1. Introduction
In Sivakasi, Tamil Nadu, and India, firecrackers have been associated with empire, tradition, and festival celebrations. Typically, people burn firecrackers at weddings, temples, social gatherings, Diwali, Dusshera, New Year, etc. During the festivities mentioned above, people show their happiness by bursting. In general, firecrackers are prepared using pyrotechnic chemical composition, and they are capable of producing heat, light, smoke, noise, and gas during their bursting (Conkling & Mocella, 1985; Palaneeswaria, 2012). The standard firecracker is made using chemical compositions like 60% potassium nitrate, 20% sulfur, and 20% aluminium. The cracker made by using this chemical composition creates environmental and human health issues when bursting. The air pollution caused by anthropogenic activities worldwide is high; burning of firecrackers during festivals like Diwali, Yanshui festival, Lantern festival, and Bonfire night in India releases a high concentration of pollutants beyond the permissible limits (Chang et al., 2011; Kulshrestha et al., 2004; Pope et al., 2016; Yang et al., 2014). A chemical mixture such as potassium nitrate (KNO3) acts as an oxidizer, aluminium (Al) acts as fuel and the role of sulfur (S) is igniting the chemical mixture. The primary source of environmental pollution from this cracker chemical mixture is sulfur which oxidizes with KNO3 and releases SO2 and other suspended particles. The primary constituent of particulate matter is sulfur which is known to play a crucial role in the formation of smog, acid rain and causes environmental pollution (Sawlani et al., 2019) and can directly impact human health (Pope III et al., 2006). The concentration of SO2 is increased 10 times and 23 times during the Diwali festival (Nasir & Brahmaia, 2015; Ravindra et al., 2003). The emission of harmful gases like SO2 and the release of suspended and metal particles cause adverse effects on human respiratory health (Gouder & Montefort, 2014). It is observed from the tropical urban region of Hyderabad during Diwali (2001-2011) that the emission rates of nitrogen oxides (NOx), sulfur dioxide (SO2), ozone (O3), and black carbon aerosol are higher and have a great impact on the quality of air (Yerramsetti et al., 2013). It is noted from the literature the environment has been contaminated more, particularly during the Diwali season compared to a typical season. Due to the presence of sulfur powder in a normal firecracker, the release of SO2 gas emission during the bursting of firecrackers in Diwali season exceeds the permissible limits framed by Central Pollution Control Board (CPCB), India, and World Health Standards. In the last ten years, the emission of SO2 in some of the major cities during the Diwali festival was two to six times greater in Delhi city (Singh et al., 2010), five times greater in Kolkatta city (Chatterjee et al., 2013), and also 6.59 times greater in Lucknow city (Barman et al., 2008). From the incidents above mentioned, it is observed that even though the fireworks industry gave job opportunities to many employees, the environment has been further polluted, and the health effects on human beings are considerable as a consequence of sulfur powder.
Although safety guidelines are given in the Environment Protection Act 1986, Environment Protection Rule 1986, and Fireworks Research Development Centre (FRDC) (Punna & Ramesh, 2019) some of the industries violated the norms, created accidents, and polluted the environment. Three methods could achieve improvements in the pyrotechnic formulation. The first method is by changing their percentage of chemical composition (Selvakumar et al., 2013), the second one is the addition of new chemicals/additives (Witkowski et al., 2012), and the last method is by lowering the particle size (Kwok et al., 2002) of ingredients. Some of the authors have taken the initiation to reduce the pollution rate by preparing the chemical powders with nano levels. The nano chemical powder is added along with the chemical mixture and research work carried out. The result observed that nanopowder influences reduce the pollution rate (Azhagurajan et al., 2011; Azhagurajan & Selvakumar, 2014). The SO2 emission is diminished when the chemical mixture is prepared using a nanoscale (Azhagurajan et al., 2014). The work is done by changing the existing chemical combination to reduce environmental pollution (Junghare et al., 2022). After going through the literature, it is found that there is scope for using biofuel materials to replace the existing chemical composition. The focus of the study is to understand the effect of replacing sulfur with TSP, and the performance of the modified chemical composition is studied and presented.
2. Materials and methods
The required quantity of potassium nitrate, aluminium, and sulfur for making a firecracker is purchased from Sri Kaliswari Metal Powder Company, Sivakasi, Tamilnadu. The tamarind seed is collected from the local supplier and processed further for making firecrackers. As per the statistics, in India, more than 46 lakhs tons of tamarind seeds are produced (Rao et al., 2015), and the cost of the tamarind seed is also meagre compared to sulfur (El-Siddig et al., 2006; Kader et al., 2015; Sakthivadivel & Iniyan, 2020). The tamarind seed is pulverized into TSP, and it is used as a chemical for making a firecracker. The chemical composition of the TSP is as follows, Carbon (47.76 %) and Oxygen (42.39 %) (Parveen et al., 2011). It also contains polysaccharides, tannins, proteins and has a gross calorific value of 21 MJ/Kg. The tamarind seed is crushed into fine particles, and moisture content is removed by heating the pulverized powder to 110⁰C for 2 hours in an electric induction furnace before making firecrackers. The TSP and firecracker chemical ingredients' particle size was measured using a particle size analyser made by Shimadzu Model no. SALD 2300. Regular firecracker (NF) uses 60% of potassium nitrate (60%), 20 % of aluminium (20%), and 20 % of sulfur (20%) for making the firecrackers. In contrast, in modified chemical composition, 5% of TSP is used, and sulfur content is reduced to 15%, and it is mentioned as MCC-1 in the subsequent section. Similarly, the modified chemical composition sample 2 (MCC-2) is made by using sulfur (10%), potassium nitrate (60%), aluminium (20%), and TSP (10%). Three different samples are made to understand the effect of TSP on emission rate and emitted gases. The samples are made as per the industry standard within fireworks by following the safety protocols. The required quantity of chemical is properly mixed using a non-conducting surface, and sieving is done as per ASTM standard using Mesh. No. 325. The steps suggested in (Rajendran et al., 2021) are followed for making the firecrackers. The size of the paper tube is 4cm in length and 0.6 cm in diameter 0.6cm. The dimension of the rolled tube is made as per the guideline recommended by Petroleum and Explosive Safety Organization (PESO) (Sharma, 2017). The chemical composition of various samples used to make firecrackers is depicted in Table 1.
Table 1 Details of the chemical mixture used for making the cracker.
| Name of the chemical powders |
Sieve size (nm) |
Particle size (nm) |
Purity (%) |
|---|---|---|---|
| ASTM | |||
| Potasium nitrate (KNO3) | 325 | 43 | 97.6 |
| Aluminium (Al) | 325 | 45 | 85.0 |
| Sulfur (S) | 325 | 43 | 99.9 |
| Tamarind seed powder (TSP) | 325 | 46 | 91 |
For easy implicit, the modified chemical composition sample 1 is labelled as MCC-1, and the modified chemical composition sample 2 is marked as MCC-2. The details of chemical composition are represented in Table 2.
Table 2 Details of the chemical composition.
| Samples | KNO3 (%) | S (%) | Al (%) | TSP (%) |
|---|---|---|---|---|
| NF | 60 | 20 | 20 | - |
| MCC-1 | 60 | 15 | 20 | 5 |
| MCC-2 | 60 | 10 | 20 | 10 |
An auto exhaust/multi-gas analyser (Model: NPM-MGA-1) is used to study the emission of gases after the firecrackers' bursting and burning. This analyser can measure various gases such as CO, CO2, HC, O2, and Propane Equivalent Factor (PEF) (Rajendran et al., 2021).
The performance tests like noise level, impact, and friction sensitivity tests are carried out as per the standard procedure. The noise level of the firecracker is observed by using Noise Level Meter made by Larson and Davis, USA, Model no.824L. The distance of 4m from the point of bursting should be maintained, and it is denoted in the dB(AI) and dB(C) scale (Sharma et al., 1999). The impact sensitivity of the firecracker chemical composition is measured by the BAM fall hammer setup supplied by Electro ceramic Pvt Ltd, India. 10 mg of sample were taken for testing, and 2 kg of the cylindrical block (deadweight) struck the piece from the different height levels, and limiting impact energy was noted. The measurement was done according to the German Federal Institute for Testing Materials. The friction sensitivity of the chemical mixture was measured with a friction sensitivity tester. 10 mg of the sample was taken for testing the friction sensitivity. The different load weights were hung in the various notches using the loading arm available in the test setup. The procedure for performing all these tests is detailed (Rajendran et al., 2021). Figure 1 depicts the various processes involved to carry out the emission analysis.
Figure 2a and b shows the closed container setup used to find firecrackers' chemical composition emission analysis.
3. Results and discussion
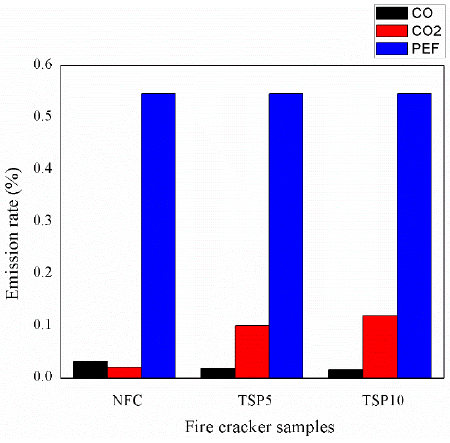
The emission rate and presence of various gases after the bursting of the sample are discussed and presented in the subsequent section. It is observed from the emission analysis, during the bursting of the regular firecracker, the following emissions are observed, CO is 0.032%, CO2 is 0.020%, HC 15%, O2 is 28.13%, and PEF is 0.546%. In modified firecracker (MCC-1) the observed emission rate of CO is 0.018%, CO2 is 0.10%), HC is 6%, O2 is 22.12% and PEF is 0.546%. From the results, the emission rate of the modified firecracker sample 1 (MCC-1) and the regular firecracker is compared, and it was observed that the generation rate of CO is 44% lesser. In case of MCC-2, the emission rate of CO is 0.016%, CO2 is 0.12%, HC is 4%, O2 is 21.12% and PEF is 0.546%. Table 3 and Figure 3a and 3b depicts the comparative result of all the samples.
Table 3 Exhaust gas analyser result analysis.
| Samples | CO | CO2 | HC | O2 | PEF |
|---|---|---|---|---|---|
| NFC | 0.032 | 0.020 | 15 | 28.13 | 0.546 |
| MCC-1 | 0.018 | 0.10 | 6 | 22.12 | 0.546 |
| MCC-2 | 0.016 | 0.12 | 4 | 21.12 | 0.546 |
The addition of TSP significantly reduces the emission of CO, and it is due to the presence of more carbon content in the TSP. But the addition of TSP along with regular chemical composition irrespective of weight. % increases the amount of CO2 in the environment, though it is higher than the standard chemical composition but it does not harm the environment. Because CO2 is a greenhouse gas, the higher concentration of CO2 in the atmosphere only produces excessive heat to the environment. It is well known that the combustion of chemicals is always associated with the liberation of CO2 and the release of heat energy. Still, it positively indicates and ensures the completed combustion. Comparatively, the emission of CO is more harmful and dangerous than CO2. According to Occupational Safety and Health Administration (OSHA) standard, the permissible level of CO2 gas to the atmosphere is 5000ppm (TWA) and 0.5% of CO2 in the air. The National Institute for Occupational Safety and Health (NIOSH) standard prescribed the limit for CO2 is 30000 ppm (STEL) and 3% of CO2 in the air (Occupational Safety and Health Administration, 2015). The time weighted average and exposure period are 8 hours. STEL is a short-term exposure limit, and the exposure period is 15 minutes. In the present work, the release rate of CO2 for MCC -1 and MCC-2 is 0.1% and 0.2%. This value is less than the prescribed limit given by OSHA and NIOSH.
By comparing standards, the addition of TSP and the existing chemical composition enhanced the chemical reaction for better combustion. The complete combustion also helps to reduce the HC, and O2 emission rate in Sample 2 (MCC-2) compared to MCC-1 and NFC. The addition of TSP with a reasonable amount of carbon helps balance the requirement of sulfur.
Noise test level results revealed the sound performance of the modified chemical composition firecrackers results, and it is compared with the regular chemical composition firecrackers. It is observed from the noise level test, the generated noise level for NFC is 107.9dBA and 131.6dBC. The noise level of the modified sample MCC-1 is 93.9 dBA and 118.9 dBC and for the MCC-2 sample is 98.7 dBA and 123.7 dBC. The difference in the noise level result for NFC and MCC-1 is 14dBA and 12.7dBC. Similarly, the noise level variation for the NFC and MCC-2 is 9.2dBA and 7.9dBC. It is understood that the addition of TSP in the firecracker reduced noise level compared to NFC. But the noise level range for all the samples is within the prescribed group given by the noise pollution control board. Hence, the performance of the modified cracker is not affected. The summarized noise level result is represented in Table 4.
Table 4 Noise level test result.
| Samples | Chemical Mixture quantity (g) |
Noise level result (dB) | |
|---|---|---|---|
| dB(AI) | dB(C) | ||
| NFC | 0.3 | 107.9 | 131.6 |
| MCC-1 | 0.3 | 93.9 | 118.9 |
| MCC-2 | 0.3 | 98.7 | 123.7 |
The recommendation and guidelines given for India's pollution and by the Control Board is in the range of 125 dB (AI) and 145 dB(C) and observed result for all the samples are within the prescribed limit. Therefore, the addition of TSP would help to reduce environmental pollution significantly with cheaper biofuel materials.
It is observed from the impact sensitivity test result, the limiting impact energy for the NFC and MCC -1 sample is 3.532 Joules, and limiting impact energy for MCC-2 is 4.905 Joules. The reason for increasing the power for the MCC-2 sample is the percentage of sulfur content (10%) in the chemical mixture. Because a minimum of 10% to 17% of sulfur content is required to sustain the cracking performance of the cracker (Sivapirakasam et al., 2004), in this MCC-2 sample minimum percentage was only added. So that the sensitivity of this sample chemical mixture is reduced, if the sensitivity property of the chemical mix is declined, it will enhance the safety aspect during handling. If increasing more than 10% of TSP, the sensitivity of the chemical composition is decreased. Then it will affect the performance of the cracker.
The limiting impact energy values for all the samples are less than 5 joules, showing the susceptible category. According to Andrejev and Beljajev (1965), if the limiting impact energy range is less than 5 Joules, it might be categorized as a compassionate chemical mixture under class IV. So, the modified sample does not alter the sensitivity property of the chemical mixture.
It is observed from the friction sensitivity test, the safe friction load for NFC and MCC -1 sample is 324N. Therefore, the flash will occur when the pack is above 324N. For the MCC-2 sample, the safe frictional load is 360 N. Because the moment does not happen at the maximum load of 360 N, the safety hazards may be reduced when using the MCC-2 sample chemical mixture. But some time due to this less frictional property, chances to failure in the cracker.
4. Conclusions
The modified chemical composition firecrackers are made without comprising the performance of the firecrackers.
The addition of TSP with regular chemical composition substantially reduced the harmful gases such as CO, SO2, and other suspended particles.
The other observation is an increase in the amount of CO2 in modified chemical composition firecrackers; though it is higher, the level concentration is within the prescribed limit.
The addition of TSP within 5% would be beneficial to produce firecrackers with reduced emission and expected sound performance.
The cost of the firecracker also significantly reduces, and storage of sulfur can be minimized, which in turn lowers fire accidents.
The sensitive property of the chemical mixture for the NFC, MCC-1 sample does not change. However, for the MCC-2 sample, the sensitivity property is lesser. Thus, it may affect the performance of the cracker.
Conflict of interest
The authors have no conflict of interest to declare.
Financing
The authors received no specific funding for this work.











 nueva página del texto (beta)
nueva página del texto (beta)