Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista odontológica mexicana
Print version ISSN 1870-199X
Rev. Odont. Mex vol.17 n.1 Ciudad de México Jan./Mar. 2013
Original research
Citotoxicity assessment of different endodontic-use sealing cements in gingival fibroblast cultures
Claudia Cortázar Fernández,* Raúl Luis García Aranda,§ Inés Willershausen,II Brita Willershausen,II Benjamín Briseño MarroquínII
* Universidad Autonoma de Nuevo Leon.
§ Universidad Autonoma de Mexico.
II Johannes Gutemberg University, Mainz, Germany.
ABSTRACT
The aim of the present study was the in vitro evaluation of the response, within 96 hours, of gingival fibroblast cultures with respect to different endodontic sealers. Results obtained at time intervals of 0, 1, 2, 3, 6, 24, 48, 72 and 96 hours were used to determine sealers' cytotoxicity. Gingival fibroblasts cultures without root canal sealer and with Sealapex were used as negative and positive controls respectively. Results were compared with negative controls and statistically analyzed with t Dunnett test (p ≤ 0.05). Assessed sealing cements were: ProRoot MTA, grey and white, CPM, MTA Angelus, Sealapex and GuttaFlow. Results showed that even though ProRoot MTA (grey and white) MTA Angelus, CPM and GuttaFlow exhibited lower cytotoxic potential than Sealapex, no statistical significant differences were established.
Key words: Cell culture, endodontic sealers, ProRoot MTA CPM, MTA Angelus.
INTRODUCTION
Several materials such as amalgam, zinc oxide-eugenol, IRM, Super EBA calcium hydroxide as well as glass ionomener have been used for endodontic treatment of iatrogenic lesions such as root perforation and/or perforation of the pulp chamber.1 Nevertheless, none of these materials has been able to achieve satisfactory sealing of the aforementioned lesions. Therefore, up to the present date, it has not been possible to promote regeneration of periapical tissues2 damaged by the perforations. To achieve this goal, in our days, the use of a tri-oxide aggregate based cement (ProRoot MTA) has been promoted.
ProRoot MTA was approved by the Food and Drug Administration in 1998. Lee & al were the first to describe it in endodontic scientific literature,3 since that time, ProRoot MTA has been used in surgical and non-surgical treatments. ProRoot MTA's chemical composition has been studied in different research endeavors. It has been studied with the help of X-rays, scattered energy spectrometer as well as electronic microscope.4-7 Trioxide aggregate mineral (ProRoot MTA) is a hydrophilic, fine particle powder, which hardens when humidity is present. The result is a colloidal gel which solidifies into solid structure in less than 4 hours.5 ProRoot MTA consists of tri-calcium silicate, tri-calcium aluminum, tri-calcium oxide and silicate oxide, as well as lesser amounts of mineral oxides. According to the manufacturer, these are responsible for this material's specific chemical and physical properties.8 Bismuth oxide present in ProRoot MTA imparts suitable radio-opacity to this product. According to Torabinejad et al,5 this radio-opacity is greater (7.17 aluminum) than that presented by Shah et al4 for gutta-percha (6.1 aluminum) and dentin. Its identification in X-rays is therefore easily discernible. According to Torabinejad et al,9 ProRoot MTA's physical-chemical composition allows for a better adaptation and therefore, better marginal seal of this material when compared to Super EBA and amalgam. In our days, there are two ProRoot MTA cement types: grey and white. One of the disadvantages of grey ProRoot MTA placed in a cavity to be filled is the fact that this material could compromise the aesthetics of the treated tooth. This was primarily the reason for the introduction of white ProRoot MTA cement, which was done with the aim of eliminating possible discoloration of adjacent tissues and teeth.8
Torabinejad et al5 analyzed ProRoot MTA under electron microscope, where it showed specific structure divided into calcium oxide and calcium phosphate. Calcium oxide was observed as discreet crystals, calcium phosphate appeared as an amorphous structure, apparently free of crystals and with granular appearance. ProRoot MTA does not contain calcium hydroxide, but calcium oxide, upon reacting with tissue fluids, can produce an in situ formation of calcium hydroxide.10
Ability to promote tissue regeneration, in situations such as in apex treatment, perforations, retrogade filling of any other clinical procedure used to seal communication between root canal systems and periapical tissue, is a desirable effect of any material used as endodontic sealer. Histological analyses11-13 have reported the fact that there are few dental materials which, when placed in contact with periodontal tissue, induce cementogenesis, and therefore, periapical tissue regeneration as is the case of ProRoot MTA.
ProRoot MTA behavior in cellular cultures has been assessed with cells similar to osteoblasts, called Mg-63.14 Authors informed that the material seemed to offer a substrate suitable for osteoblast activation. It therefore stimulateed calcium phosphate formation which in turn favored communication with cellular content. During this phase, no hydroxiapatite crystals were observed in the material when it was analyzed under scanning electron microscope. This elicited a change in cell behavior, and stimulated bone growth over the substrate.14 Pisotrius et al15 demonstrated in cellular cultures that when gingival fibroblasts were in direct contact with ProRoot MTA, they showed an apparently unaltered ability to synthesize protein when compared to controls. This ability was similar to that of titanium, therefore, they deduced that its biocompatibility was similar to that of titanium. Besides, authors reported normal cellular proliferation potential, contrary to that observed in amalgam.
In addition to analyzing possible toxic effects of root canal filling materials in cellular cultures14-22 there were observations made on dog and monkey teeth13,23,24 as well as in bone and subcutaneous implants in animals.6,10,25,26 Torabinejad et al25,26 examined bone reaction after having implanted ProRoot MTA, amalgam, IRM and EBA in guinea pigs tibiae and jaws. They observed ProRoot MTA's high biocompatibility degree, since it presented a favorable biological response. This was due to an observed absence of inflammation in the region surrounding the implants as well as greater bone tissue apposition around implants. The same group of researchers asserted that results of this research could be corroborated as well as those obtained with ProRoot MTA in previous research when it was studied as retrograde filling material9,27,28 as well as pulp capping material.29 Torabinejad et al23 conducted research on ProRoot MTA and amalgam as retrograde filling materials in dog root canals. They histologically analyzed reactions of tissue surrounding the root at 10 and 18 weeks. They observed lesser extension and severity of inflammation surrounding the roots of teeth treated with ProRoot MTA. They observed greater bone apposition adjacent to ProRoot MTA when compared to amalgam. They also frequently found presence of root cement over the ProRoot surface. Based on these results, authors recommended use of ProRoot MTA as the most suitable material for retrograde filling.
ProRoot MTA high retail cost has encouraged production of similar products such as MTA Angelus and CPM. Manufacturers asserted these materials exhibited the same characteristics, at a lesser cost. The aim of this research was to analyze cytotoxic potential of CPM and MTA sealing cements in comparison with grey and white MTA in gingival fibroblast cultures.
MATERIALS AND METHODS
This research encompassed the following cements: Portland type ProRoot MTA grey and white (Dentsply Tulsa, Tulsa, OK, USA) CPM (Medix DF, Mexico) MTA Angelus (Londrina/Larana Brasil). Negative controls were the following: root canal sealants Sealapex (Kerr Sybron/Romulus, MI USA) and Guttaflow (Roeko Coltene Whaledent/Langenau, Germany).
Human gingival fibroblasts used in this research were harvested from a connective tissue biopsy obtained during periodontal surgery of a healthy patient with no clinical history. 4th to 9th passage cell lines were used which, being younger passages, guaranteed higher metabolism and division rate without significant mutations.
Culture was conducted according to a known and established method (ING).30,31 Biopsy was fixated in small segments with Vaseline on plastic Petri dishes, 8.8 cm2 (Nunc/Wiesbaden, Germany) and were covered with a nutrient solution. To ensure preservation, cultures were preserved in tissue culture jars (185 cm2/Greiner/Frikenhausen, Germany) or 25 cm2 (Nunc, Wiesbaden, Germany) in a laminar flow bell (Function Line/Heraeus, Hanau, Germany) at 37 °C constant temperature, 95% relative humidity atmosphere, 5% sodium, and 5% CO2 saturation. In order to guarantee repeatable and constant cell growth, culture medium was kept at an 11 pH. Culture medium was changed after assessing cellular growth under a model microscope (Diavert Letiz/Wetzlar, Germany). Cellular growth control included a subjective evaluation of cellular layer density as well as probable morphological changes of cell cultivated as nuclear anomalies such a vacuolization and cellular death.32 Once they reached a confluent consistency forming a single cell layer (mono-layer) they were separated and used to form passages. A confluent cellular mono-layer, formed in the span of three to four days, was used for the experiments. At a later point, an enzymatic activation was conducted. This was performed with a EDTA trypsinized solution (200 mM, Life Technologies, Paisley, Scotland) under microscope control (40X). Medium changes were performed strictly observing hygiene measures.
Materials (10 mg) were mixed with a sterile spatula according to manufacturers indications. They were allowed to set for at least 15 hours under ultraviolet light to avoid possible contamination. They were later placed in cellular culture Petri boxes. They were covered with 30,000 cells and 200 µL of DNEM/F12 medium (Dulbecco's modified Eagle basal medium) supplemented with calf fetus serum at 10% (Grand Island Biological Company, Grand Island, New York, USA). 50 UI/mL of penicillin and 50 mg/mL streptomycin (Seromed; Seromed Biochrom-Produkte, Wiesbaden, Germany) were incorporated as nutrients. Cell were under-cultivated through trypsinization with a previously described method.19 Gingival fibroblasts cultures devoid of sealant and with Sealapex, were respectively used as negative and positive controls. Viability, or cellular proliferation rate was determined measuring tincture intensity with Alamar Blue. This procedure was performed with fluorescence micro-plate fluorometer (FLX 800 Reader, Bio-Tek Insstruments/Winooski, VA, USA) with 538-nm excitation and 600-nm wave length emission, during a four day span. Cell cultures were kept in a gas cultivator (Functionline, Heraeus Kulzer/Hanau) at 37 °C constant temperature. Cell structure was observed with the help of Phalladicin and DAPI fluorescent dyes. DAPI (4', 6- diamide-2-phenylindole) is a fluorescent dye which colors in blue the DNA of cells nuclei. Phallacidin is also a fluorescent dye which colors in green the cell cytoplasmatic skeleton. Both dyes allow observation of cellular reactions in cultures under fluorescence microscope.
CELLULAR PROLIFERATION POTENTIAL EVALUATION
Measurement of cellular proliferation potential was conducted at nine different incubation times (0, 1, 2, 3, 6, 24, 48, 36 and 96 hours). During this period the cellular culture medium did not require changing, since cells survived with unaltered alamar Blue function.
HO null hypothesis considered the fact that gingival fibroblast proliferation did not experience changes during four days (96 hours). Comparison was conducted with negative controls. Descriptive analysis of results contained averages and standard deviations of measurements performed with different cements (n = 15). Possible significant differences were established with Kolmogroff-Smirnoff and Kruskall-Wallis tests, with ≤ 5% confidence level.
RESULTS
Descriptive analysis was reflected in Table I. After three hours of incubation time, it was observed that the highest fibroblast proliferation recorded took place with CPM cement. Nevertheless, from 6 hours up to 96 hours a very similar reaction of CPM with other cements was observed. Results showed there was no significant statistical difference among researched cements and negative controls. Only Sealapex showed significant difference with respect to other cements and controls from the 48 hours incubation point and up to the end of the test (Figure 1). Figures 2A-J show gingival fibroblast reactions to materials researched after having dyeing cultures with Phallacidin and DAPi tinctures.
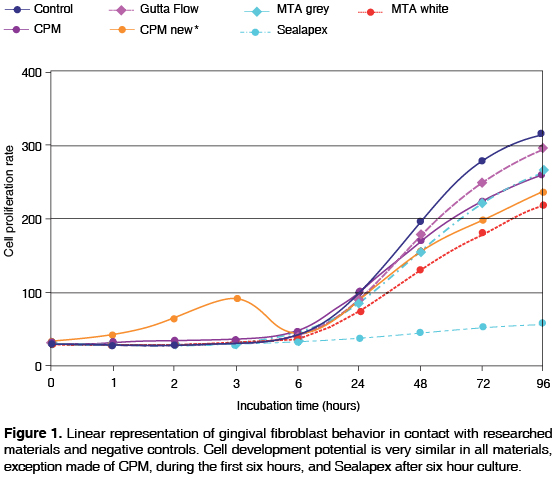
Cellular proliferation potential was a fundamental parameter to ascertain whether researched cements develop cytotoxic effect. To undertake statistical analysis, control of fibroblasts without cement contact was taken as well as incubation with cements in a 96 hour span.
DISCUSSION
Materials used in endodontic practice are placed in direct contact with periodontal tissue. This is particularly true when handling materials used for retrograde filling. For this reason, it is essential to verify the fact that sealing materials, besides meeting appropriate requirements, are biocompatible and not toxic to periodontal tissues.33 Cytotoxic potential is one of the most common parameters used in in vitro studies to determine the biocompatibility degree of an endodontic sealer. In general, there are simple and rapid tests that can provide quantifiable results. These results are valuable in order to assess the possibility of clinically using a given material. Several methods of cell cultures, such as assessment of cellular growth inhibition, cell membrane permeability tests, enzymatic activity tests, injury and/or cellular death recording34,35 have been used to determine cytotoxicity of different dental materials.
The present research conducted an in vitro study of the cytotoxicity of several cements used in endodontic practice. When the research material elicits high and constant cytotoxic reaction with some method of cell culture in vitro research, it is possible to assume this material might well exert high toxicity on vital tissues.16,21 The toxicity degree of a material used for retrograde filling can also be researched through its implantation on animal sub-cutaneous tissue, either bone or muscle, so as to assess local tissue reaction caused by in vivo reaction of the soft tissue induced by the tested material either in humans or animals.35
Results of in vitro cytotoxicity tests cannot fully be extrapolated to an in vivo situation, where multicellular systems and immunological processes take place which are impossible to fully replicate in the laboratory. In similar studies,15,16,19-22,36,37 human gingival fibroblasts have also been used to assess cytotoxicity of different dental materials. Due to the fact that dental materials are in close proximity to oral cavity gingival fibroblasts, authors confer them greater clinical relevance. Different authors emphasize as well the fact that these cells are sensitive as well as easily isolated and cultivated. Willerhausen et al22 conducted a study to research biocompatibility of several materials used as endodontic sealers. To this end, they used three cell lines (nasal fibroblasts, gingival fibroblasts and epithelial tumor cells). Cellular growth, morphology and protein content as well as PGE2 prostaglandin release were the parameters used to determine cytotoxicty of assessed sealers. Authors, based on observations achieved in the study, recommended use of a sensitive and diploid human cell line, as well as gingival fibroblasts, since this cell type proved to be the most suitable for this type of research.
Use of Alamar Blue dye is recommended as Redox indicator. Since the dye is non toxic, it becomes a suitable method to determine color changes and fluorescent signals as response to a given metabolic activity. Therefore, it is possible to quantify proliferation of animal or human cellular lines, bacteria and fungi. Alamar Blue is a sensitive and simple method to use when compared to other indicators employed in other research projects.16,18 Results of this research project show the fact that fluorescent levels achieved with Alamar Blue increased almost lineally after six hours of culture, up to the final time of the cultures, exception made of Sealapex. Results achieved by Willershausen et al22 with Sealapex and gingival fibroblasts are similar to those obtained in this study. Results achieved in this study are apparently contrary to those obtained in similar investigations18,21 where relatively low cytotoxicity to Sealapex has been reported. We are obliged to point out the fact that direct comparison among results achieved in this research project with other research previously mentioned in scientific literature is problematic, since this research used Sealapex as positive control and it was used as comparison with materials which, due to their chemical composition, were known, or presumed to have, lower cytotoxcity than Sealapex.
Camp et al36 report that incubation time is an important factor for the quantification of ProRoot MTA's cytotoxicity. Authors suggest there is a relationship between filling material solubility and its dissolution in the culture medium. In this research project, no dissolution of materials in culture medium was observed. One of the disadvantages in the method used in this research could be the possibility that materials' cytotoxicity during setting phase could be higher than the observed one. Nevertheless, the advantage of this methodology is that materials, once set, cannot dissolve in the medium, and for this reason it was possible to quantify their cytotoxicity during longer periods, without incurring in the doubt that results might not be fully true due to dissolution of materials in the culture medium. Our results show that the degree of fluorescent obtained with CPM showed to be relatively high immediately after incorporation of Alamar Blue and up to three hours after its incubation, this tends to suggest low cytotoxicity of the material. Nevertheless, during the three initial incubation hours, cellular proliferation was not possible. Therefore, in spite of everything, it was not possible to rule out the possibility that CPM elicited some sort of cytotoxic reaction during the first hours of incubation with gingival fibroblasts. In other studies17,21,38 where setting of materials was excluded as parameter, research was conducted on cytotoxicity of sealing cements based on calcium hydroxide used for retrograde filling, after being allowed to set during 48 hours in cell cultures with human gingival fibroblasts. Results reported in those studies were similar to ours, even though in some of them there was research conducted on cements with similar chemical properties to those researched in this study, only the manufacturers were different.
Osorio et al16 conducted a study assessing sealing and retrograde filling cements in root canals. The study was conducted with mice and human gingival fibroblast cell cultures (L-929) to determine mitochondrial enzymatic activity as well as viable cells numbers. Authors reported the fact that ProRoot MTA did not cause cytotoxic reactions. These results were similar to those observed in this research project. ProRoot MTA showed relatively high cytotoxicity at the beginning of the experiment, but at its completion, due to the incubation period, it decreased noticeably. Nevertheless, according to results obtained in this research project, it was not possible to assert that the material was inert and therefore did not cause any cytotoxicity. Holland et al10 conducted research on the reaction of periapical tissue in dog teeth filled with gutta-percha and Ketac-Endo as well as ProRoot MTA. After a six month period they performed a histopathological exam. Results revealed complete absence of periapical tissue inflammatory response as well as total closure of apical foramen in all teeth filled with ProRoot MTA. Teeth filled with Ketac-Endo presented two cases of partial apical closure and mild chronic inflammatory reactions in periapical tissue in over-obturated cases. In non over-obturated cases, total lack of inflammation was observed in some cases, and mild inflammation in others. Authors concluded that even though ProRoot MTA presented more favorable results than Ketac-Endo, both materials could be considered as biocompatible with periapical tissue. Histological results of the aforementioned research, as well as those obtained from the present one (even though it was conducted with cell cultures) suggested the fact that materials based on trioxide aggregate mineral present low toxic and/or cytotoxic potential and therefore, their practically unrestricted use in endodontics can be recommended.
Results of this research project were similar to those obtained in other research endeavors16,21 and showed that all materials, CPM excepted, elicited certain cellular irritation during the first six incubation hours. After the seventh incubation hour, constant cellular proliferation increase was observed, until reaching the 96 incubation hours. This same behavior was observed in gingival fibroblasts used as control. Exception had to be made with Sealapex, which showed no marked cellular proliferation increase when compared to the other materials subject of the research.
CONCLUSIONS
Results of the present research project showed the following:
• CPM, ProRoot MTA (grey and white), MTA Angelus and GuttaFlow did not inhibit gingival fibroblast proliferation potential,
• Sealapex presented a tendency to be more cytotoxic than the other materials under investigation and,
• Based on cytotoxic effect of researched materials, their use could be recommended as sealing cements in endodontic practice.
REFERENCES
1. Seltzer S, Sinai I, August D. Periodontal effects of root perforations before and during endodontic procedures. J Dent Res 1970; 49(2): 332-339. [ Links ]
2. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod 1999; 25(3): 197-205. [ Links ]
3. Lee S, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod 1993; 19(11): 541-544. [ Links ]
4. Shah PM, Chong BS, Sidhu SK, Ford TR. Radiopacity of potential root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 81(4): 476-479. [ Links ]
5. Torabinejad M, Hong C, McDonald F, Pitt Ford T. Physical and chemical properties of a new root-end filling material. J Endod 1995; 21(7): 349-353. [ Links ]
6. Campos QI, Llamosas HE, Morales de la Luz R. Evaluación de la biocompatibilidad del cemento Portland implantado en tejido conectivo subepitelial de ratas. Rev ADM 2003; 60(2): 45-51. [ Links ]
7. García-Aranda RL, García-Garduño MV. Análisis químico por difracción y fluorescencia de rayos X de los cementos Portland, blanco, ProRoot gris y blanco, Angelus blanco y CPM. Endodoncia Actual 2009; 4(2): 6-11. [ Links ]
8. Dental DT. ProRoot® MTA (mineral trioxide aggregate) root canal repair material. Material safety data. Dentsply Tulsa Dental 2002: 1-2. [ Links ]
9. Torabinejad M, Smith P, Kettering J, Pitt Ford T. Comparative investigation of marginal adaptation of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod 1995; 21(6): 295-299. [ Links ]
10. Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabé PF, Dezan JE. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod 1999; 25(3): 161-166. [ Links ]
11. Baek S, Plenk H, Kim S. Periapical tissue responses and cementum regeneration with amalgam, SuperEBA, and MTA as root-end filling materials. J Endod 2005; 31(6): 444-449. [ Links ]
12. Thomson T, Berry J, Somerman M, Kirkwood K. Cementoblasts maintain expression of osteocalcin in the presence of mineral trioxide aggregate. J Endod 2003; 29(6): 407-412. [ Links ]
13. Torabinejad M, Pitt Ford T, McKendry D, Abedi HR, Miller D, Kariyawasam S. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod 1997; 23(4): 225-228. [ Links ]
14. Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res 1997; 37(3): 432-439. [ Links ]
15. Pistorius A, Willershausen B, Briseño Marroquin B. Effect of apical root-end filling materials on gingival fibroblasts. Int Endod J 2003; 36(9): 610-615. [ Links ]
16. Osorio R, Hefti A, Vertucci F, Shawley A. Cytotoxicity of endodontic materials. J Endod 1998; 24(2): 91-96. [ Links ]
17. Keiser K, Johnson C, Tipton D. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod 2000; 26(5): 288-291. [ Links ]
18. Camps J, About I. Cytotoxicity testing of endodontic sealers: a new method. J Endod 2003; 29(9): 583-586. [ Links ]
19. Briseño B, Willershausen B. Root canal sealer cytotoxicity on human gingival fibroblasts. 1. Zinc oxide-eugenol-based sealers. J Endod 1990; 16(8): 383-386. [ Links ]
20. Briseño BM, Willershausen B. Root canal sealer cytotoxicity on human gingival fibroblasts: 2. Silicone- and resin-based sealers. J Endod 1991; 17(11): 537-540. [ Links ]
21. Briseño BM, Willershausen B. Root canal sealer cytotoxicity with human gingival Fibroblasts. III. Calcium hydroxide-based sealers. J Endod 1992; 18(3): 110-113. [ Links ]
22. Willershausen B, Marroquín BB, Schäfer D, Schulze R. Cytotoxicity of root canal filling materials to three different human cell lines. J Endod 2000; 26(12): 703-707. [ Links ]
23. Torabinejad M, Hong C, Lee S, Monsef M, Pitt Ford T. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod 1995; 21(12): 603-608. [ Links ]
24. Pitt Ford TR, Torabinejad M, McKendry DJ, Hong CU, Kariyawasam SP. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79(6): 756-763. [ Links ]
25. Torabinejad M, Hong C, Pitt Ford T, Kaiyawasam S. Tissue reaction to implanted super-EBA and mineral trioxide aggregate in the mandible of guinea pigs: a preliminary report. J Endod 1995; 21(11): 569-571. [ Links ]
26. Torabinejad M, Ford T, Abedi HR, Kariyawasam S, Tang H. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. J Endod 1998; 24(7): 468-471. [ Links ]
27. Fischer E, Arens D, Miller C. Bacterial leakage of mineral trioxide aggregate as compared with zinc-free amalgam, intermediate restorative material, and Super-EBA as a root-end filling material. J Endod 1998; 24(3): 176-179. [ Links ]
28. Torabinejad M, Rastegar A, Kettering J, Pitt Ford T. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod 1995; 21(3): 109-112. [ Links ]
29. Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc 1996; 127(10): 1491-1494. [ Links ]
30. Martin GM. Human skin fibroblasts . 1973: 39-43. [ Links ]
31. Ian Freshney R. Tierische Zellkulturen: Ein Methoden-Handbuch . 1990: 404. [ Links ]
32. Dutrillaux B, Couturier J. Praktikum der Chromosomenanalyse . 1983: 77. [ Links ]
33. Hession RW. Long-term evaluation of endodontic treatment: anatomy, instrumentation, obturation--the endodontic practice triad. Int Endod J 1981; 14(3): 179-184. [ Links ]
34. Milleding P, Wennberg A, Hasselgren G. Cytotoxicity of corroded and non-corroded dental silver amalgams. Scand J Dent Res 1985; 93(1): 76-83. [ Links ]
35. Wennberg A, Hasselgren G. Cytotoxicity evaluation of temporary filling materials. Int Endod J 1981; 14(2): 121-124. [ Links ]
36. Camp M, Jeansonne B, Lallier T. Adhesion of human fibroblasts to root-end-filling materials. J Endod 2003; 29(9): 602-607. [ Links ]
Note This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam Mailing address:
Mailing address:
Benjamín Briseño Marroquín
Poliklinik fuer Zahnerhaltung
Johannes Gutemberg Universitaet-Mainz
Augustusplatz 2
55131 Mainz Germany
Telephone +496131 173079
E-mail address: briseno@uni-mainz.de











 text in
text in 


