Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista odontológica mexicana
Print version ISSN 1870-199X
Rev. Odont. Mex vol.20 n.4 Ciudad de México Oct./Dec. 2016
https://doi.org/10.1016/j.rodmex.2016.11.005
Original research
Proposal for experimental in vitro model to assess morphological alterations in erythrocytes exposed to 5.25% NaOCl
* DDS, Endodontics Specialist, Type «B» Professor. School of Dentistry, San Nicolas Hidalgo Michoacan University (UMSNH).
§ Delivery (childbirth) physician, Specialist in Hematology, Type «B» Professor. School of Dentistry, San Nicolas Hidalgo Michoacan University (UMSNH).
II DDS, Specialist in Endodontics, Type «B» professor (intern). School of Dentistry, San Nicolas Hidalgo Michoacan University (UMSNH).
¶ DDS, Endodontics Specialist, CUEPI Endodontics Masters Degree Graduate. School of Dentistry, San Nicolas Hidalgo Michoacan University (UMSNH).
** Professor of Basic Pharmacology, Type «B» Professor. School of Dentistry, San Nicolas Hidalgo Michoacan University (UMSNH).
Introduction:
Sodium hypochlorite (NaOCl) is the chemical agent most frequently used as irrigation solution during endodontic therapy. When extruded to periapical tissue, it is highly toxic. In endodontics, hemolysis caused by NaCOl has been proven using different models, nevertheless, there is little or no evidence of morphological alterations in the cellular membrane of erythrocytes.
Objective:
To propose an experimental model which might allow to assess morphological alterations suffered by erythrocytes when they are exposed to NaOCl used in the dental practice by means of high resolution scanning electron microscopy (SEM).
Materials and methods:
In the present study, 20 mL of peripheral blood were obtained and deposited in tubes with EDTA (ethylenediaminetetraacetic acid) anticoagulant. Rinses were conducted with a phosphate buffer solution (Evan’s solution). Several dilutions of the erythrocyte sample were prepared (1:1, 1:2, 1:4, 1:8 and 1:16); 100 μL of each of these dilutions was obtained to be then confronted with 100 μL of dental use 5.25% NaOCl (Viarzoni-T, Medental®); 0.5 μL of these samples were taken to then be deposited in a sample holder made of Zn-Cu alloy which was subjected to a process of Cu ion metallization bath, following the old Spluttering method. Microphotographs were obtained with SEM.
Results:
Erythrocytes with alteration type anisocytosis and poikilocytosis (stomatocytes, elliptocytes and discocytes) were observed. Some structural characteristics of NaOCl crystals were equally observed.
Conclusion:
This experimental model allowed assessment of morphological changes experienced by erythrocytes when exposed to 5.25% NaOCl.
Key words: Experimental model; sodium hypochlorite; erythrocytes
Introducción:
El hipoclorito de sodio (NaOCl) es el agente químico más utilizado como solución irrigadora durante la terapia endodóntica. Es altamente tóxico cuando se extruye a tejido periapicales. En endodoncia la hemolisis causada por el NaOCl ha quedado demostrada utilizando diferentes modelos, sin embargo poca o ninguna evidencia se tiene de las alteraciones morfológicas en la membrana celular de los eritrocitos.
Objetivo:
Proponer un modelo experimental que permita evaluar las alteraciones morfológicas que sufren los eritrocitos cuando son expuestos a NaOCl utilizado en la práctica odontológica mediante microscopia electrónica de barrido de alta resolución (MEB).
Material y métodos:
Se obtuvieron 20 mL de sangre periférica y se depositaron en tubos con anticoagulante EDTA (ácido etilendiaminotetraacético). Se realizaron lavados con solución amortiguadora de fosfatos (solución Evan’s). Se prepararon diferentes diluciones de la muestra de eritrocitos (1:1, 1:2, 1:4, 1:8 y 1:16). Se obtuvieron 100 μL de cada una de estas diluciones y se confrontaron con 100 μL de NaOCl 5.25% de uso odontológico (Viarzoni-T, Medental®). Se tomaron 0.5 μL de estas muestras para depositarse en un portamuestra de aleación Zn-Cu, el cual se sometió a un proceso de metalización de baño de iones de Cu por el método antiguo llamado Sputtering. Obteniendo microfotografías por MEB.
Resultados:
Se lograron observar eritrocitos con alteración de tipo anisocitosis y poiquilocitosis (estomatocitos, eliptocitos, esferocitos y discocitos). También se observaron algunas características estructurales de cristales de NaOCl.
Conclusión:
Este modelo experimental permitió evaluar los cambios morfológicos que sufren los eritrocitos cuando son expuestos a NaOCl 5.25%.
Palabras clave: Modelo experimental; hipoclorito de sodio; eritrocitos
INTRODUCTION
Sodium hypochlorite (NaOCl) is the agent most used as irrigating solution during endodontic therapy,1,2 this is due to its wide-spectrum antimicrobial activity3 and its ability to dissolve vital and necrotic tissue;4,5 it additionally exhibits low viscosity thus facilitating penetration into the root canal system.6 It is used at different concentrations ranging from 0.5% up to 6.0%.7 In scientific literature, the most recommended concentrations for irrigation use are 2.5% and 5.25%.8-10 Its antimicrobial action mechanism is explained by the reactions of aminoacid neutralization and chloramination, while the dissolution of organic matter is caused by saponification processes which are a product of the degradation of lipids and fatty acids.11
Contrary to its advantages, NaOCl by itself does not possess the ability to remove smear layers from the canal walls, moreover, it demineralizes dentin and induces corrosion of endodontic instruments.12-14 Nevertheless, NaOCl cytotoxicity is the property that requires greatest attention from the clinical operator. Extrusion of this irrigating material towards periapical tissue causes hemolysis, ulceration, inhibition of neutrophil migration as well as damage to endothelial cells and fibroblasts,15-17 which clinically manifests itself as pain, burning sensation, edema and hematoma.18
In endodontics, biocompatibility of material is determined by several parameters such as genotoxicity, mutagenicity, carcinogenicity, histocompatibility, antimicrobial effects and mutagenicity. For over 30 years, cell culture studies have been used to assess cytotoxicity reactions induced by endodontic materials.19
Among methods used to determine cytotoxicity we can count the following: determination of cell morphology alterations by means of light microscopy, confocal microscopy and scanning electronic microscopy (SEM).19 Evaluations conducted with SEM have been restricted to mainly showing alterations on periodontal and fibroblast cell lines.20-23
In other medical areas, scanning electronic microscopy has enabled evaluation of abnormalities of the red blood cell membrane.24 In endodontics NaOCl -caused hemolysis has been fully demonstrated using different models,25 nevertheless there is scarce or no evidence of morphological alterations in the cell membrane of erythrocytes when they come in contact with NaOCl solutions of dental use. Therefore, the aim of the present article was to propose an experimental model which might allow SEM assessment of morphological alterations experimented by erythrocytes when they are exposed to NaOCl used in dental practice.
MATERIAL AND METHODS
Collection of biological simple
A 31 year old male was selected; he reported non- contributory pathological data; 20 mL of peripheral blood were obtained and deposited in sterile glass tubes with EDTA anti-coagulant. Samples were centrifuged at 2,000 rpm/5 minutes, plasma was withdrawn with 100-1,000 μL micro-pipettes (Spinreact®). For the cellular package, three washes were performed with Evan’s solution (PBS phosphate buffer solution Allerstand®, registry 0027 R98 SSA, lot 407-360, Mexico DF) in a 2:1 proportion. A final red blood cell suspension of 2:1 was conducted with PBS.
Red blood cell dilution preparation
The following different dilutions with PBS were conducted with the red blood cell suspension: 1:1, 12:2, 1:4, 1:8 and 1:16, in 1.5 mL capacity propylene microtubes (Eppendorf®) (Figures 1 and 2). After this, 100 μL of each of these solutions were obtained and confronted to 100 μL of 5.25% NaOCl n (dental use); 5 μL were taken and then deposited in a Zn-Cu alloy sample holder (1 cm in diameter and height). Samples were tempered for 30 days.

Figure 2 A) biological sample in An-Cu alloy sample holder of 1 cm height and diameter. B) Vacuum chamber of High Resolution Scanning Electron Microscope C) JEOL JSM-7600F High Resolution Scanning Electron Microscope.
After this, samples were subjected to a Cu ion metallization bath for 15 minutes following the old Sputtering technique. This technique consisted on Cu ion bombardment on the surface of sample’s blood red cells so as to achieve thin sheets, aiming at allowing the flow of electrons emanating from the filament beam to thus favor morphological analysis of red blood cells. To this effect equipment Vaccum coating model s150, Sputter Coating brand was used. This process of Cu ion metallization is a fundamental part of the proposed cellular experimental model development in order to evaluate the morphology of red blood cells exposed to dental use 5.25% NaOCl.
In order to analyze morphology of red blood cells, different magnitude images (110x, 250x, 500x, 1,000x, and 5,000x) were taken in the retro-dispersed secondary electron mode, a high resolution field electron scanning microscope JEOL JSM-7600F was used. Moreover, different spectrograms of the surface of different areas of the blood cells, Dispersive Energy Specters (DES), were obtained by means of Bruker Xflash 6130 Micro-analyzer (Figure 3) Likewise, weight percentage of elements forming the red blood cells was obtained. The aforementioned analyses were only determined at 1,000x images.
Physicians with expertise in the area of hematology (or better hematologically trained physicians) analyzed images obtained of all samples.
RESULTS
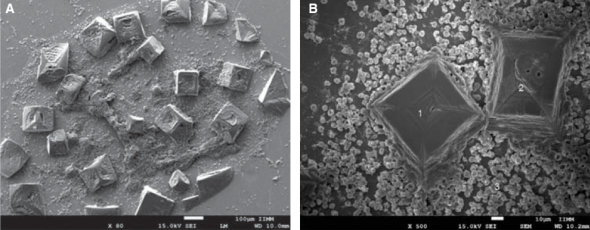
Within anomalies suffered by erythrocytes when exposed to dental use 5.25% NaOCl, red blood cells of different sizes were observed, which would indicate anisocytosis; abnormal shapes characteristics of poikilocytosis were equally observed (Figure 4). Among main poikilocytosis variations, stomatocytes, elliptocytes, spherocytes and discocytes were identified (Figure 5) Moreover, the present experimental model allowed identification of some structural characteristics of NaOCl crystals (Figure 6).

Figure 4 A) Morphological alterations of erythrocytes induced by exposition to dental use 5.25% NaOCl: with predominance of poikilocytosis characteristic forms. B) Anisocytosis characteristic forms.

Figure 5 Morphological alterations of erythrocytes exposed to dental use 5.25% NaOCl, through images obtained with high resolution scanning electron microscopy. A) Discocyte: bi-concave dis lacking nucleus. B) Elliptocyte: elliptic form with rounded tips and small bulge in lateral walls. C) Spherocyte: spherical erythrocyte with dense hemoglobin content without central halo. D) Stomatocyte: erythrocyte with a mouth-shaped elongated central zone, E) Discocyte at 10,000x where structural damage can be observed at the level of the plasmatic membrane.
DISCUSSION
It has been observed that in vitro experimental models have provided valuable information allowing thus a better understanding of several biological phenomena. The present experimental model which used high definition scanning electronic microscopy permitted the assessment of morphological alterations of the membranes of erythrocytes exposed to dental use 5.25% NaOCl; it revealed greater frequency of anomalies characteristic of anisocytosis and poikilocytosis (stomatocytes, elliptocytes, spherocytes and discocytes). According to Wang et al,26 erythrocytes are a relatively simple in vitro model to assess cytotoxicity of chemical substances. Studies conducted on erythrocytes during the last decades, have enabled the development of detailed knowledge of alterations in the function of red blood cells, as well as of disorders in their membrane due to either external, hereditary or pathological factors.27 For this reason, it is important to assess morphological alterations of erythrocytes when they are exposed to dental use NaOCl solutions.
Ionescu-Zanetti et al,28 and Bierbaum et al29 mention the lysis process experienced by erythrocytes through the saponification process, where it has been observed that lysophospholipids have shown to porate the membrane until causing its death when extra-cellular means are added. This poration allows small ions to permeate the membrane, thus, larger anionic proteins will be found in the cytoplasm. Small ions associated to water molecules, penetrate into the cell, creating thus positive osmotic pressure which will cause colloidal osmotic lysis; this leads to the transition of discocytes, and then of sperocytes, before finalizing in cell death. The aforementioned forms were equally reported in the present study. On the other hand, characteristics shapes of elliptocytes and stomatocytes were found. These erythrocyte anomalies could depend on the viscosity of the cytoplasmic fluid and the rigidity of its cellular membrane, which are affected by changes in the Redox potential and/or decrease of oxygenation in some of its cyto-skeletal proteins: spectrin and ankryn.30 These proteins are considered to be of the utmost importance to preserve membrane architecture.31
The present experimental model using high resolution scanning electron microscopy allowed identification of alterations experienced by the erythrocytes’s plasmatic membrane, this model could be implemented to assess biocompatibility among several conventional irrigating solutions used for root canal treatment
CONCLUSION
The present experimental model allowed the evaluation of morphological changes experienced by erythrocytes when they are exposed to 5% NaOCl. It is therefore considered that this model could be used to analyze biocompatibility of future chemical solutions which might be used in the field of endodontics as well as in other stomatological areas, since it can be considered a viable and easy to replicate model.
REFERENCES
1. Gül S, Savsar A, Tayfa Z. Cytotoxic and genotoxic effects of sodium hypochlorite. Cytotechnology. 2009; 59 (2): 113-119. [ Links ]
2. Kovac J, Kovac D. Effect of irrigating solutions in endodontic therapy. Bratisl Lek Listy. 2011; 112 (7): 410-415. [ Links ]
3. Radcliffe CE, Potouridou L, Qureshi R, Habahbeh N, Qualtrough A, Worthington H et al. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii, A. naeslundii, Candida albicans and Enterococcus faecalis. Int Endod J. 2004; 37: 438-446. [ Links ]
4. Clarkson RM, Moule AJ, Podlich H, Kellaway R, Macfarlane R, Lewis D. Dissolution of porcine incisor pulps in sodium hypochlorite solutions of varying compositions and concentrations. Aust Dent J. 2006; 51 (3): 245-251. [ Links ]
5. Stojicic S, Zivkovic S, Qian W, Zhang H, Haapasalo M. Tissue dissolution by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactant. J Endod. 2010; 36 (9): 1558-1562. [ Links ]
6. Spencer HR, Ike V, Brennan PA. Review: the use of sodium hypochlorite in endodontics-potential complications and their management. Br Dent J. 2007; 202 (9): 555-559. [ Links ]
7. Retamozo B, Shabahang S, Johnson N, Aprecio RM, Torabinejad M. Minimum contact time and concentration of sodium hypochlorite required to eliminate Enterococcus faecalis. J Endod. 2010; 36 (3): 520-523. [ Links ]
8. Marion JJC, Manhães FC, Bajo H, Duque TM. Ef?ciency of different concentrations of sodium hypochlorite during endodontic treatment. Literature review. Dental Press Endod. 2012; 2 (4): 32-37. [ Links ]
9. Cardenas BA et al. Hipoclorito de sodio en irrigación de conductos radiculares: sondeo de opinión y concentración en productos comerciales. Rev Odontol Mex. 2012; 16 (4): 252-258. [ Links ]
10. Siqueira JF Jr, Rôças IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000; 26 (6): 331-334. [ Links ]
11. Estrela C, Estrela CRA, Barbin EL, Spanó JCE, Marchesan MA, Pécora JD. Mecanismo de ação do hipoclorito de sódio. Braz Dent J. 2002; 13 (2): 113-117. [ Links ]
12. Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, De Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97: 79-84. [ Links ]
13. Slutzky-Goldberg I, Maree M, Liberman R, Heling I. Effect of sodium hypochlorite on dentin microhardness. J Endod. 2004; 30 (12): 880-882. [ Links ]
14. Poggio C, Arciola CR, Dagna A, Chiesa M, Sforza D, Visai L. Antimicrobial activity of sodium hypochlorite-based irrigating solutions. Int J Artif Organs. 2010; 33 (9): 654-659. [ Links ]
15. Kerbl F, DeVilliers P, Litaker M, Eleazer PD. Physical effects of sodium hypochlorite on bone: an ex vivo study. J Endod. 2012; 38 (3): 357-359. [ Links ]
16. De Sermeño RF, da Silva LA, Herrera H, Herrera H, Silva RA, Leonardo MR. Tissue damage after sodium hypochlorite extrusion during root canal treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108 (1): e46-49. [ Links ]
17. Witton R, Henthorn K, Ethunandan M, Harmer S, Brennan PA. Neurological complications following extrusion of sodium hypochlorite solution during root canal treatment. Int Endod J. 2005; 38: 843-848. [ Links ]
18. Gernhardt CR, Eppendorf K, Kozlowski A, Brandt M. Toxicity of concentrated sodium Hypoclorite used as an endodontic irrigant. Int Endod J. 2004; 37: 272-280. [ Links ]
19. Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 1. Intracanal drugs and substances. Int Endod J. 2003; 36: 75-85. [ Links ]
20. Navarro-Escobar E, González-Rodríguez MP, Ferrer-Luque CM. Cytotoxic effects of two acid solutions and 2.5% sodium hypochlorite used in endodontic therapy. Med Oral, Patol Oral Cir Bucal. 2010; 15 (1): e90-94. [ Links ]
21. Hidalgo E, Bartolome R, Dominguez C. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal ?broblasts and its bactericidal effectiveness. Chem-Biol Interact. 2002; 139: 265-282. [ Links ]
22. Malheiros CF, Marques MM, Gavini G. In vitro evaluation of the cytotoxic effects of acid solutions used as canal irrigants. J Endod. 2005; 31 (10): 746-748. [ Links ]
23. Bajrami D, Hoxha V, Görduysus Ö, Muftuoglu S, Zeybek N, Küçükkaya S. Cytotoxic effect of endodontic irrigants in vitro. Med Sci Mon Basic Res. 2014; 20: 22-26. [ Links ]
24. Ciccoli L, De Felice C, Paccagnini E, Leoncini S, Pecorelli A, Signorini C. Morphological changes and oxidative damage in Rett syndrome erythrocytes. Biochim Biophys Acta. 2012; 1820 (4): 511-520. [ Links ]
25. Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effects of NaOCl on vital tissue. J Endod. 1985; 11 (12): 525-528. [ Links ]
26. Wang C, Qin X, Huang B, He F, Zeng C. Hemolysis of human erythrocytes induced by melamine-cyanurate complex. Biochem Biophys Res Commun. 2010; 402 (4): 773-777. [ Links ]
27. An X, Mohandas N. Disorders of red cell membrane. Br J Hematol. 2008; 141 (3): 367-375. [ Links ]
28. Ionescu-Zanetti C, Wang LP, Di Carlo D, Hung P, Di Blas A, Hughey R et al. Alkaline hemolysis fragility is dependent on cell shape: results from a morphology tracker. Cytometry A. 2005; 65 (2): 116-123. [ Links ]
29. Bierbaum TJ, Bouma SR, Huestis WH. A mechanism of erythrocyte lysis by lysophosphatidylcholine. Biochim Biophys Acta. 1979; 555 (1); 102-110. [ Links ]
30. Muravyon AV, Tikhomirova IA. Role molecular signaling pathways in changes of red blood cell deformability. Clin Hemorheol Microcirc. 2013; 53 (1-2): 45-49. [ Links ]
31. Ciccoli L, De Felice C, Paccagnini E, Leoncini S, Pecorelli A, Signorini C et al. Erythrocyte shape abnormalities, membrane oxidative damage, and b-actin alterations: an unrecognized triad in classical autism. Mediators In?amm. 2013; 2013: 432616. [ Links ]
****This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam
Received: August 01, 2015; Accepted: March 01, 2016











 text in
text in