Services on Demand
Journal
Article
Indicators
Related links
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.56 n.4 Ciudad de México Oct./Dec. 2012
Article
Kinetics and Hydrodynamics of Silver Ion Flotation
Martín Reyes,*1 Francisco Patiño,1 Ramiro Escudero,2 Miguel Pérez,1 Mizraim U. Flores,1 and Iván A. Reyes1
1 Universidad Autónoma del Estado de Hidalgo, Carretera Pachuca Tulancingo km 4.5 42186. Mineral de la Reforma, Hidalgo, México. mreyes@uaeh.edu.mx
2 Universidad Michoacana de San Nicolás de Hidalgo, Instituto de Investigaciones Metalúrgicas, C.P. 45000 Morelia, Michoacán, México.
Received: Mayo 8, 2012;
Accepted: Septiembre 24, 2012.
Abstract
This paper studies and determines the dispersion properties (Jg, Eg and Db), kinetics parameters and hydrodynamics of the process and its effect on the recovery of silver contained in spent diluted fixers by techniques of ion flotation in columns. The experimental results show silver recoveries of 97 % using sodium isopropyl xanthate (SIX) 0.06 g-L-1 and 0.04 g-L-1 of frother, at a Jg of 1.0 cm-s-1 and Jl of 0.72 cm-s-1. Xanthate-promoter combinations do not improve the separation; however, recoveries of 91.5% are achieved. The control of pH at 6.0 during the ion flotation of silver helps to improve the separation, compared to pH values of 7.0 and 8.0. The kinetic constant of apparent flotation k (min-1) maintains a linear behavior with the recovery and the dispersion properties, until reaching a slope change in the plot of Eg Vs Jg, where the system changes bubble flow regime, from homogeneous to turbulent.
Key words: Silver, Ion Flotation, Gas Holdup, Kinetics, Hydrodynamics.
Resumen
Este estudio investiga y determina las propiedades de la dispersión (Jg, Eg y Db), los parámetros cinéticos y la hidrodinámica del sistema y su efecto en la recuperación de plata contenida en líquidos fijadores diluidos agotados, mediante la flotación iónica en columnas. Los resultados experimentales muestran recuperaciones de plata del 97 %, usando xantato isopropílico de sodio (XIS) en concentración de 60 mg L-1 y 40 mg L-1espumante, Jg de 1.0 cm·s-1, y Jl de 0.72 cm s -1. Combinaciones de xantato y promotor no mejoran la separación, sin embargo, se logran recuperaciones del 91.5%. El control del pH en 6.0durante la flotacióniónica de plata contribuyea mejorar la separación respecto a los pH de 7.0 y 8.0. La constante cinética de flotación aparente mantiene un comportamiento lineal con la recuperación y las propiedades de la dispersión, hasta el cambio de pendiente en el grafico de Eg Vs Jg, donde el sistema cambia de régimen de flujo de homogéneo a turbulento.
Palabras clave: Plata, flotación iónica, gas retenido, cinética, hidrodinámica.
Introduction
The treatment of effluents is an important aspect in the protection of the environment; wastewater treatment is based on the elimination of pollutants by the use of physical, chemical and biological processes [1]. A common effluent is the one generated in radiographic laboratories; these spent fixers are mainly made of silver and sodium thiosulfate Ag(S2O3)2-3, Na(S2O3)2-3 respectively, as well as other harmful elements, such as metals, halides, among other pollutants [2].
The development of X-ray plates might be carried out in any urban zone, and there is a great number of businesses specialized on this kind of process. Once the fixers are used and spent, they are disposed of, or at the best, stored for further treatment; however, they are mostly spilled into the public sewage and on the crust. The industry of silver recovery of the spent fixers was developed several decades ago [3]. However, since the X-ray labs are enormously distributed in the cities, great volumes of effluents are not collected and treated.
This is due to the lack of a versatile technology for the treatment of considerable amounts of solution with diluted concentrations of the metal to be recovered. Silver is considered, in all its forms and compounds, a toxic metal [4]. Public sewage spilling of liquid wastes containing this element is highly forbidden; from an ecological point of view, it is convenient to treat these effluents in order to comply with the increasingly strict requirements of ecological regulations. It is worth mentioning that the concentration in these solutions exceeds the Mexican ecological regulations. The technical ecological regulation NTE-CCA-031, establishes that the highest permissible concentration of silver for sewage spilling is between 0.001 and 0.002 g L-1. From an economic point of view, it is convenient to recover the silver from the spent fixers of the X-ray plate fixing process. There are several techniques for the recovery of the silver contained in the spent fixers, such as chemical precipitation, metallic substitution, electrolysis, ion exchange, inverse osmosis, distillation, evaporation, etc. [3].
Nevertheless, these techniques present serious problems when treating great volumes of effluents in relatively short periods, making it necessary to develop a highly efficient and selective system, with low investment costs. An innovative technique that could help in solving this problem is the ion flotation [5]. The flotation process was originally applied for the selective separation of ores of interest, but this technique has broadened its application field towards non metallurgical operations, such as environmental protection: separation of oil, grease, and ink from recycled paper, as well as ion flotation of heavy metals [6].
Ion flotation can be defined as a technique that makes use of the special properties that characterize the interfaces for the concentration of ions or other entities with electric charge contained in aqueous phase [5] modifying the chemical conditions of the medium and adding proper collectors (anionic or cationic). The dissolved substance is combined with the collector, and it becomes a soluble metal-collector product with hydrophobic sites provided by the hydrocarbonated chain of the collector, which is adsorbed on the bubbles, then floats to the surface and concentrates according to the original medium [6]. Ion flotation has been used in order to recover numerous heavy metal ions [7]. Recently, interest has been drawn to the use of ion flotation for the recovery of precious metals from leaching liquors [8, 9] as well as for the removal of trace pollutants from considerable effluent volumes and other waste solutions [10].
A review on the studies on ion flotation and its possibilities in hydrometallurgical operations has brought out this technique's potential compared to other traditional techniques, such as chemical precipitation and extraction with solvents, among others [11]. Thermodynamic studies have been also carried out on the adsorption phenomena in ion flotation; the results have shown that the adsorption density lasts from several seconds to several minutes, depending on the used cationic collector: Sodium dodecyl, tetradecyl and hexadecyl sulfate [12].
The found recovery kinetics shows that, in order to achieve a 100% separation, it takes around 300 min indicating that the system follows a completely chemical control [12]. These studies have made a great thermodynamical contribution, but they do not present the hydrodynamic effect of the gas dispersion on the system and on the recovery of the ion of interest. Besides, cationic organic collectors of high molecular weight were used, as well as long hydrocarbonated chains, which are not practical because of their relative low solubility in water, and they are also considered as toxic, polluting reagents that damage the environment.
In other studies, ion flotation of metals, such as nickel and copper has been carried out using anionic organic collectors of short hydrocarbonated chains, common to the mining industry, such as xanthates, which are biodegradable at low concentrations. It was found that the recovery of metal is strongly affected by the modification of the dispersion properties and the system's hydrodynamics [6, 13, 14].
The object of this research paper is to carry out the recovery of silver contained in diluted waste solutions of very dilute thiosulfate (spent fixers) by ion flotation techniques, using anionic organic collectors as reagents, such as xanthates, dithiophosphate and dithiophosphinate type promoters, as well as studying the kinetics and hydrodynamics properties.
Results and Discussion
Residence Time Distribution (RTD)
It is very important to establish the mixing conditions in flotation equipment in order to determine the mixing pattern, the experimental residence time. These tests consisted of injecting a volume of 0.5 L of a 2.5 mol L-1 KCl solution (tracer) in the feeding flow of the flotation column, feed backing the solution during the whole test and monitoring the electrical conductivity of the solution for 25 minutes in the discharge flow (tails) by means of an electrical conductivity flow cell described in the literature [15] a reading was taken every 10 s by using a data acquisition card.
Figure 1 shows the results obtained for the cylindrical sparger at different superficial liquid velocities, Jl: 0.42, 0.84 and 1.13 cm s-1 and a constant Jg of 0.8 cm s-1, with a frother concentration of 0.04 g cm-3. The found curves are similar to those of a typical mixing plug flow transport [16]. It was observed that the mixing time of the solution decreases with the Jl. Based on these results, equation 1 was used in order to calculate the kinetic constant of flotation k (min-1).


Where R is the recovery, k is the flotation rate constant (min-1) and t, is the residence time (min).
Silver ion flotation
The reaction mechanism that occurs in the ion flotation process between the silver ion and the sodium isopropyl xanthate is the following: first, the silver ion Ag2+ reacts with the xanthate, forming silver dixantogen complex, equation 2.

Then, silver dixantogen complex, which is unstable especially at pH values below 7.0, decomposes into silver xanthate equation 3.
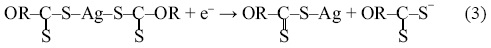
Thus, the silver xanthate is continually oxidized by the oxygen that is injected in the flotation column, forming again silver dixantogen, well as silver hydroxide and OH- ions equation 4; it is worth mentioning that in the ion flotation tests, the pH of the solution increased during the entire experimental test.

Subsequently, the silver hydroxide reacts with the sodium isopropyl xanthate, forming silver dixantogen, equation 5.

Then, silver dixantogen which is stable to alkaline pH values and thus achieve high separation efficiency.
Collector concentration effect
Figure 2 shows the percentage of silver recovery vs. superficial gas velocity Jg (cm s-1), with Jl 0.72 cm s-1, 0.04 g cm-3 frother and sodium isopropyl xanthate concentrations of 0.020.06 g cm-3 using a cylindrical sparger. It was observed that, with the increment of Jg and xanthate concentration ([X]), the recovery of silver increases progressively, achieving a maximum separation of 97% for Jg of 0.8 and 1.0 cm s-1 and 0.06 g cm-3 xanthate.

Jg plays a very important role, because it helps to increase the amount of small-diameter bubbles in the flotation column, which are available for capturing the desired species. In ion flotation, it is essential that the species modified and complexed by the collector is absorbed into a bubble; it must float towards the surface and then concentrate with respect to the original medium.
As it can be observed in figure 2, in order to achieve a solution with a silver concentration below the limits established by the ecological regulations, the necessary xanthate amount is higher than the stoichiometric amount. This is due to the collector's adsorption of other species contained in the industrial waste solutions, for example: aluminum, iron, copper and manganese among others.
As seen on figure 3, which presents the energy dispersive microanalysis (EDS) of the concentrate of silver ion flotation, as well as the morphology of the floated product (silver-xan-thate), by SEM; it is made of long colloidal particles that are visible in the froth bed. The semi-quantitative analysis confirms the presence of additional elements in the silver concentrate, which constitute the structure of the collected metal-organic product.
Figure 4 presents the behavior of the properties that characterize a dispersion: Jg, Eg, Db and the bubble specific surface area flux Sb (cm2 bubble surfaces/s)/(cm2 column area), that is to say, the amount of bubble surfaces that flow per unit of column cross sectional area expressed with equation 6 [15, 16] as well as the % w/w of silver ion flotation vs. Jg. It can be observed that with the rise of Jg, these properties increase progressively. At Jg of 0.8 cm s-1, the maximum aeration in the column and the maximum recovery are achieved. Increasing the Jg to 1.0 cm s-1 does not help in achieving better silver recoveries. The ascending behavior of the recovery results in function of Jg is lost at Jg 0.3 cm s-1. This is due to the regime change of the bubbly flow.

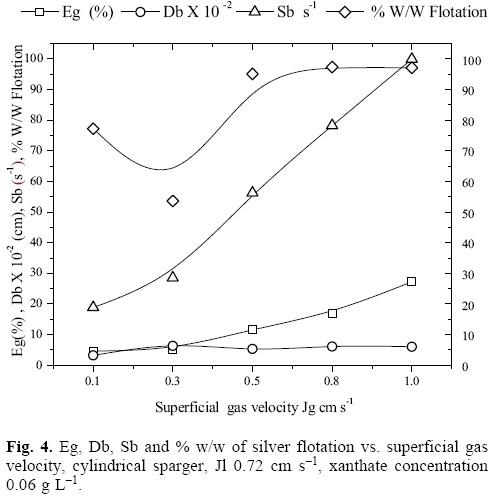
Figure 4 also shows the behavior of the gas holdup, the bubble size Db (cm) and the Sb (s-1) vs. Jg, for a xanthate concentration of 0.06 g cm-3and Jg values of 0.1 cm s-1 generate the lowest bubble sizes, which would allow considerably high recoveries; however, the relatively low buoyant rate of these bubbles, and the low value of Sb do not allow to achieve higher recoveries (as seen on the figure). With the Jg rise, increases the amount of surfaces available for carrying out the ion flotation process; at Jg of 0.8, the highest recovery values and higher gas rates are achieved. Therefore, a higher Sb does not contribute in recovering more silver because of the bubble saturation inside the column, which causes higher recirculation intensity of the continuous and disperse phases.
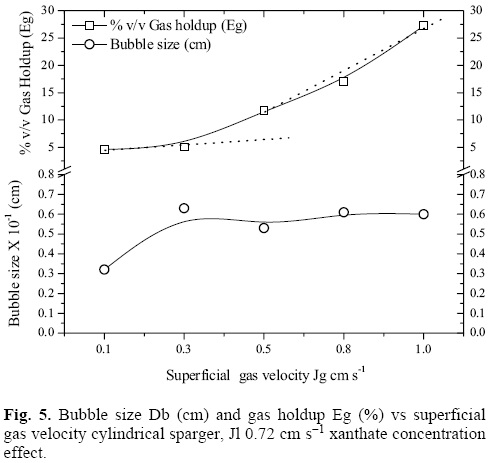
Figure 5 presents the results of bubble size (Db) and % v/v of gas holdup for a xanthate concentration of 0.06 g cm-3. It can be observed that, at Jg > 0.3 cm s-1, the hydrodynamics of the system are significantly modified, and the homogeneous, size-uniform flow regime changes to turbulent. The loss of the linear behavior of Eg vs. Jg indicates the change in the flow regime [15, 16]. Nonetheless, higher gas rates allow a significant improvement of the recovery of silver, which is a result of the competition between the hydrodynamic conditions of the medium and the chemistry of the process (as seen on figure 4). On the other hand, it can be observed that the bubble size Db (cm) increases with the Jg, and does not change significantly above a Jg of 0.3 cm s-1, with which an optimal bubble size of 0.06 cm is reached, achieving the best recoveries.
A change in the dispersion properties, for example the Db at Jg of 0.3 cm s-1, modifies the behavior of the flotation apparent rate kinetics, as shown in figure 6, which is a plot of the Db and the kinetic constant of apparent flotation rate k (min-1) vs. the Jg. However, a higher bubble flow and their decrease in bubble size increase the bubble - species density that opposes to the system's hydrodynamics caused by recirculation and mixing currents, and k (min-1) rises, which is a result of the higher transference of the silver-xanthate complexes from the collection zone into the frother zone; therefore, k (min-1) and the % w/w of recovery increases with the Jg.

Collector-promoter combination effect
The previously described results prove the significant influence of the process chemistry on the transference rate of the xanthate-silver species from the collection zone into the froth zone, surpassing the hydrodynamics of the gas-liquid dispersion generated by the cylindrical sparger.
Figure 7 shows the results of the collector-promoter combination and promoter alone in the silver ion flotation in function of Jg. These mixes don't achieve an improvement in silver recovery compared to the results of using only xanthate (0.06 g L-1). However, the recoveries are significantly high when using combinations of 0.03/0.03 g L-1 xanthate/XL 2945 promoter with values of 91% at Jg of 0.8 cm s-1, Jl 0.72 cm s-1, while for the combination of xanthate and XL 2945 promoter, the highest recovery was 84% at Jg of 0.3 cm s-1.

Combinations of promoter alone present the lower recoveries, and even the % w/w of silver flotation decreases with the Jg increment. This is due to the poor dilution of the used anionic promoters, as well as to the preference of these promoters towards some other species with lower ionic radius contained in the spent liquid fixers (such as Al, Fe, Cu). On the other hand the xanthate is highly selective for ionic silver, forming the metal-organic complex, as previously described. The chemistry of the system clearly plays an important role in the kinetics of the elimination of the silver ion from the solution at a degree higher than the hydrodynamic conditions of the system. However, both conditions complement each other for reaching the best separation efficiencies.
Figure 8 shows the behavior of the dispersion properties, % v/v Eg and Sb (s-1) in function of Jg for the process with collector-promoter and promoter combinations. It can be observed that the hydrodynamic characteristics of the system do not vary significantly with regards to those of the collector alone. However, for the latter, both the gas holdup and the Sb are slightly higher.

The rise in the Jg increases the volumetric percentage of gas holdup, and therefore, this is increased by the Sb, permitting the essential mechanism for the ion flotation of silver. However, due to the low electrostatic attraction of the organic reagents to silver, the recoveries do not improve compared to the process with xanthate alone, even with the proper hydro-dynamic conditions.
Figure 9 shows the behavior of the bubble size Db (cm), the fraction of gas holdup and the kinetic constant of apparent flotation rate (min-1) in function of Jg cm s-1 of the xanthate-promoter XL 2945 system at Jl 0.72 cm s-1. It can be observed that the % v/v Eg increases linearly with the Jg, until changing at 0.3 cm s-1 stopping at. Higher values change the flow regime, from homogeneous and uniform to turbulent, where big-sized and small-sized bubbles are found together. This has a direct effect on the bubble size and on the kinetics of the elimination of the silver ion. High gas rates, besides incrementing the bubble diameter, increase also the amount of bubble surfaces, which helps to slightly increase the kinetics of apparent flotation; therefore, there is a strong relation between Jg and optimal bubble size. Under the experimental conditions described for carrying out the silver ion flotation, the optimal Db is 0.06 (cm) at Jg 0.8 cm s-1. Nevertheless, the hydrodynamics generated at Jg 1.0, exceeds the chemical conditions, and the kinetics of apparent flotation decreases.

pH effect
It is clear that the % w/w of silver flotation of the diluted spent fixers is affected, first, by the chemistry of the process, and second, by the hydrodynamics of disperse and continuous phases.
Another important factor in the silver ion flotation is the pH. Of the previously described tests, the pH was constantly monitored in every experiment, and it was found that it increases along with the flotation time. For example, for the system containing 0.03/0.03 g L-1 xanthate/XL 2945 promoter, to Jg of 0.1 cm s-1, the pH was 7.25, while for a Jg of 1.0 cm s-1, it was of pH 8.6. This is due to the aeration to which the solution is subject in the column flotation, and to the chemical reaction of the elements and molecules contained in the diluted spent fixers, providing for the formation of metallic hydroxides.
In order to assess the solution pH effect, in the silver ion flotation the pH was kept constant during the entire test with H2SO4 or NaOH according to the case. The obtained results are shown in figure 10 for a system containing 0.03/0.03 g L-1 xanthate/XL 2945 promoter, Jl 0.72 cm s-1 and 0.04 g L-1 frother. The studied pH values were 6.0, 7.0 and 8.0. Figure 10 plots the kinetic constant of apparent flotation k (min-1) in function of Jg; it can be clearly seen that there is a higher transference rate of the hydrophobic species from the collection zone to the frother zone at pH (6.0) compared to the other analyzed values, and it is even higher than the system without pH control, worth mentioning that the decomposition of xanthate occurs faster at acidic pH values.

The lower recoveries at pH 7 and 8 are the result of the preferential adsorption of the organic anionic reagents for formed species hydroxide, reducing the collector molecules available for the formation of the silver-xanthate complexed. In general, for all the tests, the values of the % w/w of silver flotation present a sinusoidal behavior with respect to Jg by effect of the hydrodynamic modification of the involved phases (continuous and disperse), which causes the following: At certain values of Jg (for example 0.5 and 1.0 cm s-1) the recirculation and mixing of phases affect the flotation of the hydrophobic species at a higher degree due to the movement of the bubbles flowing in a sense opposite to their normal movement.
This effect can be observed in figure 11, which is a plot of the bubble size and % w/w of silver flotation in function of Jg. It is observed, that for the tests at constant pH 8.0, the bubble size Db (cm) is larger and presents a sinusoidal behavior similar to the recovery percentage. Larger bubble sizes (for example at pH 8.0) mean higher buoyant rate and lower residence time of the bubbles in the column because of their low recirculation degree. This helps to improve the recovery at Jg 0.3 cm s1.

However, these bubbles move a larger volume of liquid in their ascent, affecting the lower-diameter bubbles, which are sent downward and their ascent is slowed down, which reduces the transference of the collected species towards the froth zone, and therefore, the recovery. pH control, particularly in 8.0, requires the addition of alkali (sodium hydroxide), which modifies the surface tension of the solution; according to the literature, the increment in the concentration of alkali in solution increases the surface tension [17], due to the polarity of the sodium hydroxide molecules.
For the pH value of 6.0, it is observed that the Db increases gradually with the Jg, and the maximum recovery (93.5%) is reached when the bubble diameter is 0.06 cm at Jg of 0.8 cm s-1, while for the tests carried out at a constant pH of 7.0 the recovery decreases along with the bubble size; this is due to the hydrodynamic effects caused by the increase of the recirculation of small-sized bubbles for this pH value. The hydro-dynamic change in the phases present in the flotation column alters the bubble flow regimen, as seen on figure 12, which shows the behavior of the % v/v of gas holdup in function of Jg for the described systems.

Relatively high values of Eg are observed for the system with constant pH of 6.0, even the linearity of the behavior of the bubble flow regime is lost at Jg 0.5 cm s-1, and changes from homogeneous to turbulent, while for pH values of 7 and 8, this characteristic is lost at Jg 0.3 cm s-1. On the other hand, lower values of gas holdup are observed for the test with a constant pH of 8.0, and therefore, a higher bubble size is also observed, as seen on figure 11.
The values of gas holdup for the tests that had no pH control, 6.0 and 7.0, up to a Jg of 0.5 cm-s-1, are very similar among themselves. The recoveries at pH 6.0 and 7.0 are noticeably different due to the chemical environment of the solution, which is modified by the addition of H2SO4 o NaOH; this affects the adsorption of the species of interest by the organic chemical agents, and therefore, different silver recovery values are obtained, as seen on figure 11.
Conclusions
The recovery by column ion flotation of silver contained in diluted solutions of spent fixing liquids was carried out. The dispersion properties, hydrodynamic conditions and the kinetic constant of apparent flotation were obtained. The experimental results show silver recoveries of 97% with 0.06 g-cm-3 of sodium isopropyl xanthate, 0.04 g-cm-3 of frother, Jg 1.0 cm-s-1, and Jl 0.72 cm-s-1. Xanthate-promoter combinations do not improve the separation of silver from the continuous medium. pH values of 6.0 in the solution helps in the improvement of the separation. The kinetic constant of apparent flotation keeps a linear behavior with the recovery and the dispersion properties up to the slope change in the Eg vs. Jg plot, where the system undergoes a regime change. The removal of silver ions by column ion flotation techniques using anionic collectors is strongly influenced by the xanthate [X] and frother [e] concentrations, as well as Jg, Db, Sb.
Experimental
Equipment and materials
The silver ion flotation experiments were carried out in a transparent acrylic flotation column, 9.5 cm diameter and 360 cm height. On top of it an outlet was placed in order to collect the froth. Figure 13 shows the schematic representation of the used equipment. The intake was placed at 250 cm height (measured from the base of the column). This proportion represents an elevated zone for carrying out the collection and flotation of species.

The discharge flow or tail was installed in the base of the column. The feeding flows and tails were regulated with two peristaltic pumps (one for each flow) which were previously calibrated. The concentrate was carried out by gravity. The most important considered parameters in gas-liquid dispersion s in column flotation equipments are: superficial gas velocity Jg (cm-s-1). It is better to use volumetric flows, [15, 18]. Equation 7:
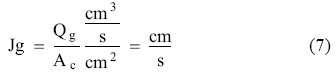
Where Qg is the liquid volumetric flow cm3-s-1 and Ac is the area of transversal section of the column in cm2. The gas was regulated with a flowmeter of 0-16 LPM (liters per minute) and introduced into the column via porous ceramic spargers made with moulding sand and sintered at 200 oC, for two hours, with cylindrical geometry, 3.5 cm diameter, and 8.0 cm height, with a surface area of 90 cm2. The object of using non metallic material is to avoid the possible transference of electrons and the deposit of the noblest metal on the less noble metal.
The flotation column was provided with four water manometers in order to measure the hydrostatic pressure inside it. The gas holdup Eg (% v/v) was calculated from the height differences in the manometers (ΔP) and the separation between pressure measurements (ΔH) by using equation 8 [16].

The pH of the solution was measured with a potentiometer, and was stirred during the whole test at 6.7 s-1 with a plastic propeller. For this work we used an effluent obtained from an X-ray laboratory located in Pachuca, Hidalgo, Mexico with a silver concentration of 0.038 g L-1. The samples collected in the concentrate and feeding flows, as well as in the tails, were analyzed for their silver concentration in an atomic absorption spectrophotometer. Some of the colloidal collected particles were characterized by scanning electron microscopy (SEM) along with microanalysis by energy dispersive microanalysis (EDS).
Experimental procedure
Air-water-frother-silver-collector
The experimental method consisted of continually feeding and discharging the solution containing 0.038 g cm-3 silver into the column, generally at a Jl of 0.72 cm s-1 for both flow currents, 0.04 g L-1 of glycol PM 425 frother were also added, as well as sodium isopropyl xanthate (SIX) C3H7OCS2Na in concentrations between 0.02 and 0.06 g cm-3. In some tests, combinations of collector-dithiophosphate XL 2945 (C10H18NaO2PS2) and dithiophosphinate XL 245 ((C4H9)2PSSNa) type promoters were used, as well as mixes of both promoters. During all of the experiments, the pH of the solution was measured.
Once the liquid level in the column was stabilized, air was injected into the column through the sparger at Jg values ranging from 0.1 to 1.0 cm s-1. For each studied Jg value, a fitting out period of twice the residence time (τ) in the collection zone was given, equation 9 [16]. Where VZC is the collection zone volume (cm3), Ql is the liquid volumetric flow (cm3), and Eg is the gas holdup.
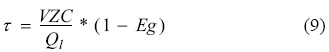
After the conclusion of this period, the movement, in cm (ΔP), in the water manometers was measured. Then, the concentrate currents, tails and feeding were sampled in that order, taking the sampling time and weighing the sample in order to estimate the recovery (% R) in the form of mass flow rate using equation 10. Where % R is the percentage of recovery of silver; FMC is the mass flow rate of the concentrate; FMT is the mass flow rate of the tails.

The bubble diameter Db (cm) was estimated through the interactive calculation procedure known as Drift Flux [19, 20], using the gas holdup data and phase relative rates Jg and Jl. According to the literature, in order to determine the kinetic constant of apparent flotation k (min-1) for a plug flow transport [16, 21] equation 1 can be used. Where the residence time (t) of all the elements is the same in all the flotation device; there is no mixing in the direction of the bubble flow, but lateral mixing does occur. The plug flow transport is equivalent to the batch processing [16].
References
1. Ruza T. F. In: Tratado universal del medio ambiente. Ed., Rezza, España, 1998. [ Links ]
2. Matulionytė, J.; Vengris, T.; Ragauskas, R.; Padarauskas, A. De-salination. 2007, 208, 81-88. [ Links ]
3. http://www.kodak.com/ek/uploadedFiles/J215ENG.pdf, accessed in march, 2012. [ Links ]
4. Chae, Y. J.; Pham, C. H.; Lee, J.; Bae, E.; Yi, J.; Gu, M.B. Aquat. Toxicol. 2009, 94, 320. [ Links ]
5. Sebba, F. Ion flotation, Ed., Elsevier, London 1962. [ Links ]
6. Reyes, M.; Tavera, F. J.; Escudero, R.; Patiño, F.; Salinas, E.; Rivera, I. Rev. Metal. 2010, 46, 109-120. [ Links ]
7. Hualing, D.; Zhide, H. Talanta 1989, 36, 633-637. [ Links ]
8. Galvin, K. P.; Engel, M. D.; Nicol, S. K. Int. J. Miner. Process. 1994, 42, 75-98. [ Links ]
9. Kinoshita, T.; Akita, S.; Ozawa; S.; Nii, S.; Kawaizumi, F.; Takahashi, K. JOM 2003, 3, 53-63. [ Links ]
10. Doyle, F.M.; Fuerstenau, D.W.; Duyvesteyn, S.; Sreenivasarao, K. EPD Congress '93. 1993, Ed. J.P. Hager, TMS, Warrendale, PA. 45-56. [ Links ]
11. Doyle, F. Int. J. Miner. Process. 2003, 72, 387-399. [ Links ]
12. Zhendong, L.; Doyle, F. Colloids and Surfaces A 2001, 178, 93-103. [ Links ]
13. Tavera, F. J.; Escudero, R.; Uribe, A.; Finch, J. A. Afinidad. 2000, 490, 415-423. [ Links ]
14. Reyes, M.; Patiño, F.; Tavera, F. J.; Escudero, R.; Rivera, I.; Pérez, M. J. Mex. Chem. Soc. 2009, 53, 15-22. [ Links ]
15. Tavera, F. J.; Ramiro, E.; Gomez, C.O.; Finch, J.A. Int. J. Miner Process. 2001 61, 23-40. [ Links ]
16. Finch, J. A.; Dobby, G. S. Column flotation, Ed., Pergamon Press, Oxford, 1990. [ Links ]
17. Orhan, O.; Stoyan, I.; Karakashev, A.; Nguyen, V.; Jan D. M. Miner. Eng. 1993, 22, 263-271. [ Links ]
18. Ityokumbol, M. T. Miner. Eng. 1993, 6, 12, 1279-1286. [ Links ]
19. Dobby, G. S.; Yianatos, J. B.; Finch, J. A. Can. Metall. Q. 1988, 27, 85-90. [ Links ]
20. Escudero, R.; Gomez, O. C.; Finch, J. A. Can. J. Chem. Eng. 2000, 78, 785-792. [ Links ]
21. Lelinski, D.; Allen, J.; Redden, L.; Web, A. Miner. Eng. 2002, 15, 499-505. [ Links ]














