Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ingeniería mecánica, tecnología y desarrollo
Print version ISSN 1665-7381
Ingenier. mecáni. tecnolog. desarroll vol.5 n.3 México Sep. 2015
Artículos
Vanadium carbide coatings produced on gray cast iron using the thermo-reactive deposition/diffusion technique
Ariel Augusto Amaya Avila1, Oscar Edwin Piamba Tulcan2, Jhon Jairo Olaya Florez3
Department of Mechanical and Mechatronic Engineering, Faculty of Engineering Universidad Nacional de Colombia. 1aaamayaa@unal.edu.co, 2oepiambat@unal.edu.co, 3jjolayaf@unal.edu.co
Fecha de recepción: 08-02-2015.
Fecha de aceptación: 23-05-2015.
Abstract
Vanadium carbide (VC) coatings were produced on gray cast iron (GCI) with a pearlitic matrix, randomized lamellar graphite, and 3.5% of total carbon. The process was carried out in a molten borax bath at temperatures of 1173 K, 1223 K, and 1273 K for 2, 4, and 6 hours. Ferro vanadium was used as the carbide-forming element and aluminum as the reducing agent. Layer growth parameters were determined on the basis of classical kinetic theory.
The coatings were characterized via scanning electron microscopy (SEM), Auger electron spectroscopy (AES), and X-ray diffraction (XRD). In general, it was possible to produce a compact, continuous, homogeneous coating, and a smooth interface with the substrate was formed. Via XRD, the FCC phase of VC was observed, with a mixed orientation along the (111) and (200) crystal planes. Using AES, the presence of mainly vanadium at 437 eV and carbon at 272 eV was verified. Finally, by means of the electrochemical technique of potentiodynamic polarization in a 3% NaCl solution, it was found that the corrosion resistance of the VC-GCI system exhibited better performance than the substrate.
Keywords: Vanadium Carbide, corrosion, cast iron, coating.
Resumen
Recubrimientos duros de Carburo de Vanadio (VC) fueron producidos sobre fundición de hierro gris (GCI) con matriz perlítica, grafitos laminares distribuidos aleatoriamente y 3,5% de carbono total. El proceso se realizó en un baño de sales de bórax, fundido a temperaturas de 1173 K, 1223 K y 1273 K, para 2, 4 y 6 horas. Se usó ferro Vanadio como elemento formador de los carburos y aluminio como agente reductor. A partir de la Teoría Cinética Clásica se determinaron los parámetros de crecimiento de capa.
Los recubrimientos obtenidos se caracterizaron por microscopia electrónica de barrido (SEM), espectroscopia de electrones Auger (AES) y difracción de rayos X (DRX). En general se logró producir un recubrimiento de morfología compacta, continua, homogénea y de una interface lisa. A partir de DRX se observó la fase FCC del VC con una orientación mixta en los planos (111) y (200). A partir de AES se verificó la presencia de Vanadio a 437 eV y carbono a 272 eV. Finalmente se encontró mediante técnicas electroquímicas de polarización potenciodinámica en solución de NaCl al 3%, la resistencia a la corrosión del sistema VC-fundición gris presento mejor comportamiento con respecto al sustrato.
Palabras clave: Carburo de Vanadio, corrosión, fundición, recubrimiento.
Introduction
Cast iron is a material widely used in the industry because of its economic benefits, ease of casting, dimensional stability, and mechanical strength, among other benefits [1], [2]. Additionally, its applicability in the automotive sector as raw material for auto parts shows its great importance in the current international environment [3]. However, its low chemical resistance makes it vulnerable to corrosion processes, thus affecting its lifespan and increasing maintenance costs. In industrial environments, the use of surface treatments allows its use in mechanical parts that must meet such requirements for operation as high surface hardness, low friction, and resistance to highly corrosive environments in general. Currently, there exist processes of efficient surface engineering to improve the surfaces properties, such as physical vapor deposition (PVD), chemical vapor deposition (CVD), thermal spray, and thermo-reactive diffusion (TRD). Among these, TRD is an economical alternative that compares well with other processes and results in the application of hard coatings with good wear and corrosion resistance.
The TRD process in a borax bath has been efficiently used for obtaining hard layers of carbide with high wear resistance on substrates having a carbon content above 0.3 wt%. These hard carbide layers exhibit excellent adhesion to the substrate, a low friction coefficient, and good corrosion resistance, with thicknesses ranging between 5 and 15 microns, depending on the carbon content of the substrate, the temperature, and the duration of treatment [4]. The range of deposition temperatures is between 1123 and 1323 K, and the immersion time ranges from 0.5 to 10 h. In TRD, the part to be coated is immersed in a bath of borax deca-hydrate, which loses its binding water at the treatment temperature (Na2B4O7 • 10H2O). In addition, the carbide forming element (CFE) for the case under study of vanadium ferroalloy is previously dissolved in the bath. These compounds react chemically with the carbon atoms diffusing from the substrate to the surface, forming a new layer on the interface based on the carbide-forming element selected. [4], [5].
The formation of the carbide layer by chemical reaction occurs when the CFE has a low free energy of carbide formation. Also, the free energy of formation of the transition metal oxide must be greater than the free energy of B2O3, favoring formation carbide and avoiding the formation of oxides in the layer. Vanadium belongs to this group, since the free energy of formation of VC is -25 kcal/mol for C and V2O3 is -146 kcal/mol for O, thus being higher than B2O3, which is -154 kcal/mol for O, fulfilling the basic requirement for the formation of carbide [6].
The literature on this topic reports the existence of a wide variety of carbides: carbides of niobium [7], vanadium [8], chromium [9], titanium [10] [11] and others have been produced. They generally are deposited on AISI H13, M2, and D2 steel, and there have been characterization studies of wear resistance [12], [13], [14], the growth kinetics of boride layers on AISI 5140 and AISI 4340 steel [15], and vanadium carbides deposited on AISI 1045 and H13 [16], [17]. Additionally, studies have been done on the microstructural characterization of vanadium carbides deposited on H13 steel, determining the grain geometry based on the carbon activity in each treated steel [18], and furthermore electrochemical characterization studies of borides of iron deposited on three different steels and vanadium carbides deposited on AISI H12 steel have been reported [19], [20].
As already mentioned, there are studies of the binary carbides of vanadium, chromium, and niobium focused mainly on wear resistance and mechanical properties, and there are some studies of microstructural and growth kinetics. However, studies of vanadium carbides deposited on GCI using the TRD technique have not been reported, and there has been no evaluation of the behavior of the growth kinetics and the corrosion resistance [7] [21]. For this reason, it is important to gain more knowledge about the dynamics of formation of these carbides, and it is a fundamental aim of the present paper to produce hard vanadium carbide coatings on gray cast iron via the TRD technique and to carry out a study of the growth kinetics as a function of the temperature and the duration of treatment, as well as of their corrosion resistance.
Experimental Procedure
Materials and methods
The material used as a substrate was gray cast iron, obtained from diesel auto parts (Piston Liner). Square samples were used (2 cm), polished to mirror finish. Random measurements of the average roughness of the samples were performed using a Bruker brand 3d optical profilometer, finding approximate values 0.5 ± 0.04 microns. The cleaning of the samples was carried out through ultrasound with acetone, isopropanol, and deionized water separately for 18 minutes. The experimental density exhibited an average value of 7.05 g/cm3, similar to that reported in the ASTM standard Section X1-G1 of 7.2 g/cm3 [22]. The chemical composition of the material used was determined using an optical emission spectrometer (Baird-Spectrovac 1000), and consisted of 93% iron, 3.4% carbon, and 1.6% silicon. It was defined according to ASTM A48M-03 as a cast Class 30 because of the predominance of perlite, with lamellar graphite type A and size No. 5 [2].
The TRD coating process was carried out in a borax bath perfused at 1123 K at 81%wt, and subsequently the temperature was raised to that of the process and ferro-vanadium (16%) and aluminum (3%) were added as reducing agents. The process was performed in containers of stainless steel, due to its low carbon content, thus avoiding interaction with the process. The coating was prepared separately at 1173 K, 1223 K, and 1273 K for 2, 4, and 6 hours for each temperature. The samples were withdrawn upon completion of the treatment time, and were rapidly cooled in order to avoid passing through the temperature that causes substrate embrittlement. They were then immersed in boiling water for 30 minutes in order to remove excess borax produced by the bath [4], [23].
Characterization
The preparation of the samples for metallographic observation was done by cutting them transversely, encapsulating them with edge protective resin, polishing them with alumina until a scratch-free surface was obtained, and finally subjecting them to ultrasonic cleaning. In order to compare the coating samples, they were etched by a chemical agent (Nital - ASTM E407-07) [24]. Multiple measurements of coating thickness were taken in different directions for each time and temperature, thus determining the average layer thickness.
The XRD profiles of the coatings were obtained using X-pertPro Panalytical high-power equipment at 45kV and 40 mA. The scan was conducted within the angular range of 10° to 90° with a step of 0.02° and a monochromatic CuKa radiation of 15.94 nm. In addition, in order to verify presence of the coating, AES surface tests were performed on an Oxford Instruments Omicron Nanotecnology CMA 169 with an evaluation range between 40 and 800 eV at intervals of 0.5 eV.
Finally, the electrochemical behavior was evaluated via potentiodynamic polarization tests on a Gamry R600 potentiostat, using a cell with 100ml of NaCl solution at 3%. The work area was 19.6 mm2, and platinum was used as an auxiliary electrode and calomel as a reference electrode. The evaluated potential was ± 0.5 V with respect to the potential of the open circuit at a rate of 0.5 mV/s.
Aalysis of the results
Microstructure of the coatings
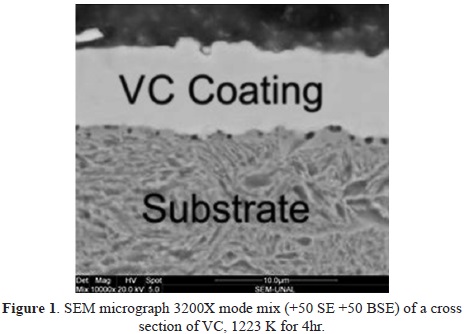
The microstructure of the cross section of vanadium carbide on the GCI substrate is shown in Figure 1. The carbide layer and casting are clearly defined and separated by a planar interface without the presence of a transition zone between the coating and the substrate. The coating exhibits a compact, continuous, and homogeneous structure with constant thickness and is free of macrodefects. The results obtained for the morphology of the interface are consistent with previous studies [17], [25] and grown with a columnar morphology [18]. On the substrate surface, a layer of supersaturated solid solution is formed, from which nucleation of the carbides begins, growing outwards from the substrate through the reaction between the CFE, the salt bath, and the carbon atoms diffused from the substrate [26],[28],[29]. The microstructure of the coatings produced with a TRD system depends on the surface mobility of atoms in the substrate surface, which is controlled by the deposition parameters and the chemical properties of the substrate. Depending on the value of the carbon activity on the surface, different morphologies can modify the mechanical performance and corrosion resistance of the coating. The coating can exhibit a microstructure of elongated grains when it grows on a substrate with low values of carbon activity, which allows a decrease in the diffusion of the carbon atoms to the surface. It can also exhibit a microstructure of equiaxed grains when the substrate has sufficient carbon activity to increase the nucleation density, which produces a decrease in the grain size and the formation of a compact microstructure [18].
XRD analysis of the layer of carbide produced on the GCI at 1223 K for 4 h is shown in Figure 2. The cubic phase of VC was confirmed according to the JCPDS 03 065 8822 card, showing high intensity peaks with a mixed orientation of mainly crystal planes (111), (200), (220), and (311). No significant effect of the composition of the salt bath or the substrate was observed in the orientations present. Vanadium carbide has a cubic crystal system similar to NaCl (Fm3m) and falls under space group number 225.
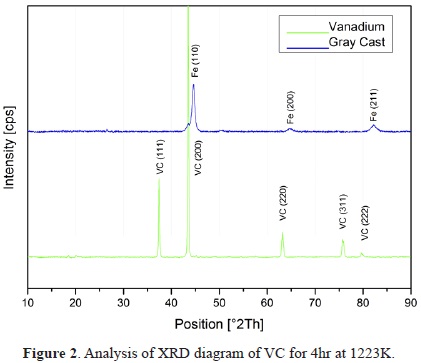
The X-ray diffraction spectra obtained are consistent with those found by Castillejo [23] and Orjuela [30], who grew their coatings on AISI H13 and AISI D2 tool steels with lower carbon content. The diffraction planes are consistent with (111), (200), (220), and (311), exhibiting the highest intensity for planes (200) and (111) [23], [30]. Its structure has the vanadium atoms occupying positions of FCC structure, and carbon occupies interstitial positions between vanadium atoms (space group 225). Unlike what was reported by Orjuela [30], no presence of the substrate used, primarily due to a thicker compact formation, is observed.
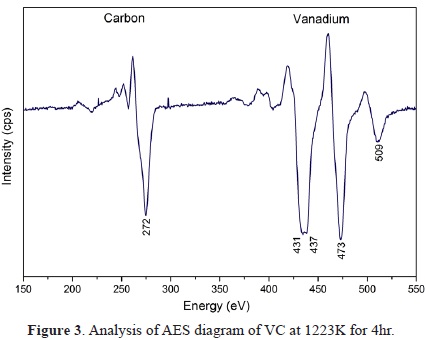
The results obtained from AES testing were analyzed using the database of the National Institute of Standards and Technology (NIST). The presence of mainly vanadium (473 eV) and carbon (272 eV) was verified. The analysis of the atomic concentration showed 13.37% carbon with KLL transition and 86.63% vanadium with LMM transition (Figure 3)[27].
Growth kinetics of vanadium carbide on GCI.
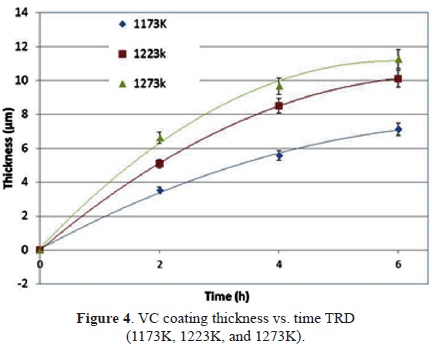
The TRD treatment was analyzed as a function of the duration of treatment and the temperature. Vanadium carbide coating thicknesses ranging from 3.52 ± 0.41 to 11.25 ± 0.37 microns at 1173 K to 1273 K for 2 h and 6 h, respectively, were obtained. Assuming that the rate of diffusion of carbon in the coating layer controls the speed of the growth and that the layer grows perpendicularly to the surface of the GCI, we can use the Arrhenius equation to determine the thickness of the layer as a function of the temperature and the duration of the treatment based on classical kinetic theory with Equation (1):

where x is the coating thickness (m), K is the diffusion coefficient of carbon in vanadium carbide coatings (m2/s), and t is the duration of the treatment. As shown in Figure 4, the coating thickness increases with duration of the treatment and the temperature.
According to the equation, the square of coating thickness (m2) is calculated depending on the duration of the treatment. These values are plotted and the K values are obtained for each temperature from the slopes in Figure 5.
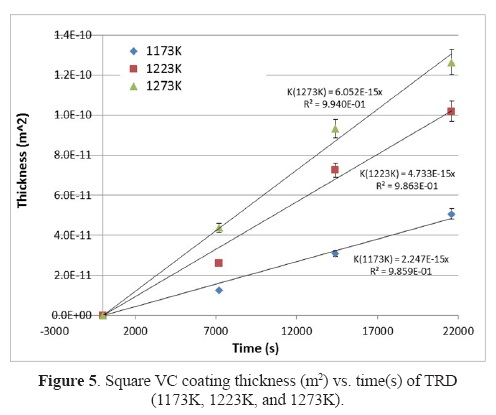
On the basis of classical theory, we see the relation between the constant growth rate, the activation energy, and the process temperature, which is expressed as an Arrhenius equation [28], [29] :

where K0 is the frequency factor (pre-exponential constant), Q the activation energy (kJ/mol), R the gas constant (8.314 J/mol.K), and T the absolute temperature. Expressing the previous relationship with logarithms:

By plotting ln K versus 1/T, we can determine the experimental value of the activation energy Q and the constant K0 as Q = 123.48 kJ/mol and K0= 7.866E-06. Replacing these values in equation 2 gives:

Replacing the information in Equation (1) and calculating the square root, an expression for calculating the coating thickness (microns) for an absolute temperature in a given time (h) can be obtained:
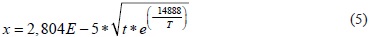
This is an experimental equation that predicts the layer growth based on treatment time and temperature, in which kinetic results are very close to the experimental results. Thus these expressions can be used with confidence in predicting the thickness of thermochemical coating deposition processes on an industrial level.
The growth kinetics of vanadium carbide was modeled by Fan et al. [18], finding that the diffusion of carbon atoms is influenced by the potential energy of the free carbon atoms when transformed from the base structure in an activated state to the treatment temperature. This potential energy can be negative for alloys with high carbon content, which means that there is no energy barrier for the formation of activated carbon atoms, understood as an increase in the activation energy of diffusion of carbon in the vanadium carbide coating, thus slowing carbon diffusion, which explains the behavior of the coating on gray cast iron with 3.5% carbon [16], [17].
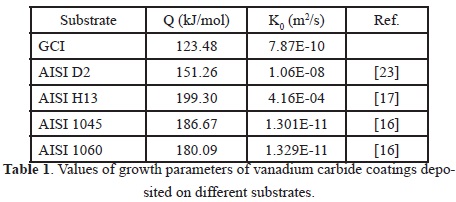
Some growth kinetic parameters of vanadium carbide reported in the literature are shown in Table 1. It can be seen that in the case of gray cast iron it has a low activation energy (Q) factor compared with AISI H13 steel (tool steel for hot work with 0.39% C, 1.1% Si, and 5.2% Cr ), AISI D2 steel (tool steel for cold work with 1.55% C, 0.3% Si, and 12% Cr), and AISI 1045 steel (carbon steel with 0.49% C, 0.33% Si, and 0.69% Mn), attributable to the chemical composition substrate [16], [17], [23]. The difference can probably be attributed to the content of carbon atoms for the formation reaction of a vanadium carbide coating. Although the carbon content in gray cast iron is higher than that in steel, the carbon activity in steel is higher than that in cast iron, whereas higher carbon activity in the substrate tends to be a larger driving force for carbon diffusion from the substrate at the initial stage of coating growth, which allows improving the nucleation density [18].
The corrosion behavior was evaluated for the substrate and the coating for comparison. The corrosion potential, the corrosion current, and the polarization resistance were evaluated. The results showed that the corrosion potential of the substrate was on average 748 mV and for the coating 438 mV, with respect to the reference potential. The corrosion current showed a reduction from 1391 nA to 462.2 nA, implying a lower flow of ions to the coating. Finally, polarization resistance was consequently lower for the substrate, at 1.72 kohm, than for the coating, at 56.12 kohm (Figure 6). Within the range of potential evaluated, no pitting or passivation processes were observed for the tests performed. According to Castillejo [23] and Orjuela et al. [30], as was evident from techniques such as XPS formation of vanadium oxides on the surface, these oxides tend to increase the corrosion resistance due to their high chemical stability. Reported failure mechanisms for the coatings are mainly related to the porosity. These defects allow the passage of electrolyte from the surface to the interface, thus exposing the substrate to electrochemical etching [23].
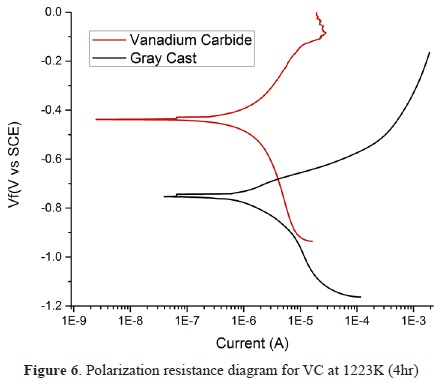
Corrosion parameters obtained in this investigation for the case of Ecorr are -438 mV less negative than those reported by Orjuela [30] for vanadium carbide deposited on AISI H13 steel (-655 mV) and AISI D2 steel (-605 mV); however, Icorr values of 462 nA for CGI are higher than the report for AISI H13 steel (82.7 nA) and AISI D2 steel (37 8 nA). This can be explained due to differences in the coatings thickness, the porosity percentage, and the electrochemical properties of each substrate.
Conclusions
Hard coatings of vanadium carbide can be produced via the TRD technique on gray cast iron, obtaining thicknesses of more than 10 microns.
The TRD process produces coatings with a defined, continuous, dense, and homogenous interface at temperatures above 950° C after 4 h of treatment.
The value of the activation energy of vanadium carbide on GCI (123.48 kJ/mol) is lower than those reported for steel substrates with considerably lower carbon contents, which implies that there is no proportional relationship between the carbon content and the rate of formation of the coating.
Hard coatings of vanadium carbide deposited on GCI exhibit better electrochemical behavior than the substrate, shown in the higher values of corrosion potential and lower values of interchange current for the coating, due to its ceramic composition and chemical stability.
Acknowledgments
The authors gratefully acknowledge the financial support granted by the Departamento Administrativo de Ciencia, Tecnología e Innovación — COLCIENCIAS through project No. 338-2011, and the Fundación para la Promoción de la Investigación y la Tecnología (FPIT) through project number 2608. The authors acknowledge the support of the Universidad Nacional de Colombia (DIB-UNAL) through project No. 203010021364.
Bibliography
[1] J. Apráiz Barreiro, Fundiciones. Madrid: Limusa/Noriega; Dossat, 1998. [ Links ]
[2] A04 Committee, "Specification for Gray Iron Castings," 2012. [ Links ]
[3] A. S. M. A. Haseeb, M. A. Fazal, M. I. Jahirul, and H. H. Masjuki, "Compatibility of automotive materials in biodiesel: A review," Fuel, vol. 90, no. 3, pp. 922-931, Mar. 2011. [ Links ]
[4] T. Arai, "Thermoreactive deposition/diffusion process," ASM Handbook, vol. 4. pp. 448-453, 1991. [ Links ]
[5] F. A. P. Fernandes and S. C. Heck, "DIFFUSION TREATMENTS," no. September, pp. 49-52, 2009. [ Links ]
[6] T. Arai, G. Baker, and C. Bates, ASM Handbook, Vol. 4: Heat Treating. ASM International, 1991. [ Links ]
[7] C. K. N. Oliveira, R. M. M. Riofano, and L. C. Casteletti, "Micro-abrasive wear test of niobium carbide layers produced on AISI H13 and M2 steels" Surf. Coatings Technol., vol. 200, no. 16-17, pp. 5140-5144, Apr. 2006. [ Links ]
[8] T. Arai, H. Fujita, Y. Sugimoto, and Y. Ohta, "Vanadium Carbonitride Coating by Immersing into Low Temperature Salt Bath," Heat Treat. Surf. Eng., pp. 49-53, 1988. [ Links ]
[9] Y. L. Su and S. H. Yao, "On the performance and application of CrN coating," Wear, vol. 205, no. 1-2, pp. 112-119, Apr. 1997. [ Links ]
[10] J. L. He, Y. H. Lin, and K. C. Chen, "Wear performance of CAP-titanium nitride-coated high-speed steel in different dry sliding conditions," Wear, vol. 208, no. 1-2, pp. 36-41, 1997. [ Links ]
[11] S. C. Lim, C. Y. H. Lim, and K. S. Lee, "The effects of machining conditions on the flank wear of tin-coated high speed steel tool inserts," Wear, vol. 181-183, pp. 901-912, Mar. 1995. [ Links ]
[12] C. K. N. Oliveira, C. L. Benassi, and L. C. Casteletti, "Evaluation of hard coatings obtained on AISI D2 steel by thermo-reactive deposition treatment," Surf. Coatings Technol., vol. 201, no. 3-4, pp. 1880-1885, Oct. 2006. [ Links ]
[13] C.-Y. Wei and F.-S. Chen, "Characterization on multi-layer fabricated by TRD and plasma nitriding," Mater. Chem. Phys., vol. 90, no. 1, pp. 178-184, Mar. 2005. [ Links ]
[14] S. Sen and U. Sen, "Sliding wear behavior of niobium carbide coated AISI 1040 steel," Wear, vol. 264, no. 3-4, pp. 219-225, Feb. 2008. [ Links ]
[15] S. Sen, U. Sen, and C. Bindal, "An approach to kinetic study of borided steels," Surf. Coatings Technol., vol. 191, no. 2-3, pp. 274-285, Feb. 2005. [ Links ]
[16] D. Rast, K. Plasti, N. A. Povr, I. N. I. Jekla, B. Matijevi, and M. Stupni, "THE DIFFUSION GROWTH OF CARBIDE LAYERS ON STEEL SURFACES," vol. 34, no. 6, pp. 425-428, 2000.
[17] X. S. Fan, Z. G. Yang, C. Zhang, Y. D. Zhang, and H. Q. Che, "Evaluation of vanadium carbide coatings on AISI H13 obtained by thermo-reactive deposition/diffusion technique," Surf. Coatings Technol., vol. 205, no. 2, pp. 641-646, Oct. 2010. [ Links ]
[18] X. S. Fan, Z. G. Yang, Z. X. Xia, C. Zhang, and H. Q. Che, "The microstructure evolution of VC coatings on AISI H13 and 9Cr18 steel by thermo-reactive deposition process" J. Alloys Compd., vol. 505, no. 1, pp. L15-L18, Aug. 2010. [ Links ]
[19] H. Tavakoli and S. M. Mousavi Khoie, "An electrochemical study of the corrosion resistance of boride coating obtained by thermo-reactive diffusion," Mater. Chem. Phys., vol. 124, no. 2-3, pp. 1134-1138, Dec. 2010. [ Links ]
[20] T. Arai and N. Komatsu, "Carbide Coating Process by Use of Salt Bath and its Application to Metal Forming Dies," Proc. 18th Int. Mach. Tool Des. Res. Conf., pp. 225-231, 1977. [ Links ]
[21] U. Sen, "Friction and wear properties of thermo-reactive diffusion coatings against titanium nitride coated steels," Mater. Des., vol. 26, no. 2, pp. 167-174, Apr. 2005. [ Links ]
[22] C. A. Environments, "Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test," vol. 03, no. Reapproved 2011, pp. 1-9, 2012. [ Links ]
[23] F. E. Nieto Castillejo, "Recubrimientos de Carburos Ternarios Depositados con la Técnica TRD.," Tesis de doctorado, Universidad Nacional de Colombia, 2013. [ Links ]
[24] E04 Committee, "Practice for Microetching Metals and Alloys," 2007. [ Links ]
[25] C. K. N. Oliveira, R. M. Muñoz Riofano, and L. C. Casteletti, "Formation of carbide layers on AISI H13 and D2 steels by treatment in molten borax containing dissolved both Fe-Nb and Fe-Ti powders" Mater. Lett., vol. 59, no. 14-15, pp. 1719-1722, Jun. 2005. [ Links ]
[26] T. Arai, H. Fujita, Y. Sugimoto, and Y. Ohta, "Diffusion carbide coatings formed in molten borax systems," J. Mater. Eng., vol. 9, no. 2, pp. 183-189, Jun. 1987. [ Links ]
[27] K. D. Childs and C. L. Hedberg, Handbook of Auger electron spectroscopy: a book of reference data for identification and interpretation in Auger electron spectroscopy. Eden Prairie, Minn.: Physical Electronics Inc., 1995. [ Links ]
[28] M. Aghaie-Khafri and F. Fazlalipour, "Kinetics of V (N,C) coating produced by a duplex surface treatment," Surf. Coatings Technol., vol. 202, no. 17, pp. 4107-4113, May 2008. [ Links ]
[29] Z. J. Shan, Z. G. Pang, F. Q. Luo, and F. D. Wei, "Kinetics of V(N,C) and Nb(N,C) coatings produced by V-Nb-RE deposition technique," Surf. Coatings Technol., vol. 206, no. 19-20, pp. 4322-4327, May 2012. [ Links ]
[30] F. A. Orjuela G, "Resistenca a la corrosión en recubrimientos de carburo de vanadio y niobio depositados con la técncia de TRD" Tesis de maestría, Universidad Nacional de Colombia, 2014. [ Links ]














