solvent A) and 100% methanol (solvent B). The elution initiated with 98% of solvent A and 2% of solvent B, until 32% and 40% of solvent B were reached at 30 min and 40 min respectively, at a flow of 0.7 mL/min for 45 min. The identification of the phenolic compounds was achieved by comparing the retention times of the correspondent standards at 280 and 330nm. Forthe quantification of the phenolic compounds, calibration curves (concentration of 0-0.2 mg/mL) were constructed for each of the phenolic compounds identified. Individual phenol and caffeine content was expressed as mg of the specific compound pergram of sample. Phenolic compounds and caffeine of coffee extracts were analyzed by triplicate.
Quantification of melanoidins
Melanoidins were quantified spectrophotometrically. Before the determination of melanoidins in the coffee samples, a standard calibration curve was done at 420 nm, the wavelength melanoidins absorb [8]. Since the molecular structure of melanoidins has not been determined yet, there is not a melanoidin standard available. Hence, the standard calibration curve was constructed by using a roasted coffee extract as a source of melanoidins. A stock solution was prepared by making a dilution (2:1) of the roasted coffee extract. This stock solution was diluted afterwards five times. After reading the absorbance of every dilution, the concentration of melanoidins in each dilution was determined using the Lambert-Beer modified formula:
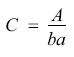
where C is the melanoidins concentration, A is the absorbance of the dilution. b is the length of the spectrophotometer`s cell (1 cm) and a is the specific extinction coefficient expressed as Lg-1cm -1. The value for "a" used was 1.1289 L g-1cm -1[16]. The standard calibration curve was constructed by plotting absorbance values as a function of the melanoidins concentration. For each sample, a 1:9 dilution was done. Melanoidins were spectrophotometrically determined at 420 nm. The melanoidins content was expressed as g of melanoidins per 100 g of sample.
Antioxidant activity of brewed coffee samples measured by ABTS•+ method
This assay was performed according to the method described by Molina et al. [15]. To generate the ABTS•+ cation, 19.2 mg of 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) were dissolved with 5 mL of distilled water. Then, 88 mL of potassium persulfate (0.0387 mg/mL) were added to the first solution. The resulting solution was homogenized and incubated at room temperature (25 + 1 °C) at darkness for 16 h. Once the ABTS•+ radical was formed, it was diluted with ethanol to obtain an absorbance value of 0,70 + 0,1 at 754 nm. Afterwards, 0.1 mL of the coffee extract was added to 3.9 mL of the solution containing the ABTS•+ radical. The absorbance was read immediately (Abst=0 min.), and every minute for a total of 7 min (Abstt=7 min.). Inhibition percentage was calculated as follows:

Standard calibration curves (concentration of 0-0.25 mg/ mL) of Trolox (soluble vitamin E analogous) were constructed by plotting percent of inhibition values as a function of the concentration of Trolox. The antioxidant activity was calculated with a standard calibration curve. The antioxidant activity of the samples were calculated in terms of Trolox (micromolar), using the calibration curves. The antioxidant activity was expressed as µmol of trolox per g of sample.
Antioxidant activity of brewed coffee samples measured by DPPH• method
The determination of the antioxidant activity by the DPPH• method was done according to the method described by Materska & Perucka [17]. A volume of 3.9 mL of the radical 2,2diphenyl-1-picrylhidrazyl (DPPH•) (0.025 mg/mL methanol) were mixed with 0.1 mL of each of the dilutions of the coffee extracts (0,25 g/mL concentration). The reaction took place for 30 min was spectrophotometrically determined at 515 nm with a UV-VIS spectrophotometer (Varian Cary 100). The results were expressed as µmol of trolox per g of sample.
Statistical Analysis
All results were presented as mean ± SD. Statistical differences between means were detected by one-way ANOVA followed by multiple comparisons using the LSD test. Differences were considered to be significant when p < 0.05. Statistical analysis was performed to know the correlation between the antioxidant activity determined by the ABTS•+ and DPPH• assays with total phenols, melanoidins, chlorogenic acid, caffeic acid, ferulic acid and caffeine. All statistical analysis was performed using SPSS 17.0 statistical package.
Acknowledgements
We express our sincere appreciation to Cafe del Pacífico for providing the samples analyzed in this research and to CONACyT for their financial support.
References
1. Gomez-Ruiz, J. A.; Ames, J. M.; Leake, D. S. Eur. Food Res. Technol. 2008, 227, 1017-1024. [ Links ]
2. http://faostat.fao.org/site/339/default.aspx accessed in July, 2012. [ Links ]
3. Gomez-Ruiz, J. A.; Leake, D. S.; Ames, J. M. J. Agric. Food Chem. 2007, 55, 6962-6969. [ Links ]
4. Joon-Kwan, M.; Yoo, A. S.; Shibamoto, T. J. Agric. Food Chem. 2009 57, 5365-5369. [ Links ]
5. Vignoli, J. A.; Bassoli, D. G.; Benassi, M. T. Food Chem. 2011, 124, 863-868. [ Links ]
6. Votavova, L.; Voldrich, M.; Sevcik, R.; Cizkova, H.; Mlejnecka, J.; Stolar, M.; Fleisman, T. Czech J. Food Sci. 2009, 27, S49-S52. [ Links ]
7. Hernandez, J. A.; Heyd, B.; Irles, C.; Valdovinos, B.; Trystram, G. J. Food Eng. 2007, 78, 1141-114. [ Links ]
8. Del Castillo, M. D.; Ames, J. M.; Gordon, M. H. J. Agric. Food Chem. 2002, 50, 3698-3703. [ Links ]
9. Nicoli, M. C.; Anese, M.; Manzocco, L.; Lerici, C. R. Lebensm Wiss Technol. 1997, 30, 292-297. [ Links ]
10. Borelli, R. C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. J. Agric. Food Chem. 2002, 50, 6527-6533. [ Links ]
11. Sánchez-González, I.; Jiménez-Escrig, A.; Saura-Calixto, F. Food Chem. 2005, 90, 133-139. [ Links ]
12. Awika, J. M.; Rooney, L. W.; Wu, X.; Prior, R. L.; Cisneros-Zevallos, L. J. Agric. Food Chem. 2003, 200351, 6657-6662. [ Links ]
13. Ozgen, M.; Reese, R. N.; Tulio, J. R. A. Z.; Scheerens, J. C.; Miller, A.R. J. Agric. Food Chem. 2006, 54, 1151-1157. [ Links ]
14. Budryn,G.;Nebesny,E.;Podsddek,A.;Zyzelewicz,D.;Materska, M.; Jankowski, S.; Janda, B. Eur. Food Res. Technol. 2009, 228, 913-922. [ Links ]
15. Molina-Quijada;D.M.A.;Medina-Juarez,L. A.; Gonzalez-Aguilar, G. A.; Robles-Sanchez, R. M.; Gamez-Meza, N. CyTA - J. of Food 2010, 8, 57-63. [ Links ]
16. Tagliazucchi, D.; Verzelloni, E.; Conte, A. J. Agric. Food Chem. 2010, 58, 2513-2519. [ Links ]
17. Materska, M.; Peruka, I. J. Agric. Food Chem. 2005, 53, 1750-1756. [ Links ]
18. Pérez-Martínez, M.; Caemmerer, B.; Paz de Peña, M.; Cid, C.; Kroh, L. W. J. Agric. Food Chem. 2010, 58, 2958-2965. [ Links ]
19. López-Galilea, I.; Paz de Peña, M.; Cid, C. J. Agric. Food Chem. 2007 55, 6110-6117. [ Links ]
20. Alves, R. C.; Costa, A. S. G.; Jerez, M.; Casal, S.; Sineiro, J.; Nuñez, M. J.; Oliveira, B. J. Agric. Food Chem. 2010, 58, 12221-12229. [ Links ]
21. Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.S. Food Bioprocess Technol. 2011, 4, 661-672. [ Links ]
22. Bekedam, E. K.; Schols, H. A.; Caemmerer, B.; Kroh, L. W.; Van Boekel, M. A. J. S.; Smit, G. J. Agric. Food Chem. 2008, 56, 4597-4604. [ Links ]
23. Nebesny, E. ; Budryn, G. Eur. Food Res. Technol. 2003, 217, 157-163. [ Links ]
24. Martysiak-Żurowska D.; Wenta W. Acta Sci. Pol., Technol. Aliment. 2012, 11, 83-89. [ Links ]
25. Aghdam, M. N.; Dehghan, G.; Kafshboran H. R., in: International Conference on Life Science and Technology, Vol. 3, IACSIT Press, Singapore, 2011, 55-58. [ Links ]














